It is well known that oxidative stress can be very harmful to mammalian tissues, particularly the intestine( Reference Circu and Aw 1 , Reference Yin, Ren and Liu 2 ). Health status is significantly affected and performance is decreased because of oxidative stress in pigs( Reference Lykkesfeldt and Svendsen 3 – Reference Yin, Liu and Ren 7 ). Weanling piglets are subjected to various challenges, such as change in the nutritional source, feed contamination with mycotoxins, pathogenic micro-organisms and some chemical agents as well as the use of drugs and vaccine factors, which might produce excessive amounts of reactive oxygen species (ROS) and induce oxidative stress( Reference Askew 8 , Reference He, Tang and Ren 9 ). Oxidative stress induces cell apoptosis, inhibits cell proliferation, suppresses intestinal development and disturbs intestinal function( Reference Yang, Xiong and Wang 10 – Reference Yin, Wu and Li 13 ). Further, the intestinal cells are rich in mitochondria( Reference Jeynes and Altmann 14 ), which are the main sites for ROS generation( Reference Figueira, Barros and Camargo 15 ). At the same time, the intestine is highly susceptible to oxidative damage because of its frequent interaction with O2 metabolism or luminal oxidants from the intake of nutrients, micro-organisms or through infections( Reference Duan, Yin and Wu 16 , Reference Hussain, Tan and Yin 17 ).
l-Arginine (l-Arg) is not only an essential amino acid in young piglets but also a functional amino acid. Numerous research studies show that l-Arg can function as a potential substance against oxidative stress( Reference Suschek, Schnorr and Hemmrich 18 – Reference Southern and Baker 21 ). Our previous study showed that supplementation with Arg can ameliorate the negative effect induced by diquat, an initiator of ROS production, through the enhancement of antioxidant capacity and attenuation of negative effects of feed intake in piglets( Reference Zheng, Yu and He 5 ). The intestine plays a key role in Arg absorption, endogenous synthesis and metabolism in addition to functioning as the site for maintaining Arg homoeostasis( Reference Wu, Bazer and Davis 22 ). Studies show that Arg is predominantly transported across the intestinal membrane via Na+-independent cationic amino acid transporters (CAT) including CAT-1, CAT-2 and CAT-3 isoforms( Reference Closs, Simon and kony 23 , Reference Pan, Choudry and Epler 24 ). Changes in Arg transport activity in the intestine reflect the status of Arg metabolism in intestines as well as in the whole body. Research studies also found that catabolic disease increased intestinal Arg transport in rats and in Caco-2 cell cultures( Reference Pan, Choudry and Epler 24 ). Besides, the intestine is the most important site for Arg and citrulline synthesis by pyrroline-5-carboxylate (P5C) synthase( Reference Wu, Borbolla and Knabe 25 ), because other major tissues including the liver, kidney and skeletal muscle lack P5C synthase for synthesising citrulline from glutamine or glutamate( Reference Wu, Davis and Flynn 26 ). As arginase and nitric oxide (NO) synthase use Arg as the common substrate, arginase may play a role in regulating NO synthesis by modulating Arg availability( Reference Morris 27 , Reference Mori and Gotoh 28 ). Therefore, Arg availability is regulated by many factors, and dietary Arg supplementation may be a necessary strategy to maintain Arg homoeostasis for good health and body functions under many physiological and pathological conditions( Reference Wu, Bazer and Davis 29 ).
Our previous studies already found that oxidative stress decreased the level of Arg in circulation in piglets( Reference Zheng, Yu and Lv 30 ), and supplementation with Arg could attenuate oxidative stress by enhancing the total antioxidative capacity in piglets( Reference Zheng, Yu and He 5 ). However, little is known about the effect of dietary Arg supplementation on Arg metabolism in the intestine under oxidative stress conditions in piglets. The aim of this study was to investigate the effects of supplementation with Arg on Arg transport and metabolism, and potential mechanisms underlying the Arg-induced protective effects in the intestine under the oxidative stress conditions in piglets.
