The popularity of employing dietary patterns to assess the effects of dietary intake on disease incidence has increased rapidly over recent years. These patterns are increasingly used as an alternative to, or in tandem with, the study of individual nutrients or food intake in epidemiological studies of health risk. Analysis of this kind may overcome the inherent problems of examining individual food and nutrient associations, namely the inter-correlations between these foods and nutrients(Reference Hu, Rimm, Smith-Warner, Feskanich, Stampfer, Ascherio, Sampson and Willett1). Currently, the most common method of obtaining dietary patterns is via the method of principal components analysis (PCA). This process identifies the underlying dimensions in a large number of variables based on the inter-correlations between these variables.
It is important to consider the stability of dietary measures used in longitudinal cohort studies. As Weismayer et al. (Reference Weismayer, Anderson and Wolk2) pointed out, the cost of maintaining such cohorts could be reduced if dietary measures proved to be stable over time. The need to collect regular dietary information from cohort members could be reduced if it were shown that there was little variation in dietary patterns, both at a cohort level and at an individual level over time. Knowledge of the stability of dietary patterns over time would also aid researchers in setting up their studies from the outset. If it were known that changes in dietary patterns were evident at or between specific ages, researchers could plan their follow-up times in order to incorporate dietary data collection at such ages. To date, a number of studies have approached this issue in adults and have shown reasonable stability(Reference Hu, Rimm, Smith-Warner, Feskanich, Stampfer, Ascherio, Sampson and Willett1, Reference Prevost, Wichelow and Cox3–Reference Northstone and Emmett12). However, there are inconsistencies between these studies including substantial differences in follow-up times, the number of follow-up points, sample sizes and different methodologies employed to collect dietary data. Together, these differences make it difficult to draw any firm conclusions about the stability of dietary patterns over time from the published results.
To our knowledge no study has investigated the stability of dietary patterns specifically during childhood. Any changes in patterns may reflect the modification of diet by individuals over time for a number of different reasons. These include: major life events; changes in food preferences or nutritional advice (which is constantly being updated); or by changes in the food supply (e.g. newly available foods such as soy products and probiotics). It is important to assess the effects of any changes in dietary patterns in childhood on health outcomes.
The aim of the present study is to investigate the stability of dietary patterns obtained at four time-points in a cohort study of children, considering patterns scores as both categorical and continuous variables.
Methods
The Avon Longitudinal Study of Parents and Children (ALSPAC), an ongoing longitudinal cohort study, was designed to investigate the determinants of development, health and disease during childhood and beyond(Reference Golding, Pembrey and Jones13). Eligible participants for the study were those pregnant women with an expected date of delivery between 1 April 1991 and 31 December 1992, who were resident in the former Avon health authority in south-west England. More detailed information on the study can be found on the website (www.alspac.bris.ac.uk). A cohort of 14 541 pregnant women was established resulting in 13 988 children who were alive at 12 months of age. Ethical approval for the study was obtained from the ALSPAC Law and Ethics committee and the three local research ethics committees.
The present study uses data collected from questionnaires completed when the children were 38, 54, 81 and 103 months of age, these time-points are equivalent to 3·2, 4·5, 6·8 and 8·6 years, respectively. For ease of reporting we will refer to these time-points as 3, 4, 7 and 9 years of age throughout the paper. All four questionnaires contained a set of questions enquiring about the frequency of consumption of a wide range of foods and drinks. The mother or main carer was given the following options to indicate how often her child was currently consuming a variety of food types ‘nowadays’: (1) never or rarely; (2) once in 2 weeks; (3) one to three times per week; (4) four to seven times per week; (5) more than once per day. Note that at the ages of 7 and 9 the wording of the questionnaires was slightly different such that the mother was asked about the foods she provided and was asked not to include school dinners, which were asked about separately. Mothers were also asked to record how many cups of tea or coffee, the number of glasses of cola and the number of slices of bread consumed daily. The usual type of bread (white or other) and milk (full-fat or other) usually consumed was also recorded. The data on frequency of consumption were numerically transformed into times consumed per week, in order to apply quantitative meaning to the frequency categories, as follows: (1) 0; (2) 0·5; (3) 2; (4) 5·5 and (5) 10 times per week. All data were standardised by subtracting the mean and dividing by the standard deviation for each variable; this was necessary because tea, coffee, cola and bread were measured on a different scale to the other variables.
