Low-birth weight (LBW) infants remain at much higher risk of both neonatal mortality and morbidity than infants with normal weight at birth(Reference McIntire, Bloom and Casey1). According to a report by the WHO, LBW has been defined as weight at birth of < 2500 g(Reference Wardlaw, Blanc and Zupan2). This is based on epidemiological observations showing that infants weighing < 2500 g are approximately 20-fold more likely to die than heavier babies(Reference Kramer3). There are overwhelmingly more cases of LBW in developing countries than in developed countries(Reference Wardlaw, Blanc and Zupan2). More than 20 million infants worldwide, representing 15·5 % of all births, are born with LBW, 95·6 % of them in developing countries. Half of all LBW babies are born in south-central Asia, where more than one-quarter (27 %) of all infants weigh < 2500 g at birth. LBW is closely associated with fetal and neonatal mortality and morbidity, inhibited growth and cognitive development, and chronic diseases later in life(Reference McIntire, Bloom and Casey1, Reference Eriksson, Forsén and Tuomilehto4). Many factors have been reported as determinants of LBW, e.g. low socio-economic status (SES), maternal weight and nutritional status(Reference Torres-Arreola, Constantino-Casas and Flores-Hernández5–Reference Lone, Qureshi and Emanuel7), and they play important roles in determining the birth weight and future health of infants. Birth length has also been reported to be strongly associated with the development of the infant at 12 months(Reference Morris, Victora and Barros8). Thus, newborn size has important implications for mortality, morbidity, subsequent growth and development. It is also a reflection of prenatal growth and the intra-uterine environment.
Maternal nutrition during pregnancy has a significant effect on fetal and infant growth, as does maternal health. Ca is an essential element of nutrition and is important as a primary structural constituent of the skeleton, as well as necessary for proper soft tissue functioning in muscle contraction, nerve conduction, hormone release and other physiological actions. A fetus demands Ca for growth; therefore, a pregnant woman requires more Ca than a non-pregnant woman(Reference Kovacs9). The total Ca accretion rate of the fetus increases from approximately 50 mg/d at 20 weeks' gestation to 330 mg/d at 35 weeks' gestation. For the third trimester of pregnancy, 200 mg/d is considered the average accretion rate(Reference Prentice10). Ca for the fetus is transported via the placenta, and bone mineralisation in the fetus increases during pregnancy(Reference Prentice11). Regarding fetal growth, among infants with very LBW ( < 1500 g), their bone mineral content (BMC) and bone mass are decreased, which is related to the deficiency of a mineral, e.g. Ca(Reference Abrams, Hawthorne, Weaver and Heaney12). Previous studies revealed that rickets is more common in infants with very LBW than in heavier infants(Reference Callenbach, Sheehan and Abramson13, Reference Lindroth, Westgren and Laurin14). Other findings have shown that infant BMC is correlated with birth weight(Reference Namgung, Tsang and Specker15).
Several Ca supplementation trials for pregnant women have previously been conducted to determine the effects in improving maternal Ca nutritional status. A few of them reported that higher birth weights were associated with Ca supplementation(Reference Purwar, Kulkarni and Motghare16); however, others did not find that Ca supplementation in pregnant women with low Ca intake influenced fetal somatic growth, skeletal growth or size at birth(Reference Sanchez-Ramos, Briones and Kaunitz17–Reference Abalos, Merialdi and Wojdyla19). During pregnancy, Ca and vitamin D metabolism, e.g. Ca absorption and excretion, are physiologically altered(Reference Kovacs9, Reference Kovacs and Kronenberg20, Reference Guéguen and Pointillart21). In addition, seasonal influences might also need to be considered(Reference Verity, Burman and Beadle22–Reference Walters24). Furthermore, data on the actual status of Ca or Ca-related biomarkers in utero that might affect bone metabolism and fetal growth, not those in mothers or newborns, are meagre.
In rural Bangladesh, the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) initiated a study called ‘Maternal and Infant Nutrition Interventions in Matlab’ (MINIMat), randomising all pregnant women in Matlab to receive a combination of protein, energy and micronutrient supplements and exploring combinations of pre- and postnatal nutritional interventions to address the issues of maternal, fetal and infant malnutrition. In a subcohort of infants from the main study, we aimed to investigate the association between levels of Ca and Ca-related biomarkers in umbilical cord blood and newborn size at birth, taking into account other variables. As vitamin D levels are known to change depending on the season, we also examined seasonal effects on the levels of these biomarkers.
