In China, universal salt iodisation (USI) as a national strategy has been successfully implemented since 1995( Reference Zhao and Haar 1 ). As the implementation of USI, six national iodine deficiency disorder (IDD) surveys have been conducted, and the goitre rate decreased from 9·6 % in 1997( Reference Lv, Liu and Sun 2 ) to 2·6 % in 2014( 3 ). Thus, the consumption of iodised salt is an effective means for reducing the prevalence of iodine deficiency goitre. Furthermore, the median urinary iodine concentration (MUI) of children was 197·9 µg/l in 2014, which lies within the range of ‘adequate iodine’ nutrition. In populations with long-standing iodine deficiency, a rapid increase in iodine intake is associated with the risk of iodine-induced hyperthyroidism( 4 ). This adverse condition usually occurs 5–10 years after the introduction of iodised salt( Reference Todd, Allain and Gomo 5 , Reference Stanbury, Ermans and Bourdoux 6 ). After that period, an MUI as high as 300 μg/l was not associated with thyroid dysfunction in populations consuming adequately iodised salt( 4 ). In Iran, consumption of iodised salt, containing 40 parts per million of iodine, did not cause an increase in the prevalence of thyroid dysfunction after 12 years of salt iodisation( Reference Azizi, Navai and Fattahi 7 ). Two national studies from Poland have shown that a rapid rise in iodine intake 2 years after implementation of iodine prophylaxis among adults from areas with a high prevalence rate of iodine deficiency led to an increase in thyroid autoimmunity and the prevalence of hyperthyroidism( Reference Gołkowski, Buziak-Bereza and Trofimiuk 8 ). However, investigations regarding the adverse effects of long-term USI programmes are rarely reported( Reference Clar, Wu and Liu 9 ), except for a few reports on goitre associated with excessive iodine intake in children( Reference Chen, Li and Wu 10 ).
The MUI in children has been used as a proxy indicator of iodine nutrition status for all populations. A recent multination study suggested that in school-age children, for the assessment of iodine nutrition, a single category of ‘sufficient iodine’ (MUI 100–299 µg/l, Zimmermann et al.) can be used to replace the current ‘adequate iodine’ (MUI 100–199 µg/l, WHO) and ‘above requirement’ (MUI 200–299 µg/l, WHO) statuses( Reference Zimmermann, Aeberli and Andersson 11 ). However, this study was conducted only in children and was based on the children’s thyroid function, without considering the potential effects on the thyroid function of other populations living in the same area.
The aims of this study were: (1) to assess the extent of thyroid dysfunction in Chinese children, adults, pregnant women and lactating women after 15 years of USI implementation, (2) to compare the prevalence of thyroid dysfunction in children with apparent optimal (or adequate) iodine and above requirement iodine statuses and (3) to verify whether the prevalence rates of thyroid dysfunction in adults, pregnant women and lactating women differ between areas of adequate iodine status and above requirement iodine status.
Methods
Sampling methods
A cross-sectional survey was conducted. The Yangtze River and Yellow River divide China into its southern, central and northern regions. From each region, two provinces representing urban and rural settings, respectively, were selected (southern: Fujian and Chongqing; central: Shandong and Anhui; and northern: Gansu and Jilin) (Table 1). In each province, one community (or township in a rural area) was chosen according to the economic level (medium level), coverage rate of iodised salt (>95 %) and iodine concentration in drinking water (<10 µg/l). In each surveyed community, 100 school children aged 8–10 years (girls/boys, 1:1), 100 adults aged 18–45 years (women/men, 1:1), fifty pregnant women (evenly distributed according to trimester of pregnancy) and fifty lactating women were recruited from schools (children), households (adults) and clinics (pregnant women and lactating women). All participants had lived in the selected areas for more than 12 months, did not have any self-reported thyroid diseases, and were not consuming antithyroid or thyroid hormone medicines before the investigation. The final sample size is presented in Table 1.
