The Rex rabbit is a small herbivore bred for fur and meat production and a major component of farm economies in developing countries(Reference Liu, Sun and Liu1). A major goal of meat production in farm animals is to increase skeletal muscle growth and reduce excess fat deposition(Reference Tan, Yin and Liu2). Unfortunately, meat-type animals exhibit excessive amounts of abdominal and subcutaneous adipose tissue when at market weight(Reference Mersmann, Smith, Burrin and Mersmann3). Furthermore, obesity is a growing epidemic worldwide, and identifying new means to reduce excess fat deposition is important for both human health and animal production. The nutritional manipulation of lipid metabolism remains a focus biology research on growth, and studies have demonstrated that dietary arginine supplementation is effective for reducing body fat deposition in mice(Reference Clemmensen, Madsen and Smajilovic4), obese rats(Reference Jobgen, Meininger and Jobgen5), broiler chickens(Reference Fouad, El-Senousey and Yang6), white Pekin ducks(Reference Wu, Fang and Guo7), and growing-finishing pigs(Reference Zhan, Ou and Piao8). However, because arginine and lysine compete for the same transport systems, high levels of arginine may cause lysine-arginine antagonism and hence retard growth(Reference Zhan, Ou and Piao8).
N-Carbamylglutamate (NCG) is a metabolically-stable analogue of N-acetylglutamate, which modulates key enzymes (pyrroline-5-carboxylate synthase (P5CS) and carbamoylphosphate synthase-I (CPS-I)) in arginine synthesis in enterocytes and thus ultimately regulates intestinal endogenous arginine synthesis(Reference Wu, Knabe and Kim9). In addition, NCG does not affect lysine absorption in enterocytes(Reference Wu, Knabe and Kim9). Therefore, NCG supplementation is a novel, effective strategy to improve arginine nutrition. Studies have indicated that NCG supplementation could increase the serum arginine level; improve intestinal growth in intrauterine growth-retarded suckling lambs(Reference Zhang, Zhao and Peng10); enhance the antioxidant status against oxidative stress in rats(Reference Cao, Xiao and Liu11); and increase growth performance in piglets(Reference Wu, Ruan and Gao12). However, at present, information on the effects of dietary NCG supplementation on body fat deposition is not available.
Fat deposition is determined by lipid metabolism. Recent proteomics and metabolomics analyses revealed that NCG supplementation had a significant effect on lipogenesis(Reference Zhu, Zeng and Peng13,Reference Sun, Zhang and Fan14) , but the mechanism involved is not clearly understood. Because NCG itself has no other cellular activity beyond activating CPS-I and P5CS(Reference Wu, Knabe and Kim9), this agent likely exerts its regulatory effect on lipogenesis through altering the synthesis of arginine. Many regulatory factors are involved in the process of lipogenesis. NO produced from arginine by nitric oxide synthase (NOS) can react with the active cysteine thiol group of coenzyme A to form S-nitroso-coenzyme A, which is metabolically inactive, impairing coenzyme A, which is central to the lipogenesis pathway(Reference Roediger, Hems and Wiggins15). Recent evidence indicated that growth hormone (GH) inhibited hepatic de novo lipogenesis in adult mice(Reference Cordoba-Chacon, Majumdar and List16), whereas diabetic rats given exogenous insulin had a higher rate of fatty acid synthase (FAS) gene transcription than controls(Reference Ruzzin, Petersen and Meugnier17). In addition, insulin-like growth factor 1 (IGF-1), which has both GH-like activity and insulin-like activity, has an inhibitory effect on lipogenesis(Reference Yutaka18). Although the effects of arginine in modulating NOS and hormone secretion have been previously studied(Reference Cochard, Guilhermet and Bonneau19,Reference Jobgen, Fried and Fu20) , the effects of NCG on these regulatory factors related to lipogenesis remain unknown.
Based on the aforementioned findings, we hypothesised that dietary NCG supplementation would reduce body fat deposition by altering the production of NO and hormones and thereby reducing lipogenesis. Therefore, the present study was designed to evaluate the influence of dietary NCG supplementation on body fat deposition, hepatic lipogenic enzyme activities, serum metabolites related to lipogenesis, NOS, and hormone secretion in Rex rabbits. The effects of NCG supplementation on hepatic health indicators were also evaluated as a further measure to assess the response of rabbits to different nutritional regimens in the present study.