Experimental methods
Study area
The study area, Matlab, is located 53 km southeast of the capital Dhaka. Since 1966, the ICDDR,B has organised a health and demographic surveillance system (HDSS) in the Matlab area. The HDSS covers a population of approximately 220 000. Community health research workers visit every household on a monthly basis to update information on demographic events, i.e. marriage, pregnancy, birth, death and in- and outmigration, as well as to collect information on the morbidity of children younger than 5 years of age and women of childbearing age. In 2001, the ICDDR,B initiated the MINIMat study for subsequent investigations of the effects on size at birth, gestational age at birth, fetal loss and infant mortality.
Study population and design
In the MINIMat study, through the monthly home visit by community health workers, pregnant women were identified by a history of a missed menstrual period. Upon identification of pregnancy, usually at about 6–10 weeks of gestation, women were advised to visit the respective health facility in the area for confirmation of pregnancy by ultrasound and antenatal care. If they fulfilled certain inclusion criteria(Reference Rahman, Vahter and Smith25), the pregnant women were thereafter invited to be enrolled into the MINIMat study. Around 5000 pregnant women were recruited in the main MINIMat trial from November 2001 to October 2003. The present study was nested into the MINIMat study. For the present study, we collected cord blood from all newborns delivered at all of the Matlab subcentre clinics during daytime hours (07.00–14.30 hours) of working days (Sunday–Thursday) from April 2003 to June 2004. The term of sample collection for this nested study was the second half of the main study. Because of the logistics of the cord blood collection, we did not collect blood from newborns delivered at home or those born in the evening or at night. In Bangladesh, most pregnant women give birth at home. The proportion of subcentre clinic deliveries was 11·9 % of all pregnancies at Matlab in 2004(26).
Anthropometric measurements
Birth weight and length were measured immediately following delivery in subcentre clinics. Birth weights of all infants were measured by beam scales, which are accurate to 10 g (SECA, Hamburg, Germany). Locally manufactured collapsible length boards, which are precise to 1 mm, were used to measure the recumbent length of newborn infants. The recumbent length was measured according to standard procedures(27). Maternal weight and height were measured at the first visit by pregnant mothers to the subcentre clinics (about 8 weeks of pregnancy).
Cord blood sample
At birth, a cord blood sample from the umbilical vein was collected into lithium heparin tubes by a trained research physician/nurse at subcentre clinics. Then, cord blood was transferred to the Matlab hospital. The Matlab hospital has a supporting laboratory for sample handling, freezer storage and sample transport to the Dhaka laboratory. From the obtained cord blood, Ca, albumin, 25-hydroxy vitamin D (25-OH VD), bone-specific alkaline phosphatase (BALP) and intact parathyroid hormone (iPTH) levels were determined in the nutrition biochemistry laboratory in Dhaka. All cord blood samples were measured for Ca and albumin. For 25-OH VD, BALP and iPTH, taking into account the feasibility and the available resources, 100 samples were randomly selected. The estimated sample size of 100 was based on the primary outcome variables of birth size, previous reports in studies using cord blood and the use of Student's t test(Reference Bogden, Thind and Louria28, Reference Elizabeth, Krishnan and Vijayakumar29).
Calcium and calcium-related biomarker assessment
Cord blood was fractionated using serum separator tubes (BD Biosciences, Franklin Lakes, NJ, USA), and serum was stored at − 80°C. Serum Ca and albumin were assayed using the QuantiChrom Ca assay kit (BioAssay Systems, Hayward, CA, USA). The inter-assay CV for these analyses were 8·6 % of low-dose control and 5·2 % of high-dose control. Ca values were adjusted for albumin concentrations using the method described by Klemm & Klein(Reference Klemm, Klein, McPherson and Pincus30). Serum 25-OH VD levels were measured using the 25-hydroxyvitamin D EIA kit (Immunodiagnostic Systems Limited, Boldon, Tyne and Wear, UK). The inter-assay CV were 10·5 % for low-dose control and 10·4 % for high-dose control. Serum BALP levels were determined using the Ostase BAP kit (Immunodiagnostic Systems Limited) according to the manufacturer's recommendations. The inter-assay CV for these analyses were 6·8 % for low-dose control and 4·8 % for high-dose control. Serum iPTH levels were measured using the PTH Intact ELISA kit (DRG International, Inc., Mountainside, NJ, USA) according to the manufacturer's recommendations. The inter-assay CV for these analyses were 11·0 % for low-dose control and 10·3 % for high-dose control.