Methods
Experimental animals and diets
The samples for the current study were obtained from the animal experiment conducted in our previous study. The experimental piglets and diets are described in detail in our previous study( Reference Zheng, Yu and He 5 ). In brief, a total of thirty-six crossbred weaned male piglets (8·67 (sem 0·43) kg, 28 (sem 1) d) (Pig Improvement Company, PIC) were housed individually and randomly allocated into six groups with six replicates per group (n 6). Piglets were subjected to three dietary treatments (two groups per treatment) in the 1st week and fed a basal diet supplemented with varying concentrations of Arg. The diets were the same as in our previous report( Reference Zheng, Yu and He 5 ). Dietary treatments were as follows: basal diet (ArgL), basal diet and supplementation with 0·8 % synthetic l-Arg (ArgM) and basal diet and supplementation with 1·6 % synthetic l-Arg (ArgH).
Experimental procedure
The experimental procedure was the same as in our previous study( Reference Zheng, Yu and He 5 ). In brief, at 08.00 hours on day 8, piglets in each dietary treatment were intraperitoneally injected with diquat at a concentration of 10 mg/kg body weight or with a sterile 0·9 % NaCl solution of the same amount, respectively. Diquat (Diquat dibromide monohydrate, PS365; Sigma, Co.) was dissolved in isotonic saline and filter-sterilised. The concentration of the diquat solution was 10 mg/ml. The whole trial lasted for 11 d. This study was approved by the Animal Care Advisory committee of Sichuan Agricultural University.
Blood sampling and analyses
Before injection (0 h) and at 6, 24, 48 and 96 h post injection, a blood sample (10 ml/pig) was collected from the portal vein precava into heparinised polyethylene tubes (Axygen biotechnology Co., Ltd). Plasma was prepared by centrifuging the blood (3000 g at 4°C for 15 min) and immediately stored at −20°C.
Plasma Arg concentration was assayed using the amino acid automatic analyser L8800 (Hitachi). Frozen plasma samples were thawed at 4°C and 3 ml of a 10 % (w/v) solution of sulfosalicylic acid was added to 1 ml of plasma sample and centrifuged (12 000 g for 1 h) at 4°C. The Arg concentration of deproteinised plasma was determined by ion-exchange chromatography( Reference Ertingshausen, Adler and Reichler 31 ).
Tissue sample collection
After the blood was collected at 96 h post injection, piglets were euthanised with an intravenous injection of pentobarbital sodium (50 mg/kg body weight) and then slaughtered by exsanguination protocols approved by the Sichuan Agricultural University Animal Care Advisory Committee.
A midline laparotomy was performed. The abdomen was incised, and the entire small intestine starting from the pylorus to the ileocaecal valve was removed and divided into three segments: duodenum, jejunum and ileum. The segment from the pylorus to the suspensory muscle of duodenum was defined as duodenum, the last three-fifths segment was defined as ileum, and the middle segment was defined as jejunum. Segments of 2 cm length were cut from the middle of the duodenum, jejunum and ileum, respectively. The segments were flushed gently with ice-cold PBS (pH 7·4) and then fixed in 10 % fresh, chilled formalin solution. Following this, 0·5 cm jejunum samples were removed and snap-frozen in N2 and then stored at −80°C until RNA isolation.
Intestinal morphology analysis
The samples were fixed in neutral-buffered formaldehyde, embedded and stained according to the method of Luna( Reference Luna 32 ). The sections were stained with haematoxylin–eosin. In each section, villus height, villus width and crypt depth were examined in ten well-orientated villi and crypts using an Olympus CK 40 microscope (Olympus Optical Company).
Intestinal gene expression
Total RNA was extracted from samples of the jejunum using the TRIzol reagent (TaKaRa), according to the manufacturer’s instructions. The concentration of RNA in the final preparations was calculated from the OD260. The integrity of RNA was verified using denaturing agarose gel electrophoresis. Reverse transcription was performed using the Prime ScriptTM RT reagent Kit (TaKaRa) with a 2-μg RNA sample, according to the manufacturer’s instructions. Complementary DNA was used as the template for PCR.