Materials and methods
All experimental protocols involving animals were approved by the Animal Care and Welfare Committee of the Animal Science College and Scientific Ethical Committee of Zhejiang University (no. ZJU2013105002) (Hangzhou, China).
Animals, diets, and experimental design
A total of 160 3-month-old Rex rabbits with similar body weights (BW) were housed in individual cages (60 cm × 40 cm × 40 cm) within a semicontrolled closed building (temperature, 20–25°C; photoperiod, 16 h of light per d). The rabbits were randomly assigned to four groups, with forty animals per dietary group (twenty males and twenty females). The basal diet (Table 1) used in this study was formulated to meet the recommended nutrient requirements for growing rabbits(21). The following four different concentrations of NCG (99 % purity; Yuanchang Industrial Co. Ltd) were used to supplement the basal diet: 0 (control), 0·04, 0·08, and 0·12 % (as-fed basis). NCG was used to supplement the basal diet at the expense of zeolite powder. The diets were pressure pelleted with a pellet diameter of 4 mm. All rabbits had free access to drinking water and were provided 170 g of feed (on an as-fed basis) daily (at 08.00 and 16.30 hours) during the entire rearing period. No antibiotics were added to the food or drinking water during the experiment. The feeding trial lasted 67 d, which included a 7-d adjustment period and 60-d experimental period.
Table 1. Composition and nutrient levels of basal diet
(Percentages, air-dry basis)

* Digestible energy was calculated, whereas the others were measured values.
† Premix provided the following per kg of diet: vitamin A, 6 mg; vitamin D3 0·05 mg; vitamin E, 50 mg; vitamin K3, 4 mg; Cu, 20 mg; Zn, 50 mg; Mn, 30 mg; Fe, 100 mg; iodine, 0·5 mg; Se, 0·15 mg; choline chloride, 1500 mg; nicotinamide, 100 mg; biotin, 20 mg.
Feed samples from each experimental diet were prepared in duplicate and analysed for crude protein, crude fibre, diethyl ether extract, Ca, P, lysine, and methionine following the Association of Official Analytical Chemists International procedures(22).
Growth performance
Individual weights were measured at the beginning and end of the trial, and the average daily gain was calculated. The F:G indicates the ratio of daily feed intake to average daily gain. Mortality and health status were visually assessed and recorded daily throughout the whole experimental period. The magnitude of each performance parameter was adjusted for rabbit mortality.
Sample collection and preparation
At the end of the 67-d feeding trial, forty-eight rabbits (twelve rabbits per group, six males and six females, with BW close to the average group BW) were selected for blood sampling through the ear vein before the morning feeding and watering. Sera separated by the centrifugation of blood at 3000 g for 10 min were stored in 1·5-ml Eppendorf tubes at –80°C until analysis. When blood sampling was complete, the rabbits were electrically stunned and sacrificed by exsanguination from the carotid artery. The liver and adipose tissue were harvested and weighed immediately after dissection. All weights, including those of the liver, perirenal fat, and subcutaneous fat, are expressed as a percentage relative to the live BW before slaughter. Liver samples were frozen in liquid N2, vacuum packed and stored at –80°C for lipogenic enzyme activity and apo concentration assays. Jejunal mucosa samples were collected, washed with normal saline, frozen as aliquots in liquid N2, and stored at –80°C to measure mRNA abundance and enzyme activity.
Serum amino acids analysis
Serum was deproteinised by mixing equal volumes of serum and TCA (7·5 % w/v), vortexed (30 s), and centrifuged for 15 min at 15 000 g. Subsequently, a 20-μl aliquot of the supernatant was injected into a high-performance liquid chromatography column (Hitachi L-8900 Amino Acid Analyzer). Amino acids were separated by cation exchange using lithium buffers, with the UV light detection (570 nm) of individual amino acids (440 nm for proline) performed by postcolumn ninhydrin derivatisation.