The FFQ were adapted from the one used to assess maternal diet at 32 weeks of pregnancy(Reference Rogers and Emmett14). Over time the questionnaires were modified slightly. This was in the light of analysis of data as it became available and changes in the availability of various foods. For example, separate categories were created from the age of 4, for coated poultry and fish products, vegetarian pies and tuna, which had previously been encompassed in other categories. Therefore, as time progressed, additional foods/drinks were included in the questionnaires (at 3 years ice cream, milk-based puddings, cold meats, meat and vegetarian pies, tinned pasta and nuts were not included, and at 4 years cold meats was not included). We chose to keep this extra information separate in order to maximise the available information, rather than combine into existing food groups created from the earlier questionnaires. The number of food items/groups included in the PCA were 34, 35, 41 and 41 at 3, 4, 7 and 9 years of age, respectively.
Statistical methods
PCA with varimax rotation(Reference Gorsuch15, Reference Kline16) was performed on the standardised food items. Methods for the 3, 4 and 7 years questionnaires have been described in detail elsewhere(Reference North and Emmett17, Reference Northstone and Emmett18). An identical procedure was used for the standardised food items from the 9 years questionnaire. The number of components that best represented the data at each time-point was primarily chosen on the basis of the scree plot(Reference Cattell19) and the interpretability of the components. Children were excluded from each PCA if they had more than ten dietary items missing from the respective questionnaire. If ten or fewer items were missing, we made the assumption that the child did not consume those items and they were given a value of 0. Foods with loadings above 0·3 on a component were considered to have a strong association with that component and were deemed to be the most informative in describing the dietary patterns. Labels were given to each component at each time-point. While these do not perfectly describe each underlying pattern, they aid in the reporting and discussion of the results.
For each child a score was created for each component identified at each time-point by multiplying the factor loadings by the corresponding standardised value for each food and summing across the food items. All component scores were approximately normally distributed. Spearman's correlation coefficients were calculated to measure the associations between the dietary pattern scores obtained at each time-point. Paired t tests were then applied to assess the change in mean scores between each period of questioning. The 95 % limits of agreement(Reference Bland and Altman20) were calculated as the mean difference between each pair of scores plus or minus twice the standard deviation of the differences, enabling us to assess the extent of agreement between the time-points and provide an idea of the spread of the variation of scores between the time-points. In order to make valid comparisons of the limits of agreement across time-points we calculated z scores for each dietary pattern score by subtracting the mean and dividing by the standard deviation. The mean and standard deviation used depended upon which pairwise comparison was being made. For example, when comparing the ‘processed’ mean scores at 3 and 7 years of age, both these scores were transformed using the 3 years mean and standard deviation for the ‘processed’ score; when comparing the 4 and 9 years ‘traditional’ mean scores, the mean and standard deviation for the 4 years scores were used to transform both variables. Standardising using this adjusted method meant that we could express the units of the limits of agreement in terms of standard deviations of the earlier score. Finally, component scores were split into quintiles and weighted κ(Reference Cohen21) was used to compare scores between each pair of time-points. Weighted κ was chosen due to the ordered nature of the categorical data as it takes into account partial agreement between groups.
All analyses were performed using SPSS for Windows version 12.0.1, with the exception of weighted κ, which were obtained using STATA for Windows version 9.2.
Results
Table 1 summarises the dietary components that were extracted at each time-point, in the order that they were extracted, together with the proportion of variance that was explained by each component and the number from the cohort with data available. Two patterns were consistently (in terms of the foods loading highly) obtained across time: the ‘processed’ and ‘traditional’ patterns. The ‘health conscious’ pattern was extracted at each time-point but was slightly modified at 9 years of age, where meat products were negatively associated. Table 2 summarises the foods that loaded highly on the patterns at each time-point. It can be seen that the ‘processed’ pattern was described by foods with high fat and sugar content, and processed and convenience foods. The ‘traditional’ pattern was highly associated with meat, poultry, potato and vegetable consumption at all time-points. At the ages of 3, 4 and 7 salads, fruit, vegetables, fish, pasta and rice were the foods loading highly on the ‘health conscious’ pattern. At 8 years of age slight differences were evident. Some of the foods, which loaded highly on the ‘health conscious’ pattern at earlier ages, loaded highly on the ‘traditional’ pattern at this age and negative loadings were apparent for poultry, red and cold meats. We therefore chose to label this pattern ‘health conscious/vegetarian’ but chose to make direct comparisons with the preceding ‘health conscious’ scores as, overall, the 9 years pattern was closer to the ‘health conscious’ pattern than the ‘traditional’ at previous ages.