Real-time quantitative PCR was performed in an Option Monitor 3 Real-Time PCR Detection System (Bio-Rad) using the SYBR Green Supermix (TaKaRa). Expression levels of CAT-1, CAT-2, CAT-3, arginase II (ARGII), inducible nitric oxide synthase (iNOS), endothelial nitric oxide synthase (eNOS), IL-6, TNF-α and PPAR-γ in the jejunum were analysed using real-time quantitative PCR with SYBR Green PCR reagents (TaKaRa) carried out in the Option DNA Engine (Bio-Rad) using the following cycle parameters: 95°C for 10 s, followed by forty cycles at 95°C for 5 s, annealing temperature (Table 1) for 20 s, and a final extension at 72°C for 15 s. The gene-specific primers used are listed in Table 1. All primers were purchased from TaKaRa. Fluorescence detection was carried out immediately at the end of each annealing step, and the purity of the amplification was confirmed by analysing the melting curves. Gene expression relative to the housekeeping gene β-actin was measured in order to correct for the variance in amounts of RNA input in the reactions. β-Actin was used as the reference gene according to the stability test of Hillig et al.( Reference Hillig, Jørgensen and Cirera 33 ).
Table 1 Primers used for real-time analyses

CAT, cationic amino acid transporter; eNOS, endothelial nitric oxide synthase; iNOS, inducible nitric oxide synthase; ARGII, arginase II.
Each primer pair used yielded a single peak in the melting curve and a single band of the expected size in the agarose gel. The relative gene expression levels compared with the housekeeping gene β-actin were calculated using the Pfaffl (2001) method( Reference Pfaffl 34 ).
Statistical analysis
Data were analysed by two-way ANOVA using the general linear model procedure. The main effects of the model included Arg supplementation levels (0, 0·8 and 1·6 %) and oxidative stress processing (injection of diquat or saline). P<0·05 was considered to indicate a significant difference and values between 0·05 and 0·10 to indicate a trend. Variable means for treatments showing significant differences in ANOVA were separated by Duncan’s test (P<0·05). Values were expressed as means with their standard errors. All statistical analysis was performed using SPSS17.0.
Results
Concentrations of plasma arginine
The data on plasma Arg are shown in Table 2. Compared with the basal diet, supplementation with Arg had no significant influence on plasma Arg concentration (P>0·05). Injection of diquat resulted in an acute reduction in plasma Arg concentration at 6 h after diquat injection (P<0·05). There was no significant effect of interaction between Arg supplementation and injection of diquat on Arg concentration.
Table 2 Effects of arginine (Arg) supplementation and diquat injection on the concentration of Arg in the plasma of piglets (nmol/ml)Footnote *
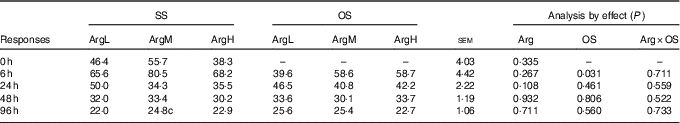
SS, injection with sterile saline; OS, injection with diquat; ArgL, basal diet; ArgM, basal diet and supplementation with 0·8 % synthetic l-Arg; ArgH, basal diet and supplementation with 1·6 % synthetic l-Arg; Arg×OS, Arg×OS interaction effect.
* 0 h, n 12, others, n 6.
Intestinal morphology
Data on morphology of the small intestine are shown in Table 3. Injection of diquat resulted in a reduction of villus height in the jejunum and ileum (P<0·05) as well as in villus width and crypt depth in the duodenum, jejunum and ileum (P<0·05). Supplementation with Arg (ArgM and ArgH) significantly decreased crypt depth in the jejunum with or without injection of diquat (P<0·05). There was no significant interaction effect of Arg supplementation and injection of diquat on intestinal morphology.
Table 3 Effects of arginine (Arg) supplementation on villus height, villus width and crypt depth of weaned piglets after 96 h oxidative stress induced by diquat, n 6

SS, injection with sterile saline; OS, injection with diquat; ArgL, basal diet; ArgM, basal diet and supplementation with 0·8 % synthetic l-Arg; ArgH, basal diet and supplementation with 1·6 % synthetic l-Arg; Arg×OS, Arg×OS interaction effect.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
Cationic amino acid transporter gene expressions in the jejunum
As shown in Fig. 1, supplementation with Arg had no effect on CAT-1 mRNA level in the jejunum (P>0·05) under non-oxidative stresses conditions. Injection of diquat significantly increased CAT-1 mRNA level (P<0·05), whereas supplementation with Arg along with injection of diquat in the jejunum significantly decreased CAT-1 mRNA level (P<0·05).