Table 1 Basic information of survey sites in six provinces
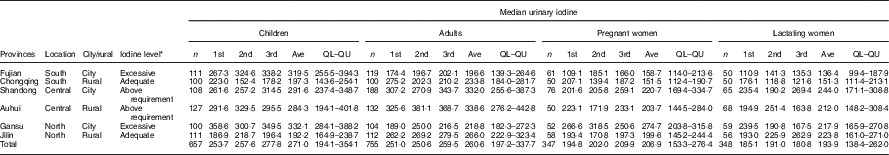
Ave, average of three times, 1st the first sample, 2nd the second sample, 3rd the third sample in 10 d; QL–QU, lower and upper quartiles.
* Based on children’s median urinary iodine for three times.
Sample collection and measurement
The participants’ spot urinary samples were collected three times during a 10-d period. Venous blood samples were obtained from all participants on the 1st day of urinary sample collection. Serum thyroid function and antibody levels were determined at the special test centre of Tianjin Medical University General Hospital. Free triiodothyronine (FT3), free thyroxine (FT4), total triiodothyronine (TT3), total thyroxine (TT4) and thyroid-stimulating hormone (TSH) were measured using a chemiluminescent immunoassay (Bayer ADVIA Centaur System); thyroglobulin (Tg), thyroglobulin antibody (TgAb) and thyroid microsomal antibody (TMAb) were measured using the RIA method (China Institute of Atomic Energy). Urinary iodine concentration was measured by the participating provincial laboratories by using the acid digestion method (WS/T107-2006)( 12 , Reference Yan and Chen 13 ); and the internal quality control samples of urinary iodine were provided by the China National Iodine Deficiency Disorders Reference Laboratory, which is a member of the Programme for Ensuring the Quality of Iodine Procedures.
Diagnostic criteria
An iodine nutrition status of sufficient iodine in children is defined as MUI 100–299 µg/l according to Zimmermann et al.( Reference Zimmermann, Aeberli and Andersson 11 ), adequate iodine is defined as MUI 100–199 µg/l, and above requirement is defined as MUI 200–299 µg/l according to the WHO guidelines( 4 ). Whether the adequate iodine and above requirement groups can be combined to form a sufficient iodine group depends on the differences in thyroid dysfunction in adults, pregnant women and lactating women between two areas with child iodine nutrition of adequate iodine and above requirement statuses. If no differences are observed, they can be combined. In the present study, the criteria for diagnosing serum thyroid dysfunction in children and pregnant women were the standard criteria established by Tianjin Medical University( Reference Lin, Sun and Li 14 – Reference Kapelari, Kirchlechner and Högler 15 ); the criteria for adults and lactating women were established by the special test centre of Tianjin Medical University General Hospital. All reference values listed in Table 2 were established based on Chinese data as well as international criteria, and thyroid function references for pregnant women were specific to the trimester of pregnancy( Reference Yan, Dong and Dong 16 , Reference Panesar, Li and Rogers 17 ). The diagnosis criteria for thyroid dysfunction included cut-offs for individual indicators and for proportions indicating high prevalence or public health significance. Regarding individual indicators, the normal reference values of FT4 in whole blood were 13·4–20·6 pmol/l in children and 11·5–23·5 pmol/l in adults and lactating women; in the first, second and third trimesters of pregnancy, the normal reference values of FT4 in whole blood were 11·8–21·0, 10·6–17·6 and 9·2–16·7 pmol/l, respectively. The normal reference values of TSH levels were 1·0–8·40 mIU/l in children; 0·3–5·0 mIU/l in adults and lactating women; and 0·03–4·51, 0·05–4·50 and 0·35–4·54 mIU/l in the first, second and third trimesters of pregnancy, respectively. The reference values for Tg status, TgAb levels and TMAb levels as individual indicators were <25 ng/ml, <30 % and <25 %, respectively, in the four groups. The prevalence of thyroid dysfunction was used for determining effects of the USI programme on public health, and prevalence of ≥5 % was defined as a public health problem( 4 ). The prevalence rates of thyroid dysfunction in adults, pregnant women and lactating women were compared between areas with child MUI in the adequate iodine and above requirement ranges to determine if it is appropriate to combine them into a single category.