Socio-economic status
SES was estimated using a wealth index based on information about household assets and estimated by principal component analysis, producing a weighted score(Reference Gwatkin, Rustein and Johnson31). Scores were categorised into quintiles, with category 1 representing the poorest and category 5 the richest.
Statistical analysis
Maternal age and season at birth were categorised into three groups. Maternal age was grouped as follows: below 20 years old ( < 20 years); from 20 to 29 years old (20–29 years); over 30 years old ( ≥ 30 years). Season at birth was divided into three seasons in Bangladesh: the hot and dry season (March–June); the monsoon season (July–September); winter (October–February)(Reference Moore, Prentice and Wagatsuma32). As an indicator of SES, a wealth index patterned in the quintile format described earlier was included in the analysis.
First, the association between size at birth, as the dependent variable, and Ca, 25-OH VD, BALP and iPTH, as independent variables, were tested by simple linear regression. Next, a linear regression analyses were performed to select the covariates for multivariate regression analyses(Reference Neel and Alvarez6, Reference Lone, Qureshi and Emanuel7, Reference Deshmukh, Motghare and Zodpey33). The covariates were maternal weight, height, BMI, category of maternal age, parity, SES evaluated by the wealth index, educational years, gestational weeks at birth, sex of newborn and season at birth. Finally, the multivariate regression analyses were performed. Categorical variables were entered in the model by producing dummy variables. Appropriate statistical methods were selected for the data being analysed. The aforementioned analyses were conducted using the statistical package R (version 2.10.1; R Foundation for Statistical Computing, Vienna, Austria)(34).
Ethics
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Research Review and Ethical Review Committees, ICDDR,B, Dhaka, Bangladesh. Written informed consent was obtained from all subjects.
Results
Characteristics of mothers and newborns
A total of 225 cord blood samples were collected. The background characteristics of 225 mothers and newborns are presented in Table 1. The age of mothers ranged from 14 to 43 years with a mean age of 25·8 (sd 5·9) years. The mean weight and height of the women at about week 8 of gestation were 46·4 (sd 8·1, range 25·0–76·4) kg and 150·6 (sd 5·5, range 134·5–167·0) cm, respectively. Approximately one-third (28·9 %) of the women were malnourished (BMI < 18·5 kg/m2); two-fifths (40·0 %) of the women were primigravidas. Approximately half (45·3 %) of the women had completed less than seven educational years. The mean size at birth (birth weight and length) was 2812 (sd 389, range 1820–4000) g and 48·1 (sd 1·9, range 43·0–58·1) cm, respectively. Approximately twenty (19·6) % of the newborns had LBW. The mean gestational age at birth was 39·2 (sd 1·5) weeks. Approximately 7 % of the infants were born pre-term ( < 37 weeks). Outliers were identified by the univariate analysis. The two outliers were excluded for further analysis because of birth length >58 cm and iPTH levels >133 pg/ml. Serum Ca levels were determined in 223 samples.
Table 1 Characteristics of mothers, newborns, calcium and calcium-related biomarkers in cord blood
(Mean values, standard deviations, medians, interquartile ranges (IQR), number of participants and percentages)

25-OH VD, 25-hydroxy vitamin D; BALP, bone-specific alkaline phosphatase; iPTH, intact parathyroid hormone.
* Ca was adjusted for albumin.
† Two participants were excluded as outliers for further analysis.
‡ The 100 samples were randomly selected for Ca-related biomarkers. One or two samples were excluded as outliers.