Intestinal pyrroline-5-carboxylate synthase and carbamoylphosphate synthase-І activity analysis
Jejunal mucosa samples were homogenised in ten volumes of cold normal saline. The homogenates were then centrifuged at 20 000 g for 20 min at 4°C, and the supernatant was collected for further analyses. The activities of P5CS and CPS-I of jejunum were analysed spectrophotometrically (UV-2000; Unico Instruments Co. Ltd) using commercial kits from Hengyuan Biological Technology Co. Ltd according to the manufacturer’s instructions. The jejunum protein concentrations were determined using Coomassie brilliant blue G-250 reagent with bovine serum albumin (BSA) as a standard, and the P5CS and CPS-I activities are expressed as units per mg of protein.
Pyrroline-5-carboxylate synthase and carbamoylphosphate synthase-І mRNA expression assay
Total RNA was extracted from jejunal mucosa samples using TRIzol reagent (Invitrogen). The quality of the total RNA was assessed by both native RNA electrophoresis on a 1·0 % agarose gel and the UV absorbance ratio at 260 and 280 nm. Then, complementary DNA was synthesised from 2 μg of total RNA with M-MLV RT (TaKaRa) at 42°C for 60 min with oligo(dT) adaptor primer using the manufacturer’s protocol.
The sequences of gene-specific primers for P5CS, CPS-I, and β-actin (an endogenous reference gene) are shown in Table 2. The mRNA abundance was determined with a StepOnePlus Real-Time PCR system (Applied Biosystems). PCR was carried out with a SYBR Premix PCR kit (TaKaRa). The PCR programme was 95°C for 10 min followed by forty cycles of 95°C for 15 s and 60°C for 60 s. The standard curve was determined using pooled samples. The efficiency of the real-time PCR primers for all the examined genes was calculated from standard curves. Each sample was run in duplicate, and a no-template control was included. The specificity of the amplification was verified at the end of PCR by melting curve analysis. The difference in cycle threshold (Ct) values for β-actin was less than 0·5 between all treatments, and β-actin was therefore considered to be an appropriate endogenous control. The average gene expression for each sample relative to expression of the endogenous control was calculated using the 2–ΔΔCt method(Reference Livak and Schmittgen23). The average ΔCt value of the control group was used to calibrate each studied gene.
Table 2. Gene-specific primers used for the analysis of rabbit gene expression
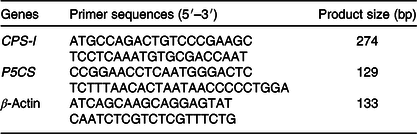
CPS-I, carbamoylphosphate synthase-І; P5CS, pyrroline-5-carboxylate synthase.
Lipogenic enzyme activity and apo concentration in the liver
Liver samples (approximately 0·5 g each) were homogenised in 5 ml of cold normal saline. The homogenates were then centrifuged at 20 000 g for 20 min at 4°C, and the supernatants were collected for further analyses. The malic dehydrogenase and FAS activities were measured using ultraviolet chromatometry with commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute). The concentrations of apo A, B, and E were analysed with a SpectraMax M5 microplate reader (Molecular Devices) using ELISA kits (Shanghai Ding Biological Technology Co. Ltd) according to the manufacturers’ instructions. The total protein concentration in each supernatant was determined using Coomassie brilliant blue G-250 reagent with BSA as a standard, and the concentrations/activities of the aforementioned parameters were expressed as units per mg of protein.
Determination of the concentrations of serum metabolites related to lipogenesis
The serum concentrations of cholesterol, TAG, LDL-cholesterol, and HDL-cholesterol were measured spectrophotometrically using commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute).
Analyses of serum nitric oxide concentration, nitric oxide synthase activity, and hormone levels
Serum NO concentration, inducible NOS activity, and total NOS activity were measured spectrophotometrically using commercial diagnostic kits (Nanjing Jiancheng Bioengineering Institute). Insulin, GH, and IGF-1 levels were determined by radioimmunoassays using kits from Nanjing Jiancheng Bioengineering Institute. The intra-assay CV for hormone levels was less than 5 %.