Table 2 The thyroid abnormal of four populations in six provinces based on universal salt iodisation for more than 15 years (Percentages and numbers)
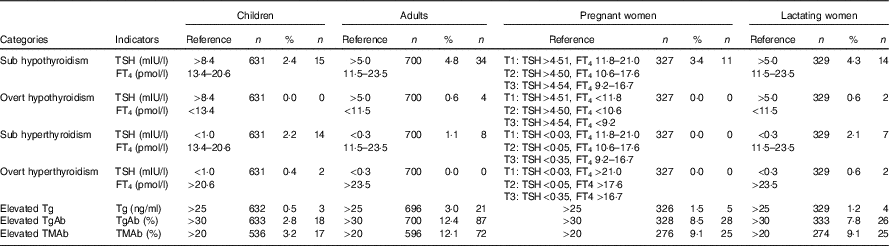
TSH, thyroid-stimulating hormone; FT4, free thyroxine; Tg, thyroglobulin; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody.
Statistical analysis
The SPSS (version 20.0; Polar Engineering and Consulting) and WPS Excel 9.1 (Beijing and Zhuhai Kingsoft Software Company) software packages were used for data analysis. The sample size of each province was determined by the variation of the urinary iodine concentration; when the variation of MUI was 10 %, the sample size was 200( Reference Andersen, Karmisholt and Pedersen 18 ). The prevalence rates of thyroid dysfunction, including subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, overt hyperthyroidism and elevated levels of Tg, TgAb and TMAb were separately calculated for the four population groups. Then, thyroid dysfunction prevalence and thyroid function results were compared between two provinces with MUI in the adequate iodine (child MUI at 100–199 μg/l) range and two provinces with MUI in the above requirement (child MUI at 200–299 μg/l) range for the four population groups. The median, upper and lower quartiles were calculated to describe the variables with a skewed distribution, such as the urinary iodine concentration and levels of TSH, Tg, TgAb and TMAb. Means and standard deviations were used to describe variables with a normal distribution, such as levels of FT3, FT4, TT3 and TT4. The χ 2 test was used to evaluate differences in thyroid abnormality prevalence. The Mann–Whitney U test and one-way ANOVA were used for comparisons of skewed and normally distributed variables, respectively. The mean difference and 95 % CI were also calculated for each comparison pair. The results were considered statistically significant when P<0·05 throughout the study.
Ethics committee approval
This study was conducted according to the guidelines established in the Declaration of Helsinki, and all procedures involving human volunteers were approved by the Ethics Committee of Harbin Medical University (no. HMUe09.n3). Written informed consent was obtained from the pregnant women, lactating women and adults, and permission was obtained from guardians of the children.
Results
Thyroid dysfunction in four populations
The basic information of each province and each population group is presented in Table 1. In total, 657 children, 755 adults, 347 pregnant women and 348 lactating women were recruited during the study. No abnormally high prevalence (>5 %) of thyroid dysfunction (subclinical or overt hyper- or hypothyroidism) was observed in any of the population groups. However, the elevated TgAb level prevalence rates in the adults, pregnant women and lactating women were 12·4, 8·5 and 7·8 %, respectively, and the elevated TMAb level prevalence rates in these populations were 12·1, 9·1 and 9·1 %, respectively (Table 2).