Calcium and calcium-related biomarkers in cord blood
The levels of Ca in cord blood are summarised in Table 1. Ca, 25-OH VD, BALP and iPTH were not normally distributed, even after log transformation. Therefore, non-parametric tests were used for those variables. The median value of Ca was 34 (interquartile range (IQR) 22–63 mg/l; n 223). For Ca-related biomarkers, one or two samples were excluded as the aforementioned outliers. The median scores and IQR for the Ca-related biomarkers were as follows: 25-OH VD (23·8 ng/ml; IQR 18·6–30·8; n 98); BALP (16·3 μg/l; IQR 13·3–19·1; n 99); iPTH (0·5 pg/ml; IQR 0·0–5·3; n 99). Serum Ca, 25-OH VD, BALP and iPTH levels were not associated with allocation to food and micronutrient randomisation groups in the MINIMat study. Ca was not contained in the micronutrient tablets used in the MINIMat study.
Relationship among size at birth, calcium and calcium-related biomarkers
The associations among size at birth, Ca and Ca-related biomarkers were assessed by a simple linear regression analysis. The associations between Ca and size at birth were examined by scatter plots that included a linear regression line (Fig. 1). The regression analysis showed a significant association between Ca levels and size at birth (birth weight: β 1·83, 95 % CI 0·27, 3·39; birth length: β 0·012, 95% CI 0·005, 0·020). Serum 25-OH VD, BALP and iPTH levels were not significantly related to birth weight and length. For the relationship between Ca and size at birth confirmed by simple linear regression, a further multivariate analysis was conducted.

Fig. 1 Scatter plot and estimated simple linear regression equation for the association between calcium in cord blood and newborn size. (a) Birth weight (Y = 2728+1·83X; n 223; P = 0·022) and (b) birth length (Y = 47·5+0·012X; n 223; P = 0·001).
Simple linear regression analysis of size at birth, calcium and selected covariates
Table 2 shows the simple regression analysis of Ca, size at birth and selected covariates for multivariate regression analysis. For birth weight, maternal height (P < 0·001), maternal BMI (P = 0·002), categories of maternal age (P = 0·032), wealth index (P = 0·046) and gestational weeks at birth (P < 0·001) were found to be correlated significantly. For birth length, maternal height (P < 0·001), maternal BMI (P = 0·025), gestational weeks at birth (P < 0·001) and the sex of newborns (P = 0·013) were significantly associated. Ca levels in cord blood were significantly related to the sex of newborns (P = 0·011). Ca levels in cord blood were significantly different among seasons at birth (P < 0·001).
Table 2 Simple regression analysis with calcium, size at birth and selected covariates
(β-Coefficients and standard errors)
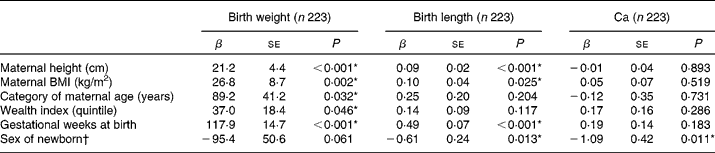
* P values were significantly different for birth weight, birth length and Ca by covariates (P < 0·05).
† Sex of newborn, females v. males.
Among covariates, maternal weight was associated with maternal height (r 0·55, P < 0·001; n 223) and maternal BMI (r 0·91, P < 0·001; n 223). Parity was significantly different in the category of maternal age (P < 0·001; n 223). The mother's educational years and wealth index were positively correlated (r 0·44, P < 0·001; n 223). Considering correlations between these covariates, maternal weight, parity and mother's educational years were excluded.
In the present study, three adjusted models were created for birth weight and birth length. Model I was controlled with the gestational weeks at birth, sex of the newborn and wealth index. For model II, covariates on maternal characteristics – maternal height, BMI and category of maternal age – were added to model I. Finally, season at birth was included in the fully adjusted multivariate model (model III).
Multivariate regression analysis between size at birth and calcium
Table 3 shows the multivariate regression analysis of size at birth (birth weight and length) and Ca in cord blood. Regarding birth weight, Ca was not correlated significantly after adjustment for gestational weeks at birth, sex of the newborn and wealth index (model I, P = 0·178). Additional adjustment for covariates on maternal characteristics (model II) did not reveal an association between Ca levels and birth weight (P = 0·138). After adjusting for season at birth (model III), no association was observed between Ca levels and birth weight (P = 0·132).
Table 3 Multivariate regression analysis of size at birth and calcium in cord blood
(β-Coefficients and standard errors)
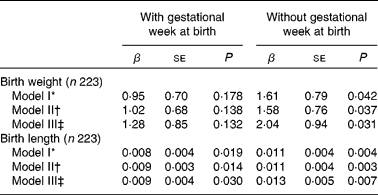
* Adjusted for gestational weeks at birth, sex of newborn and wealth index in quintile.