Fig. 1 Effects of arginine (Arg) supplementation and diquat injection on relative expressions of CAT-1, CAT-2 and CAT-3 mRNA in the jejunum of piglets. CAT, cationic amino acid transporter; ArgL, basal diet; ArgM, basal diet and supplementation with 0·8 % synthetic l-Arg; ArgH , basal diet and supplementation with 1·6 % synthetic l-Arg; OS, injection with diquat. Values are means (n 6), with their standard errors represented by vertical bars. x,y or a,b Mean values with unlike superscript letters were significantly different (P<0·05). * Mean values were significantly different between two groups (P<0·05).
ArgM significantly increased CAT-2 mRNA level in the jejunum compared with ArgL without injection of diquat (P<0·05), and injection of diquat significantly increased CAT-2 mRNA level compared with injection of saline (P<0·05).
Supplementation with Arg had no effect on CAT-3 mRNA level in the jejunum (P>0·05) but injection of diquat significantly increased CAT-3 mRNA level (P<0·05).
Arginine catabolism-related gene expressions
As shown in Fig. 2, supplementation with Arg did not affect ARGII mRNA level in the jejunum compared with supplementation with ArgL under non-oxidative stress (P>0·05). Injection of diquat significantly decreased ARGII mRNA level in the jejunum (P<0·05). Supplementation with Arg significantly reversed ARGII mRNA level induced by diquat to normal level in the jejunum (P<0·05).

Fig. 2 Effects of arginine (Arg) supplementation and diquat injection on relative expressions of arginase II (ARGII), endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) mRNA in the jejunum of piglets. ArgL, basal diet; ArgM, basal diet and supplementation with 0·8 % synthetic l-Arg; ArgH, basal diet and supplementation with 1·6 % synthetic l-Arg; OS, injection with diquat. Values are means (n 6), with their standard errors represented by vertical bars. x,y or a,b Mean values with unlike superscript letters were significantly different (P<0·05). * Mean values were significantly different between two groups (P<0·05).
However, ArgH significantly decreased iNOS mRNA level in the jejunum compared with ArgL under non-oxidative stress (P<0·05). Injection of diquat significantly decreased iNOS mRNA level in the jejunum (P<0·05) as well. ArgM significantly decreased iNOS mRNA level in the jejunum with injection of diquat (P<0·05) but ArgH did not have the same effect.
ArgM significantly increased eNOS mRNA level compared with ArgL under non-oxidative stress. Injection of diquat did not significantly affect eNOS mRNA level in the jejunum (P>0·05).
Inflammation-related gene expressions
To investigate the possible protective effects of Arg on jejunum injury caused by oxidative stress induced by diquat, we quantified mRNA levels of IL-6, TNF-α and PPAR-γ in the jejunum.
As shown in Fig. 3, ArgM and ArgH significantly increased IL-6 mRNA level in the jejunum of piglets, compared with ArgL with or without injection of diquat (P<0·05). Injection of diquat significantly increased IL-6 mRNA level in the jejunum of piglets (P<0·05).

Fig. 3 Effects of arginine (Arg) supplementation and diquat injection on relative expressions of IL-6, TNF-α and PPAR-γ mRNA in the jejunum of weaned piglets. ArgL, basal diet; ArgM, basal diet and supplementation with 0·8 % synthetic l-Arg; ArgH, basal diet and supplementation with 1·6 % synthetic l-Arg; OS, injection with diquat. Values are means (n 6), with their standard errors represented by vertical bars. x,y or a,b Mean values with unlike superscript letters were significantly different (P<0·05). * Mean values were significantly different between two groups (P<0·05).
Supplementation with Arg did not affect TNF-α mRNA level in the jejunum under non-oxidative stress (P>0·05). Injection of diquat significantly increased TNF-α mRNA level in the jejunum (P<0·05). Supplementation with Arg could effectively relieve the increment of TNF-α mRNA level induced by diquat (P<0·05).
Arg and injection of diquat had no effect on PPAR-γ mRNA level in the jejunum of pigs (P>0·05).