Table 1 Dietary patterns obtained at each time-point in childhood, showing the order of extraction and percentage of variance explained*
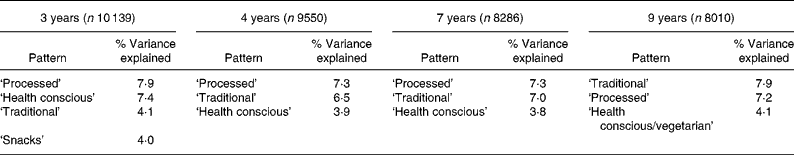
* For details of subjects and procedures, see Methods.
Table 2 Summary of the foods which had loadings >±0·3 on at least one dietary pattern at at least one time-point for the three common dietary patterns extracted at each time-point*

X, loading >0·3; − ve X, loading < − 0.3; blank cell, data on that food was not collected at that time-point.
* For details of subjects and procedures, see Methods.
† This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
‡ At 3 years, only one question was asked regarding puddings.
§ At 3 years, breaded fish products were included in this group, they were treated separately at all other time-points.
∥ At 3 years tinned pasta was not included in this group, as it was not asked about.
In total, 6177 children had data available at all four time-points and the remaining analyses are restricted to this complete case sample. Note that performing PCA at each time-point in this restricted sample did not make any difference to the interpretability of the patterns; there were only minor differences in the factor loadings (data not shown). Table 3 presents the correlations between the dietary patterns at each time-point. As expected, high correlations were evident for all three scores at each pair of time-points (all P < 0·0001). The smallest correlation for these pairwise comparisons was between the ‘traditional’ components at 3 and 9 years of age (0·35). All other correlations were similar in magnitude, falling between 0·41 and 0·69. Little correlation was seen between different patterns across time, with the exception of the ‘health conscious/vegetarian’ pattern at 9 years of age. This showed a level of negative correlation with the ‘traditional’ pattern at the preceding time-points, which emphasises the change in the ‘health conscious’ pattern by 9 years of age.
Table 3 Pearson's correlation coefficients between the dietary pattern scores obtained at each time-point in childhood (n 6177)†
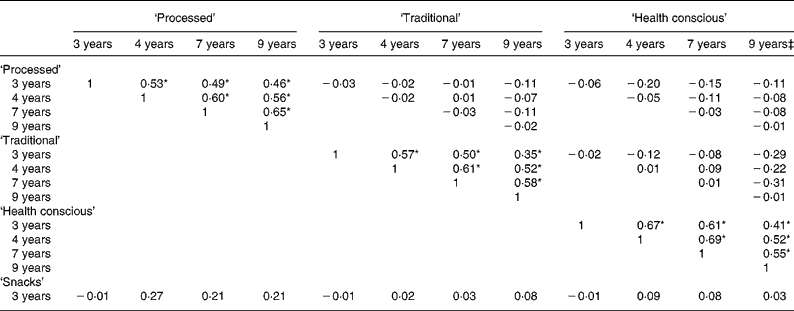
*P < 0·0001 for the correlations between the same dietary patterns at each pair of time-points.
† For details of subjects and procedures, see Methods.
‡ This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
Since the ‘snacks’ pattern from the 3 years data was not repeated at any other time-point it was not included in any further comparative analyses.
The mean pattern scores at each time-point are given in Table 4. It can be seen that the mean ‘processed’ score increased over time. Similar mean scores were evident for the ‘health conscious’ and ‘traditional’ patterns at each time-point. The differences in mean pattern scores between each pair of time-points are shown in Table 5, and compared using paired t tests. There was a consistent increase in the mean ‘processed’ score at the later ages compared to 3 years (all P < 0·0001). Little difference was seen in the mean ‘traditional’ or ‘health conscious’ scores between 3, 4, 7 and 9 years of age.
Table 4 Mean dietary pattern scores obtained at each time-point in childhood (n 6177)*
(Mean values and standard deviations)
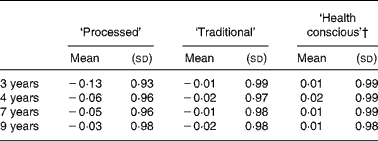
* For details of subjects and procedures, see Methods.
† This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
Table 5 Mean differences in scores across the dietary pattern scores obtained at each time-point in childhood (n 6177)*
(Mean difference values and standard deviations)

* For details of subjects and procedures, see Methods.