† Additional adjustment for maternal height, maternal BMI and category of maternal age to model I.
‡ Simultaneously adjusted for season at birth to model III.
We also examined the associations of Ca and birth length in three types of adjusted models. Ca levels were significantly associated with birth length in the multivariate model I (P = 0·019). We examined the associations of Ca and size at birth controlled for the aforementioned three covariates and covariates on maternal characteristics (model II) and confirmed a significant result (P = 0·014). Even after adjusting for season at birth, Ca levels remained significantly associated with only birth length in the fully adjusted model III (P = 0·030). Normal quantile–quantile plots were made to examine the residuals of the multivariate models. As a result, the residuals were normally distributed, and no extreme outlier existed.
Considering that the gestational weeks at birth would be an intermediate factor, the aforementioned three multivariate analyses were also conducted without the gestational weeks at birth (Table 3). The multivariate regression analysis without the gestational age at birth revealed the significant relationships between size at birth and Ca in the three models (birth weight, model I: P = 0·042; model II: P = 0·037; model III: P = 0·031; birth length, model I: P = 0·004; model II: P = 0·003; model III: P = 0·007).
Seasonal change in calcium and calcium-related biomarkers
Table 4 shows the characteristics of Ca and Ca-related biomarkers in cord blood depending on the season at birth. By using the Steel–Dwass test for multiple tests, the Ca levels in cord blood were significantly different among the three seasons. Compared with the hot and dry season, the 25-OH VD concentration in cord blood during the winter and monsoon seasons was significantly lower (Table 4). Serum 25-OH VD in cord blood was negatively correlated with Ca (ρ = − 0·21, P = 0·04; n 98). The mean BALP and iPTH concentrations in cord blood were not significantly different among the three seasons.
Table 4 Seasonal change of calcium and calcium-related biomarkers in cord blood
(Number of participants, medians and interquartile ranges (IQR))

W, winter; H, hot and dry; M, monsoon; W–H, within the winter and the hot and dry seasons; H–M, within the hot and dry and the monsoon seasons; M–W, within the monsoon and the winter seasons; 25-OH VD, 25-hydroxy vitamin D; BALP, bone-specific alkaline phosphatase; iPTH, intact parathyroid hormone.
* Ca was adjusted for albumin.
† Values were significantly different between two seasons by the Steel–Dwass test for multiple tests (P < 0·05).
Discussion
The present study found an association between the level of Ca in umbilical cord blood and infant size at birth in rural Bangladesh. In particular, the positive association of Ca levels with birth length remained after adjustment for covariates. The present study is the first to clarify the relationship between Ca levels at birth and newborn size after adjustment for other covariates. Participants of this cohort were recruited over an entire year so that seasonal variations in independent and dependent variables could be considered. In the present study, the values of Ca-related biomarkers in cord blood in rural Bangladesh were determined for the first time. Those could be considered as reference values for future studies.
Whether Ca levels in cord blood are associated with birth length are heretofore unknown. In the present study, Ca levels in cord blood had an association with birth length both by simple linear regression and by multivariate regression analysis. Birth length was also reported to be as important a factor as birth weight for infants. Morris et al. (Reference Morris, Victora and Barros8) reported that birth length was strongly associated with child development at 12 months as measured by the Denver II developmental screening test. In addition, infants who were born both short and with a low ponderal index were at increased risk of mortality and severe morbidity in infancy. Ca levels in cord blood were found to be significantly associated with birth length in the present study. These data might suggest that low Ca levels in pregnant women may negatively affect fetal bone development by limiting the amount of Ca provided to the fetus, and this would lead to a lower birth length. Chang et al. (Reference Chang, O'Brien and Nathanson35) reported that a significant association between maternal dairy intake and fetal femur growth was found among pregnant African–American adolescents. In their study, dairy intake had a significant positive effect on fetal femur growth after adjustment for gestational age, biparietal diameter, maternal age and height, and BMI. Fetal femur length was significantly lower in the lowest dairy-intake group than in the highest dairy-intake group. Even though it is not clear whether the differences in femur length during pregnancy corresponded to differences in birth length, Ca levels in pregnancy might be related to birth length in addition to bone length and fetal length. To increase newborn birth length, pregnant mothers might be recommended to consume more foods containing Ca.