Comparison of thyroid dysfunction prevalence between regions with median urinary iodine concentration in the adequate iodine and above requirement ranges
To determine whether the prevalence of thyroid dysfunction differed between populations with MUI in the adequate and above requirement ranges, Jilin (192·2 μg/l) and Chongqing (197·3 μg/l) were designated as the adequate iodine group, whereas Anhui (284·3 μg/l) and Shandong (291·6 μg/l) were designated as the above requirement group. Results for Fujian (319·5 μg/l) and Gansu (332·1 μg/l) are presented in the online Supplementary Table S1 together with the interquartile range because MUI in the school-aged children from these provinces was >300 μg/l, which is beyond the range with which the study is concerned. For comparisons of the prevalence of thyroid dysfunction (including subclinical hypothyroidism; overt hypothyroidism; subclinical hyperthyroidism; overt hyperthyroidism; and elevated Tg, TgAb and TMAb levels; upper parts of Tables 3 and 4, online Supplementary Tables S2 and S3), the public health cut-off was set for reference as 5 %( 4 ). The above requirement group did not exhibit a higher thyroid dysfunction rate in all four populations compared with the adequate iodine group. In the adults, the above requirement group had higher prevalence rates of elevated TgAb levels (>30 %) and elevated TMAb levels (>20 %) than the adequate iodine group did. However, among the pregnant women and lactating women, the above requirement group exhibited lower prevalence rates of elevated TgAb levels and elevated TMAb levels than the adequate iodine group did, although the differences were not statistically significant.
Table 3 Thyroid dysfunction and thyroid function parameters of children and adults by different iodine status areas (Percentages and numbers; mean differences and 95 % confidence intervals; medians and lower and upper quartiles (QL–QU); mean values and standard deviations)
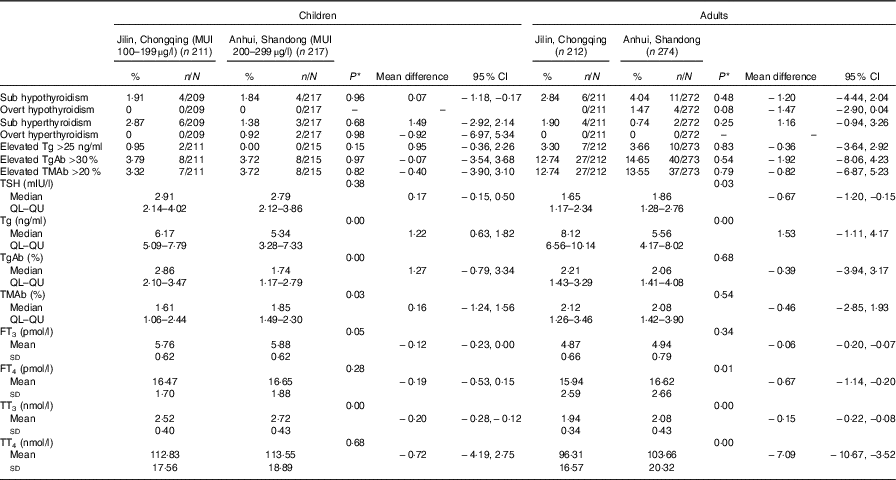
MUI, median urinary iodine concentration; Tg, thyroglobulin; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TT3, total triiodothyronine; TT4, total thyroxine.
*One-way ANOVA was used for FT3, FT4, TT3 and TT4; Mann–Whitney U test was adopted for TSH, Tg, TgAb and TMAb; χ 2 test was used for subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, overt hyperthyroidism, elevated Tg, Elevated TgAb and elevated TMAb. P<0·05 was considered significant.
Table 4 Thyroid dysfunction and thyroid function parameters of pregnant women and lactating women by different iodine status areas (Percentages and numbers; mean differences and 95 % confidence intervals; medians and lower and upper quartiles (QL–QU); mean values and standard deviations)
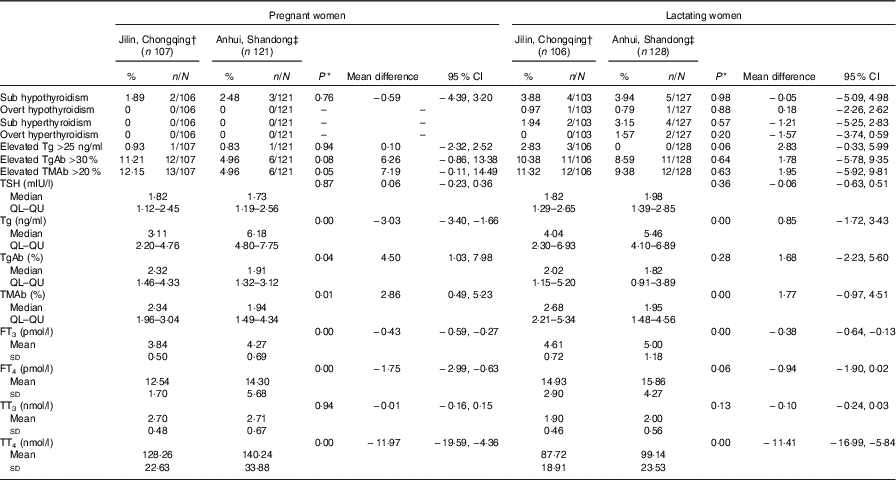
Tg, thyroglobulin; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TSH, thyroid stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine; TT3, total triiodothyronine; TT4, total thyroxine.