† This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
The narrowest limits of agreement for the adjusted scores were seen for the ‘health conscious’ pattern between both 3 and 4 years of age and 7 years of age (Table 6), while the widest were seen for all pairings between the 3 and 9 years data. When compared to all other time-point pairings, the limits of agreement between 4 and 7 years of age were the narrowest for each pattern.
Table 6 Mean difference and limits of agreement for adjusted* standardised scores across the dietary pattern scores obtained at each time-point in childhood (n 6177)†
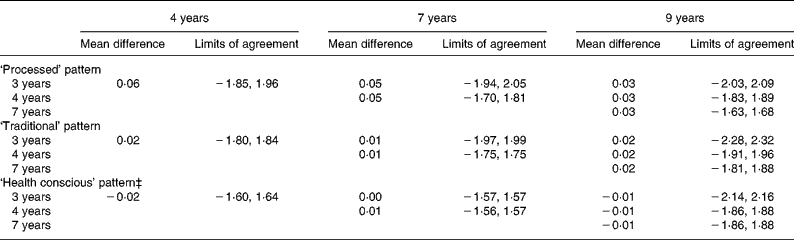
* Scores for the later time-point in each pair are transformed using the mean and standard deviation of the earlier time-point.
† For details of subjects and procedures, see Methods.
‡ This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
A reasonable level of agreement was seen with the categorised component scores from each time-point of data, ranging from 0·25 to 0·47 (Table 7). The lowest levels of agreement were seen with the ‘processed’ pattern scores between 3 and 9 years (κ 0·30) and the ‘traditional’ pattern scores between 3 and 9 years and 4 and 9 years (κ 0·28 and 0·25, respectively). The levels of agreement between 4 and 7 years of age were between 0·42 and 0·47.
Table 7 Weighted κ for quintiles of dietary pattern scores obtained at each time-point in childhood (n 6177)*
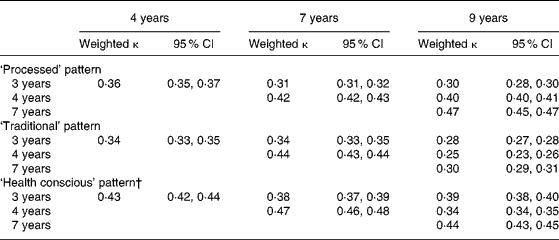
* For details of subjects and procedures, see Methods.
† This pattern was labelled ‘Health conscious/vegetarian’ at 9 years.
Given the differences in patterns obtained at 9 years of age compared to previous ages, supplementary analyses were performed to try to determine any general differences in food consumption that may explain the presence of the ‘vegetarian’ pattern at this time. There were no differences evident in the proportions of children consuming meat, fruit, vegetables or meat substitutes at 7 and 9 years of age (data not shown). There was also no difference in the proportion of children being described as vegetarian by their mothers at these ages.
Discussion
This paper presents four sets of dietary patterns obtained using PCA in the same cohort of children between 3 and 9 years of age. Two patterns were consistently obtained at each time: the ‘processed’ and ‘traditional’ patterns. A ‘health conscious’ pattern was obtained at the first three time-points while a modified ‘health conscious’ pattern was obtained at 9 years of age. This loaded highly on many of the foods associated with the previous ‘health conscious’ patterns but also meat substitutes, pulses, nuts and vegetarian pies were associated. In addition a ‘snacks’ pattern was only obtained at 3 years of age; cheese, fruit, puddings, cakes, biscuits and crisps loaded highly on this pattern(Reference North and Emmett17), it was originally labelled ‘snacks’ as the pattern could be described as a diet consisting primarily of snack and finger foods as opposed to foods where cooking is required.
We chose to compare the ‘health conscious/vegetarian’ pattern at 9 years with the ‘health conscious’ patterns obtained previously. So, for the three patterns that were consistently obtained at each time-point, ‘processed’, ‘traditional’ and ‘health conscious’, there were moderate levels of agreement and stability across the time-points; all correlation coefficients were greater than 0·35. However, the size of the correlations decreased as the time-points became further apart; this suggests that the dietary patterns change gradually over time, which is not surprising as a child's tastes develop. In particular, the ‘snack pattern’ was only obtained at 3 years of age but was related to the ‘processed’ pattern obtained at later ages. Moreover, by 9 years of age, the ‘heath conscious’ pattern had become modified; showing a negative relationship with poultry and meats, while fruit and vegetable eating was more closely associated with the ‘traditional’ pattern at this age. This change was further emphasised by the negative correlations seen with the ‘traditional’ pattern scores obtained at preceding time-points. The values of κ were smaller for longer time spans (e.g. between 3 and 9 years of age) compared to shorter time spans (e.g. between 7 and 9 years of age). Similarly, the widest limits of agreement were seen for all pairings between the 3 and 9 years data. This suggests greater variability between the 3 and 9 years of age data for this pattern. The narrowest limits of agreement were evident between the 4 and 7 years pairings.