Previous reports have shown that the Ca level of cord blood is related to birth weight(Reference Bogden, Thind and Louria28, Reference Elizabeth, Krishnan and Vijayakumar29). Bogden et al. (Reference Bogden, Thind and Louria28) found that Ca levels in cord blood were significantly lower in LBW babies than in control babies weighing more than 2500 g. Their result is consistent with that of Elizabeth et al. who also found lower Ca levels in LBW infants. Elizabeth et al. (Reference Elizabeth, Krishnan and Vijayakumar29) classified newborns as pre-term LBW, term LBW and term controls, and confirmed that pre-term and term LBW babies were born with significantly lower Ca levels in cord blood than term control babies. These studies have revealed that Ca levels in cord blood were associated with birth weight by simple bivariate analysis without adjustment for other predictors. In the present study, the association of Ca levels in cord blood with birth weight was demonstrated by simple linear regression. Ca is a primary component of bone, and BMC is related to birth weight and infant weight(Reference Avila-Díaz, Flores-Huerta and Martínez-Muñiz36, Reference Dennison, Syddall and Sayer37). Ca might lead to a higher birth weight via increasing Ca transfer to the fetus and increasing the BMC. LBW is closely associated with fetal and neonatal mortality and morbidity, inhibited growth and cognitive development, and chronic diseases later in life(Reference Wardlaw, Blanc and Zupan2). The prevention of LBW is beneficial for the life of the infant. Purwar et al. (Reference Purwar, Kulkarni and Motghare16) conducted Ca supplementation for pregnant women to determine the effects of maternal Ca nutritional status on fetal growth. Their results indicated that larger birth weights are associated with supplementation. However, in their study, larger birth weights were not explained by Ca levels in cord blood.
Several predictors concerning birth weight have already been reported in previous studies, e.g. SES, maternal nutrition, maternal age, height, BMI and parity(Reference Neel and Alvarez6, Reference Lone, Qureshi and Emanuel7, Reference Deshmukh, Motghare and Zodpey33). Taking into account previously reported predictors, the association between Ca levels and birth weight was not confirmed by the multivariate analysis. Birth weight, especially LBW, was known to be correlated with gestational age at birth. In the present study, the effect of gestational age on size at birth was obvious, and in particular, birth weight was more affected by gestational age than birth length. Ca has also been reported to reduce the risk of birth weight by prolonging gestation(Reference Merialdi, Carroli and Villar38). Compared with birth length, birth weight would be more explained by gestational age, and the direct effects of Ca on birth weight might be weaker than that on birth length. As a result, the association after adjustment could not be detected in this population. Otherwise, there might be a time lag until the change in the Ca level is reflected in the birth weight. Body length reflects skeletal growth, whereas birth weight may reflect more factors, e.g. body fat and muscle mass.
Previously, Ca levels have been reported to be positively correlated with gestational age(Reference Nelson, Finnström and Larsson39). Ca supplementation has been reported to prevent pre-term delivery and pre-eclampsia(Reference López-Jaramillo, Delgado and Jácome18, Reference Villar and Repke40). It has been suggested that the influence of extracellular Ca concentrations might reduce smooth muscle cell tone and increase its relaxation(Reference Villar and Repke40). The loosening of smooth muscle cells might prevent the pre-eclampsia and hypertension or pre-term delivery of the uterus, thereby increasing the number of gestational weeks. As a different viewpoint of this regard, Ca transport from the mother to the fetus increases skeletal growth from the second trimester to the third trimester of pregnancy(Reference Prentice, Laskey, Jarjou, Bonjour and Tsang41). With shorter gestational time, there might be still lower Ca amounts to be transferred through the placenta. Consequently, Ca in cord blood might be lower at early gestational age. If the gestational age might mediate the association between Ca and newborn size, the relationship between Ca and newborn size in the present multivariate analysis might be underestimated by the control of the gestational age at birth. In the present study, Ca was not significantly related to gestational age. Because of the low proportion of pre-term delivery in the present study, the relationship between Ca and gestational age might not be observed.