* One-way ANOVA test was used for FT3, FT4, TT3 and TT4; Mann–Whitney U test was adopted for TSH, Tg, TgAb and TMAb; χ 2 test was used for subclinical hypothyroidism, overt hypothyroidism, subclinical hyperthyroidism, overt hyperthyroidism, elevated Tg, Elevated TgAb and elevated TMAb. P<0·05 was considered significant.
† Adequate, Jilin and Chongqing.
‡ Above requirement, Anhui and Shandong.
In comparisons of the mean or median thyroid function parameters and thyroid antibody levels (lower parts of Tables 3 and 4, online Supplementary Tables S2 and S3), the adequate iodine group was considered the control group and the above requirement group was considered the experimental group. If no difference was observed between the means or medians of the two groups, they could be combined to form a single sufficient iodine group. The major significant differences between the two groups were in the Tg, TgAb and TMAb levels. For the children and adults, the Tg level was lower in the above requirement group than in the adequate iodine group; however, the level was higher in the pregnant and lactating women in the above requirement group than in the adequate iodine group. In the children, the above requirement group had a lower TgAb level and a higher TMAb level than the adequate iodine group did. Among the adults, no significant differences in the levels of the two antibodies were observed between the two groups. Among the pregnant women and lactating women, the TgAb and TMAb levels were both lower in the above requirement group than in the adequate iodine group. For other parameters, minor statistically significant differences were observed in the TSH, FT3, FT4, TT3 and TT4 levels between the two groups. Differences in the TSH levels between the groups were not consistent among the populations. In the children and pregnant women, the TSH levels were lower in the above requirement group than in the adequate iodine group; however, in the adults and lactating women, the TSH level was higher in the above requirement group than in the adequate iodine group. The FT3, FT4, TT3 and TT4 levels in the above requirement group were all higher than those in the adequate iodine group in the four populations.
Overall, among the four population groups, the above requirement group exhibited higher thyroid hormone levels (FT3, FT4, TT3 and TT4) than the adequate iodine group did; however, the thyroid dysfunction prevalence was similar between the two groups.
Discussion
Features of iodine nutrition indicators
All indicators for assessing iodine nutrition, including urinary iodine, thyroid function, thyroid volume and thyroid dysfunction rate, have three features, namely collectiveness, time and variance. The collectiveness feature determines whether an indicator can be analysed at the individual level or population level; for example, MUI is used to evaluate population iodine nutrition as a collective indicator. The time feature pertains to the duration needed to reflect a change after iodine intake modification. Urinary iodine concentration only reflects iodine intake over the past few days( Reference Nath, Moinier and Thuillier 19 , Reference Jahreis, Hausmann and Kiessling 20 ), whereas the thyroid volume changes relatively slowly( Reference Aghini-Lombardi, Antonangeli and Pinchera 21 , Reference Zimmermann, Hess and Adou 22 ), and each index of thyroid function requires different periods to respond to iodine deficiency; for example, T4 might require 4 weeks, whereas T3 might require 5 weeks( Reference Clifford, Bertram and Mark 23 ). The variation feature concerns variation among measurements when the individual iodine nutrition status is stable; for example, the urinary iodine concentration could vary by >100 µg/l from morning to evening each day. Sometimes, problems arise in analyses of the relationship between two iodine nutrition indices, which could be attributed to differences in the collectiveness, time and variation features between two analysed indices. Analysing two indices with different collectiveness features would result in biological fallacy( Reference Li 24 ), and two indices with different time and variation features would possibly exhibit a low correlation coefficient( Reference Shakya, Gelal and Lal Das 25 , Reference Hwang, Lee and Lee 26 ).