A particular difficulty inherent in attempting to assess the stability of dietary patterns over time lies in the fact that performing separate PCA at each time-point enables the researcher to identify the important patterns at each time-point. However, it is very difficult to assess the stability of the patterns obtained at earlier time-points if these patterns differ over time, as is the case with the patterns presented here. An alternative approach would be to apply the factor loadings obtained from earlier time-points to the data obtained at later time-points in order to obtain dietary pattern scores. This would enable the researcher to specifically assess the stability of the dietary patterns that were identified at baseline. However, this method is also limited, as any new patterns that may have emerged at follow-up would not be identified. Indeed, we have shown in the ALSPAC mothers that using this ‘applied’ method is inappropriate to assess the stability of dietary patterns over time when the dietary patterns are different(Reference Northstone and Emmett12). Nevertheless, in other cohorts this method may be more appropriate, particularly where the follow-up period is relatively short. For example, in adult populations where any changes in patterns cannot be explained by major life events such as pregnancy, illness or moving into a different culture where food availability may differ substantially. We would encourage researchers to investigate both methods when estimating changes and determining what drives the changes in dietary patterns over time obtained using PCA.
There have been conflicting reports of stability in dietary patterns in adults. Mishra et al. (Reference Mishra, McNaughton, Bramwell and Wadsworth8) reported ‘fair-to-moderate’ stability in their fruits, vegetables and dairy dietary patterns but poor stability in their meat, potatoes and sweet food pattern over a 17-year period. Borland et al. (Reference Borland, Robinson, Crozier and Inskip11) also reported that their prudent dietary pattern was more stable than their high-energy pattern. Their results suggest that the tracking of healthier eating behaviours is reasonably good. Meanwhile, Van Dam et al. (Reference Van Dam, Rimm, Willett, Stampfer and Hu4) reported that their ‘Western’ pattern was more highly correlated over 4 and 9 years follow-up compared to the ‘prudent’ dietary pattern. The study by Mikkila et al. (Reference Mikkila, Rasanen, Raitakari, Pietinen and Viikari6), providing the closest comparison to the present study, obtained dietary patterns in their cohort of 3596 children aged 3–18. Follow-up data were collected after 6 and 21 years. Similar dietary patterns were obtained at each time-point. Considering the younger group of 3–12 year olds, the level of tracking was less compared to the adolescent group aged 15–18 years. It is difficult to make direct comparisons with the results we have presented and these studies due to the different ages examined and the variability in follow-up times. However, the present results are similar to those of Van Dam et al. (Reference Van Dam, Rimm, Willett, Stampfer and Hu4) in that our ‘health conscious’ score had the lowest correlations and levels of agreement as measured by κ over time.
A number of studies have tracked nutrient intakes through early and middle childhood(Reference Boulton, Magarey and Cockingron22, Reference Singer, Moore, Garrahie and Ellison23) but few have examined the tracking of food intakes. Those that have started in middle childhood and followed up into early adolescence showing that fruit, vegetable, milk and fruit juice consumption drops over time, while soft drink/sweetened beverage consumption increases substantially into early adulthood(Reference Lytle, Seifert, Greenstein and McGovern24, Reference Demory-Luce, Morales, Nicklas, Baranowski, Zakeri and Berenson25).