For maintaining adequate Ca levels in utero, Ca supplementation for low Ca intake might be useful. Several studies of Ca and/or vitamin D supplementation for pregnant women have already been conducted, of which many studies have indicated a reduced risk of pregnancy-induced hypertension and pre-eclampsia(Reference Purwar, Kulkarni and Motghare16, Reference Sanchez-Ramos, Briones and Kaunitz17). Ca levels in cord blood have already been reported to be correlated with maternal Ca status(Reference Schauberger and Pitkin42). It appears that increasing Ca levels in pregnancy with those supplements will lead to an increase in Ca in utero, and that the positive effects of Ca supplementation on fetal growth or newborn size will result. In a systematic review, the overall results of trials of Ca supplementation demonstrated a significant 17 % reduction in the risk of LBW(Reference Merialdi, Carroli and Villar38). However, some trials have found that Ca supplementation in pregnant women with low Ca intake does not appear to have an impact on birth weight and length(Reference Sanchez-Ramos, Briones and Kaunitz17–Reference Abalos, Merialdi and Wojdyla19, Reference Villar and Repke40, Reference Belizán, Villar and Gonzalez43). From the present results, if the appropriate amount of Ca is transported in utero and is kept at appropriate levels, size at birth might be significantly improved. During pregnancy, physiological mechanisms involved in Ca homeostasis are also known to be altered because Ca metabolism, e.g. Ca absorption, renal Ca excretion, Ca resorption, Ca transportation via the placenta and increases in bone turnover in pregnancy and lactation, is working complexly for the fetus and the mother(Reference Prentice10, Reference Kovacs and Kronenberg20, Reference Kovacs44). Taking this into account, a method to maintain appropriate Ca levels might be important. We also need to consider the amount of Ca supplementation with respect to other factors, e.g. geography, nutritional status or cultural habits. Bangladeshi women have been reported to usually have low Ca intake status(Reference Islam, Lamberg-Allardt and Kärkkäinen45), and there is also a high prevalence of rickets in a few areas in Bangladesh(Reference Kabir, Rahman and Talukder46), although none of these areas was included in the present study field.
Other interesting findings of the present study were the significantly higher values of 25-OH VD levels in the hot and dry season (March–June) and the differences in the Ca levels among the three seasons. In the present study, even though the season at birth did not affect birth weight and length, a seasonal difference in 25-OH VD/Ca levels was confirmed. In Bangladesh, lower amounts of UV are radiated in the monsoon season, which means that synthesis and 25-OH VD stock might decrease in that season, and that Ca levels subsequently decline. The maternal level of 25-OH VD changes seasonally. 25-OH VD is transported from the mother via the placenta to the fetus(Reference Greer47). Seasonal differences in maternal 25-OH VD levels might give rise to the different maternal Ca levels. Ca is also transported from the mother via the placenta to the fetus, and transported Ca is correlated with the maternal Ca level(Reference Schauberger and Pitkin42). Consequently, the Ca levels in cord blood might be changed simultaneously with the maternal Ca level. There would be a time interval between the change in the 25-OH VD level and that in the Ca level because of the pathways of metabolism and transport. Unfortunately, maternal levels of 25-OH VD and Ca during pregnancy or maternal BMC as the indicator of total Ca status were not measured in the present study. Earlier studies have revealed seasonal variations in serum 25-OH VD concentrations in both pregnant women and their newborn infants, with low values in winter(Reference Verity, Burman and Beadle22, Reference Kuroda, Okano and Mizuno48). Namgung et al. found significant seasonal differences; summer-born infants had significantly lower BMC, higher 1,25-vitamin D levels and lower total Ca levels than winter-born infants. A positive relationship has also been reported between BMC and birth weight(Reference Namgung, Tsang and Specker15). According to the present results, the protective effects of vitamin D supplementation would be more expected for pregnant women during the winter and monsoon seasons in Bangladesh.
The present study reported Ca-related biomarker values of cord blood in Bangladesh. Even though newborns with LBW usually tend to have low Ca levels(Reference Bogden, Thind and Louria28) and 20 % of newborns were LBW in the present study, Ca levels were lower than the usually reported value of 90 mg/l(Reference Kratz and Lewandrowski49, Reference Namgung, Tsang and Specker50). Poor Ca intake by Bangladeshi women has been reported. Islam et al. (Reference Islam, Lamberg-Allardt and Kärkkäinen45) reported that more than half of the women in the low-SES group had Ca intake below 200 mg/d. In their study, 95 % of women in the low-SES group and 47 % of those in the high-SES group had a daily Ca intake on average of < 400 mg/d. Low Ca intake might influence low Ca levels in cord blood. Among different ethnicities, the metabolism of Ca and Ca-related biomarkers has been reported to be different(Reference Dibba, Prentice and Laskey51). In addition, it is known that the season, meteorology or environmental toxic materials might affect the Ca levels(Reference Namgung, Tsang and Specker50, Reference Silbergeld52).