Thyroid dysfunction after universal salt iodisation
Undeniably, the use of iodised salt has improved iodine intake, as indicated by increased urinary iodine concentrations, a considerable reduction in the prevalence of goitre, and improvement of the population intelligence quotient (IQ)( Reference Qian, Wang and Watkins 27 ). In this study, the thyroid dysfunction prevalence was assessed after more than 15 years of USI in China. The prevalence of subclinical and overt hyper- and hypothyroidism was <5 % in all population groups, suggesting that more than 15 years of USI has not increased thyroid dysfunction prevalence. This is crucial in assuring the safety of USI and ensuring that it does not cause any side effects on thyroid function. Similar results have been reported in other countries, such as India and Bangladesh( Reference Ranganathan 28 , Reference Parveen, Latif and Kamal 29 ). Furthermore, a longitudinal study conducted before and after USI in Switzerland showed that USI had no pathological side effects; similar results were observed in a study from Denmark( Reference Als, Haldimann and Minder 30 , Reference Krejbjerg, Bjergved and Pedersen 31 ). Other studies have reported the existence of an increase in thyroid autoimmunity and hyperthyroidism in adults, but these conditions were transient( Reference Gołkowski, Buziak-Bereza and Trofimiuk 8 ). A recent WHO systematic review published in 2014 demonstrated the benefit of salt iodisation, but it had little evidence of adverse effects of salt iodisation( Reference Aburto, Abudou and Candeias 32 ).
In this study, the prevalence of elevated TgAb and TMAb levels in the adult population was 12·4 and 12·1 %, respectively. A high thyroid antibody positivity rate was reported in the normal adult population; specifically, in Xinjiang, China, the prevalence of TgAb positivity was 23·2 %, and that of thyroid peroxidase antibody (TPOAb) was 16·6 %( Reference Wang, Osiman and Ma 33 ). A study in Washington, DC, USA, reported that women with MUI 100·1 µg/l had a TPOAb positivity rate of 8 % and TgAb positivity rate of 15 %. Overall, 16 % women tested positive for at least one thyroid antibody (TPOAb and/or TgAb)( Reference Stagnaro-Green, Dogo-Isonaige and Pearce 34 ). In Saudi Arabia, 26 % of people aged 13–60 years tested positive for TPOAb and TgAb( Reference Jammah, Alshehri and Alrakhis 35 ).
Although the consequences of thyroid antibody positivity in the normal adult population are not clear, some studies have suggested potential adverse consequences in pregnant women. Studies have reported that pregnant women with TPOAb positivity during pregnancy had an increased risk of postpartum thyroiditis( Reference Chen, Jin and Xia 36 ) and are possibly associated with anaemia and a high mean platelet count( Reference Gur, Karadeniz and Inceefe 37 ). In women without autoimmune disease or hereditary thrombophilia, thyroid autoantibodies might directly increase the risk of recurrent pregnancy loss and obstetric complications( Reference Mumusoglu, Beksac and Ekiz 38 , Reference van den Boogaard, Vissenberg and Land 39 ). The prevalence of thyroid antibodies (TPOAb and TgAb) is also associated with a higher incidence of adverse pregnancy outcomes, such as miscarriage, premature delivery, abruption of the placenta, and postpartum thyroiditis( Reference Negro, Formosa and Mangueri 40 ). Thyroid autoimmunity independent of thyroid dysfunction could have significant adverse outcomes in the mother and fetus( Reference Saki, Dabbaghmanesh and Ghaemi 41 ). Although the aforementioned studies present the adverse results of thyroid antibody positivity in pregnant women, some of them did not eliminate the influence of preexisting overt or subclinical thyroid disease during pregnancy, and some did not describe the iodine nutrient status of the participants; both might be confounding factors. Hence, data regarding the adverse effect of thyroid antibody positivity on pregnancy outcomes in euthyroid women remain inadequate. In the current study, the prevalence of thyroid antibodies was similar to that in the aforementioned studies. Although the prevalence of elevated thyroid antibody levels in the above requirement group was higher than that in the adequate iodine group in adults, it was lower in the pregnant women and lactating women. If thyroid antibodies are determined to be associated with the iodine status, this finding indicates that in areas where children have an adequate iodine level, pregnant and lactating women might be deficient, and adults might be above requirement. However, this assumption requires additional research for confirmation.