Discussion
l-Arg is a multifunctional substance, which is not only a precursor for the synthesis of protein, NO, urea, polyamines and creatine but also regulates gene expression and antioxidation( Reference Wu, Bazer and Davis 22 , Reference Montanez, Rodriguez-Caso and Sanchez-Jimenez 35 – Reference Ma, Zheng and Hu 37 ). Arg plays an important role in improving the growth performance and morphological development of the small intestine in suckling piglets( Reference Kim, McPherson and Wu 38 ), weanling piglets( Reference He, Tang and Ren 9 ) and growing pigs fed a mould-contaminated diet( Reference Yin, Ren and Duan 39 ). Previous research studies showed that supplementation with Arg could increase the weight of the small intestine, enhance intestinal development( Reference Yao, Guan and Li 40 ), improve microvascular development of the intestine in weanling piglets( Reference Zhan, Ou and Piao 41 ) and alleviate the impairment induced by deoxynivalenol in growing pigs( Reference Wu, Wang and Yao 42 ). Intestinal function in piglets was greatly disturbed by oxidative stress, which resulted in reductions in nutrient absorption( Reference Yin, Liu and Ren 7 ). Our previous study proved that piglets suffered oxidative stress upon diquat injection( Reference Zheng, Yu and He 5 ). Injection with diquat significantly increased the concentration of cortisol and MDA and decreased the activities of glutathione peroxidase and superoxide dismutase in the plasma of piglets( Reference Zheng, Yu and He 5 ). At present, we have found that oxidative stress induced by diquat significantly decreased villus height, villus width and crypt depth in the jejunum and ileum. Supplementation with Arg significantly mitigated jejunum morphology impairment (e.g. lower villus width). Our previous study found that supplementation with Arg had a trend to increase ADFI of piglets under oxidative stress( Reference Zheng, Yu and He 5 ). This result further proved that the increase in nutrient intake was beneficial to the health of piglets under oxidative stress. This result is also consistent with those from previous research studies that showed that supplementation with Arg can relieve dysfunction of the intestine caused by LPS-induced stress( Reference Liu, Huang and Hou 43 ). Besides, Viana et al. ( Reference Viana, Santos and Generoso 44 ) found that Arg was able to preserve barrier integrity and reduce bacterial translocation in the intestine of mice. We also detected the mRNA expression of inflammation-related genes and found that oxidative stress significantly increased IL-6 and TNF-α mRNA levels in the jejunum of piglets; supplementation with Arg could effectively relieve the increment of TNF-α mRNA level induced by oxidative stress. TNF-α is a proinflammatory cytokine involved in systemic inflammation( Reference Ma, Boivin and Ye 45 ). Further, research studies found TNF-α caused an increase in barrier permeability of the intestinal epithelium through activation of the ERK1/2 signalling pathway( Reference Al-Sadi, Guo and Ye 46 ). Therefore, it is reasonable to speculate that supplementing Arg in piglets can partially reduce oxidative injury by suppressing proinflammatory cytokine TNF-α expression in the jejunum.
Arg within the body is derived from the diet, endogenous synthesis and from turnover of proteins. The intestine plays a central role in maintaining Arg homoeostasis by providing exogenous Arg into the system. Animal studies showed that Arg was predominantly transported across the intestinal membrane via an Na+-independent system: that is, via y+ CAT isoforms( Reference White 47 , Reference Stevens 48 ). In this study, we measured CAT expression and found that oxidative stress induced by diquat significantly increased gene expression of CAT-1, CAT-2 and CAT-3 in the jejunum of piglets. Duan et al. ( Reference Duan, Yin and Ren 6 ) found that oxidative stress induced by injection of H2O2 tended to increase mRNA levels of anionic amino acid transporters and that dietary Arg supplementation enhances intestinal anionic amino acid transporter expression( Reference Yin, Ren and Duan 39 ). Supplementation with Arg increased gene expression of CAT-2 but not CAT-1 and CAT-3 in the jejunum under the non-oxidative stress condition. However, under oxidative stress situations, supplementation with Arg significantly suppressed the increase in gene expression of CAT-1 and CAT-2 induced by oxidative stress but not of CAT-3. These results suggest that Arg transport is very complicated and influenced by many factors. The regulatory mechanisms of Arg absorption could be mainly in the following two ways. First, Arg absorption was mediated by the level of substrate( Reference Morris 36 ). In this study, increasing the concentration of Arg in diet significantly increased the gene expression of CAT-2 under non-oxidative stress conditions and CAT-3 in oxidative stress conditions in the jejunum, respectively. On the other hand, the gene expressions of CAT-1, CAT-2 and CAT-3 in the jejunum were increased when the concentration of Arg in plasma was reduced by oxidative stress. Second, Arg absorption was mediated by the cytokine TNF-α pathway( Reference Pan, Wasa and Lind 49 ). In this study, oxidative stress significantly increased the expression of the TNF-α gene as well as of CAT-1, CAT-2 and CAT-3 genes, whereas supplementation with Arg significantly inhibited the expression of TNF-α and CAT-1. Research studies indicated that TNF-α increased Arg transport in a time- and dose-dependent manner in human vascular endothelium by activation of protein kinase C( Reference Pan, Wasa and Lind 49 ). PKC regulated Arg transport activity via a mechanism of activating transporter mRNA and translational processes in Caco-2 intestinal cells( Reference Pan and Stevens 50 ). The data from this study suggested that TNF-α was involved in the regulation of Arg transport mainly by affecting the activity of CAT-1 but not CAT-2 and CAT-3 in the jejunum under oxidative stress.