The mean values of 25-OH VD and iPTH were similar to previously reported data(Reference Namgung, Tsang and Specker50, Reference Fischer, Rahman and Cimma53). Concerning 25-OH VD, BALP and iPTH, there were no associations with newborn size in the present study. Nonetheless, there have been previous reports indicating the relationships between size at birth and Ca-related biomarkers. Leffelaar et al. (Reference Leffelaar, Vrijkotte and van Eijsden54) indicated that infants from women with deficient vitamin D levels had lower birth weights than those from women with adequate vitamin D levels in a large multiethnic cohort. In the vitamin D supplementation trials in the Cochrane database, mothers had a lower number of LBW infants(Reference Mahomed and Gulmezoglu55). Morely et al. (Reference Morley, Carlin and Pasco56) reported that the maternal parathyroid hormone concentration at 28–32 weeks was positively related to knee–heel length, birth weight, and mid-upper arm and calf circumferences of infants at birth independently. Regarding the relationship between bone growth and Ca-related biomarkers, previous studies suggested that maternal vitamin D status affects bone mineral accrual during the intra-uterine period and influences bone size(Reference Morley, Carlin and Pasco56, Reference Viljakainen, Saarnio and Hytinantti57). These findings suggest that Ca-related nutritional factors have an aetiological function for newborn size.
The strength of the present study is that we could utilise cord blood. By using cord blood, we were able to evaluate the actual Ca status of the newborn at birth and to analyse the association of Ca with size at birth. Increased Ca levels in utero were clarified to be directly associated with birth length. Moreover, samples were obtained from the large population under the HDSS. By utilising the HDSS, characteristics of the mother and household were obtained appropriately, and following the participants in the future will be possible.
The present study had some limitations. First, the sample size was small. Blood samples were taken from only a minority of the main study subjects. Caution should be taken in applying the present results to all newborns, and further confirmation is required. Next, we did not consider the food intake of the mothers during pregnancy. The effects of food intake and Ca status of pregnant women were not considered in the present analysis.
The present study demonstrated a significant association of Ca levels in cord blood with birth size among Bangladeshi newborns. In particular, the relationship between Ca levels in cord blood and birth length was not altered by the adjustment for gestational age at birth, maternal factor and SES. The present study also found that Ca levels in cord blood were low in Bangladesh, where the prevalence of maternal malnutrition is high. Ca may play an important role in fetal growth in this population. Further studies will be required to confirm the findings in other areas.
Acknowledgements
The MINIMat study was funded by the ICDDR,B; United Nations Children's Fund; the Swedish International Development Cooperation Agency; the United Kingdom Medical Research Council; the Swedish Research Council, Department for International Development; Child Health and Nutrition Research Initiative; Uppsala University; the US Agency for International Development; and a Grant-in-Aid for Scientific Research of the Japan Society for the Promotion of Science (no. 18256005). M. D. contributed to the design of the study, data analysis and writing of the manuscript. R. S. R. conducted the data collection, editing of the data and laboratory assessment. S. A. performed the data collection and laboratory assessment. M. O. interpreted the data. A. K. R. performed the biomarker assessment. S. E. A. contributed to the overall design and implementation of the MINIMat study. E.-C. E. provided expertise on micronutrient analysis and scientific comments to improve the manuscript. R. R. contributed to the design and implementation of the present study in Bangladesh, interpretation of the data and writing of the manuscript. Y. W. assisted in designing and implementing the study, interpreting the data and writing of the manuscript. All authors read and approved the final version of the manuscript. None of the authors has a personal or financial conflict of interest to declare. We thank the staff of the MINIMat study. We are grateful to Ms Marianne Kimura for editing the manuscript. Drs Hideto Takahashi and Embo Ma are acknowledged for their valuable comments to improve the manuscript.