Criteria for iodine sufficiency
To determine whether combining the criteria of the adequate iodine and above requirement categories based on MUI for children is appropriate, the differences in thyroid dysfunction between the two categories must be considered. In comparisons of the thyroid dysfunction prevalence in all four population groups, no statistically significant differences were found between the two categories; hence, combining the adequate iodine and above requirement ranges as sufficient iodine for children’s iodine nutrition based on MUI might not increase thyroid abnormality in children and adults, including pregnant women and lactating women. However, increases in antibody levels in adults and some thyroid hormone indices in other populations indicate some potential risks associated with changing the range of children’s urinary iodine from adequate (100–199 µg/l) to sufficient (100–299 µg/l); thus, this decision must be made cautiously.
Strengths and limitations
The data of this study were obtained from six provinces covering three broad subnational regions, providing a satisfactory representation of the Chinese situation. For comparison between adequate iodine and above requirement, two provinces together achieved a sample size above 200 for children and adults, and above 100 each for pregnant and lactating women, which according to the reference, had a variation of MUI between 5 and 10 %( Reference Andersen, Karmisholt and Pedersen 18 ). In most surveys, a single spot urine sample is collected from the participants. In this study, three urine samples were collected in a 10-d period to control for the day-to-day urinary iodine variation in an individual. The variation of MUI was decreased considerably; however, larger sample size would reduce the variation further. In this study, potential side effects on thyroid function were investigated after more than 15 years of USI in four populations of participants who reported being healthy. Those who had received diagnoses of thyroid disease were not included in the evaluation. Because of the limited volume of serum, some tests were not performed for all participants. Unlike a previous study which used children’s thyroid function as the evaluation standard to justify the combination of urinary iodine ranges( Reference Jukić, Zimmermann and Granić 42 ), the present study used thyroid dysfunction in four population groups living in the same area. Additional studies on this topic should also consider the goitre rate, thyroid volume, TSH of newborns and IQ of young children.
Conclusions
The Chinese IDD prevention and control programme has been deemed as one of the most successful programmes globally. More than 15 years after the USI programme was implemented in China, no increase in thyroid dysfunction prevalence was found in four populations. Changing the range of children’s urinary iodine from adequate to sufficient might not increase the thyroid abnormality rate in children and adults, including pregnant women and lactating women; however, it might increase their thyroid hormone level. Therefore, the range should be cautiously changed. Furthermore, adults, particularly pregnant women, positive for thyroid antibodies should be closely monitored.
Acknowledgements
The authors appreciate all organisations and all team members of the Eleventh Five-year National Support Project for Science and Technology and National nature science project; the Chongqing, Gansu, Anhui, and Fujian provincial Centres for Disease Control; the Institute for Endemic Disease Control in Shandong and Jilin; and the Institute of Endocrinology, Tianjin Medical University. The authors also appreciate the constructive suggestion concerning the manuscript from professor Cres. J. Eastman.
This study was funded by Eleventh Five-year National Support Project for Science and Technology(2006BAI06B05) and National Nature Fund (81773370). The sponsors had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
X. S., H. S. and S. L. designed the research; Y. W., Z. C., J. W., W. L., H. C. and B. X. carried out the field survey; X. S., P. J. K. and Y. H. analysed the data; P. L., M. L. and P. J. K. wrote the paper; S. L. and P. L. have primary responsibility for final content. All authors have read and approved the final manuscript.
The authors declare that they have no financial relationships with any organisations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518000570