The small intestine is the most important site not only for Arg absorption but also for metabolism of Arg. Arg homoeostasis is achieved principally through regulation of Arg catabolism. Both NOS and arginase use Arg as a common substrate, and arginase may down-regulate NO production by competing with NOS for Arg. In this study, oxidative stress significantly decreased iNOS and ARGII mRNA levels in the jejunum, which could be a result of the low availability of Arg. This conclusion is supported by the observation that Arg can regulate gene expression as a substrate for enzymes( Reference Morris 36 ) and that Arg deprivation results in decreased expression of iNOS ( Reference El-Gayar, Thuring-Nahler and Pfeilschifter 51 ). Supplementation with Arg could significantly increase eNOS and ARGII mRNA levels but not iNOS levels, probably because elevated ARGII expression inhibited the expression of iNOS ( Reference Lee, Ryu and Ferrante 52 ). A previous study reported that the large amount of NO synthesised by iNOS lead to arthritis in humans( Reference Meininger, Kelly and Li 53 ), which indicated that the role of NO in vivo depended on its origin. Hence, in this study, supplementation with Arg could significantly increase the expression of eNOS but not iNOS, which could be one of the reasons for relieving the influences of oxidative stress with Arg supplementation. In conditions such as oxidative stress, which increase the metabolic demand for Arg beyond the maximum Arg synthetic capacity, dietary Arg supplementation is necessary. Hu et al. ( Reference Hu, Li and Rezaei 54 ) also studied the safe dose of Arg supplementation in weaned pigs and indicated that supplementing up to 2 % l-Arg (as either l-Arg–HCl or l-Arg–base) in the diet for 91 d did not have any adverse effect on postweaning pigs. However, Arg is expensive; therefore, it is important to research the proper dose of Arg to be supplemented in different situations. In this study, ArgM showed the same effects as ArgH in relieving oxidative stress in the intestine of piglets; thus, ArgM could be the best choice in this model, and the related research studies deserve more attention in the future.
In conclusion, oxidative stress induced by diquat can influence absorption and metabolism of Arg in the jejunum of weaned piglets, and dietary supplementation with Arg has beneficial effects for intestinal health and functions through improvement in the morphology of the intestine, regulation of Arg availability and reduction of inflammatory cytokine levels. This study illustrates that dietary Arg supplementation in piglets is necessary for increasing the metabolic demand of Arg in piglets under oxidative stress. Adequate l-Arg provision may be a novel and effective means to ameliorate oxidative injury in the small intestine.
Acknowledgements
This project was jointly financed by the National Natural Science Foundation of China (31501963), the Science and Technology Support Program of Sichuan Province (2013NZ0056, 2016NZ006) and by the earmarked fund for China Agriculture Research System (no. CARS-36).
The author contributions are as follows: P. Z., Y. L. G. T. and Q. Z. conducted the experiments. B. Y. and D. C. participated in the design of the study. J. L. and Z. H. assisted in analyses. J. H. and J. Y. assisted in manuscript preparation. L. C and X. M. assisted with all data analyses.
The authors declare that there are no conflicts of interest.