The present sample is substantially larger than many of the studies that have been performed to date in adults. However, as with all cohort studies, there was loss to follow-up. It could be argued that any changes seen in overall dietary pattern scores over time were a result of biases in those who were followed-up. However, as the differences in mean pattern scores are small between time-points that are close together we believe the changes are real and not unduly affected by such bias. A related issue that warrants discussion is that we chose not to repeat the PCA in the sub-sample of 6177 children who had data at all four time-points. All scores derived using PCA have a mean of 0 and a standard deviation of 1 but in Table 4 it can be seen that this is not the case for our dietary pattern scores as we reduced the sample after the PCA had been performed. The mean scores for the ‘traditional’ and ‘health conscious’ patterns at all four time-points were very close to 0. However, they were slightly more negative for the ‘processed’ score and for the 3 years time-point in particular. The reduced sample had lower scores overall for this pattern, given the mean score of − 0·13, compared to the original full sample which therefore implies a level of selection bias. The mean scores for the ‘processed’ pattern are also negative at all future time-points. However, such a consistent pattern was not evident in the other two patterns, suggesting that any effects of selection bias may only be important for the ‘processed’ pattern. We reported a consistent increase in the mean ‘processed’ score at the later ages compared to 3 years and given the potential for selection bias these increases might be misleading. Nevertheless, we feel that the most important finding from the current study is that distinctly different patterns were obtained at 3 and 9 years of age compared to those obtained at 4 and 7 years of age. Selection bias is unlikely to have affected the present findings as virtually identical patterns were obtained in the reduced sample of 6177 children compared to those that were obtained in the complete case samples from each time-point.
A further potential limitation of the present study is that dietary intake was assessed using unquantified FFQ as opposed to a more individualised method such as weighed dietary records. However, studies comparing the results of PCA using FFQ and weighed dietary records(Reference Hu, Rimm, Smith-Warner, Feskanich, Stampfer, Ascherio, Sampson and Willett1, Reference Van Dam, Rimm, Willett, Stampfer and Hu4) have found the resulting dietary patterns to be comparable. Finally, mothers were asked at the ages of 7 and 9 to complete the FFQ with regard to the foods that she provided and not to include school dinners. As such there is the possibility of under-reporting of certain foods. However, we do not believe that excluding school dinners when completing the FFQ would have a major impact on the resulting dietary patterns we have obtained. Approximately a quarter of the children were having dinners provided by the school at these time-points and potential under-reporting would only be in effect if questionnaires were completed during term-time. If such under-reporting were a significant problem it would be expected that the patterns obtained at 4 and 7 years would differ as a result, however, they were virtually identical.
To reiterate the differences found in dietary patterns in childhood, we have reported the presence of a ‘snack’ pattern at the age of 3. It is likely that after this age, children are more developed in the foods that they eat and the consumption of finger foods lessens. There was also a change in the ‘health conscious’ pattern obtained at the age of 9 years. There was no obvious explanation for the modification of this pattern in terms of the frequency of the different kinds of foods that were consumed. Changes in the FFQ did not explain the differences. A ‘vegetarian’ pattern was identified in the mother's diets during pregnancy and again when their children were 47 months of age(Reference Northstone and Emmett12). It is possible that by 9 years of age, a child's diet has moved more towards that of an adult. It will be important to assess the diet of the children at later ages to determine whether this dietary pattern persists.
It will be important to follow up the present study in a number of ways. By following the children into adolescence; it will be of interest to make more detailed comparisons with other studies that have dietary data available at later ages. The next point of dietary data collected by FFQ in the ALSPAC cohort is at the age of 13. It will also be of interest to assess those factors that affect intra-individual changes in diet. We have shown that in addition to changes in overall diet, with the loss of the ‘snack’ pattern after 3 years of age and the modification of the ‘health-conscious/vegetarian’ pattern by 9 years of age, that there is slight variation in the stability of the consistent patterns over time.
It is apparent from the present study that researchers cannot assume that a cross-sectional measure of diet at one time-point in childhood is relevant to another time-point in childhood. To assess the impact of diet on the development of disease it is important to take repeated measures of diet, at least throughout early to mid-childhood. However, this is not always possible, primarily due to financial restraints. Based on the results presented here there appear to be differences in the dietary patterns obtained over a 5-year period, from early childhood at age 3 through to mid-childhood at age 9. However, these differences were relatively small between each pair of consecutive time-points, in particular between the ages of 4 and 7 years although this period was the longest. The dietary patterns obtained at these two time-points were virtually identical in terms of the factor loadings and hence the patterns obtained and it may be reasonable to suggest that data collection at one point between the ages of 4 and 7 would suffice.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. The UK Medical Research Council, the Wellcome Trust and the University of Bristol provide core support for ALSPAC. This work was also partially funded by a Wellcome Trust VIP award to K. N. and by the Arthritic Association supporting K. N. and P. M. E. This publication is the work of the authors who also serve as guarantors for the contents of this paper; we declare there is no conflict of interest.