Measurements of hepatic health indicators
Serum ammonia content, alanine transaminase, aspartate transaminase, and cholinesterase activity, served as hepatic health indicators according to previous studies with human and domestic animals(Reference Silanikove and Tiomkin24–Reference Yousef, Awad and Mohamed27), were evaluated using reagent kits purchased from Nanjing Jiancheng Bioengineering Institute following the manufacturer’s protocol.
Statistical analysis
All statistical procedures, means and standard errors of the mean were calculated using the statistical software SPSS 18.0 (SPSS, Inc.). Data are presented as mean values with their standard errors and were analysed by one-way ANOVA, in which the individual rabbit served as the experimental unit (n 40 for growth performance parameters and n 12 for all other parameters in this study). The linear and quadratic effects of NCG among the treatment groups were analysed using a contrast statement. Significant differences among treatments were tested using Duncan’s multiple range test. The relationships between variables were analysed using Pearson’s correlation coefficient. A probability value of P < 0·05 indicated statistical significance.
Results
Serum concentrations of arginine, proline, and cysteine (P = 0·01, P = 0·01, and P < 0·01, respectively) responded to increasing levels of NCG in a linear manner; their maximal levels were observed in the 0·08 % NCG group (Fig. 1). Serum phenylalanine levels decreased (linear, P = 0·04) in response to dietary NCG supplementation at 0·12 %. NCG treatment had no effect on the concentrations of the other measured amino acids (including lysine) (online Supplementary Table S1). The intestinal CPS-I activity displayed a linear (P < 0·01) and quadratic trend (P = 0·04) following NCG supplementation, with the maximum response observed in the 0·08 % NCG group (Fig. 1). No significant difference in the activity of intestinal P5CS or the mRNA expression of intestinal CPS-I and P5CS was observed between NCG-supplemented and control rabbits (Fig. 1).

Fig. 1. Effects of dietary supplementation with N-carbamylglutamate (NCG) on concentrations of serum amino acids (A), activities (B) and mRNA expression (C) of intestinal pyrroline-5-carboxylate synthase (P5CS) and carbamoylphosphate synthase-I (CPS-I) in Rex rabbits. Values are means with their standard errors of twelve rabbits. Control, rabbits fed a basal diet; 0·04, 0·08, and 0·12 % NCG, rabbits fed the basal diet supplemented with 0·04, 0·08, and 0·12 % NCG, respectively. Relative gene expression was calculated using the 2–ΔΔCt method with β-actin as the endogenous control and the average ΔCt value of the control group as the calibrator. a,b Mean values with unlike letters are significantly different (P < 0·05; linear or quadratic effects of NCG levels).  , Control;
, Control;  , 0·04 % NCG;
, 0·04 % NCG;  , 0·08 % NCG;
, 0·08 % NCG;  , 0·12 % NCG.
, 0·12 % NCG.
The final BW and growth performance (i.e. average daily gain and F:G) were not significantly affected by NCG supplementation in the diet (online Supplementary Table S2). The perirenal fat percentage decreased in a linear manner (P = 0·01) in response to dietary NCG at 0·08 and 0·12 %, while the subcutaneous fat percentage decreased (linear, P = 0·04) in response to dietary NCG at 0·08 % (Fig. 2). In addition, all rabbits were healthy, and no deaths occurred throughout the entire experimental period.

Fig. 2. Effects of dietary supplementation with N-carbamylglutamate (NCG) on subcutaneous and perirenal fat percentages in Rex rabbits. Values are means with their standard errors of twelve rabbits. Control, rabbits fed a basal diet; 0·04, 0·08, and 0·12 % NCG, rabbits fed the basal diet supplemented with 0·04, 0·08, and 0·12 % NCG, respectively. a,b Mean values with unlike letters are significantly different (P < 0·05; linear effects of NCG levels).  , Control;
, Control;  , 0·04 % NCG;
, 0·04 % NCG;  , 0·08 % NCG;
, 0·08 % NCG;  , 0·12 % NCG.
, 0·12 % NCG.
Rabbits whose diet was supplemented with 0·08 and 0·12 % NCG had lower (linear, P < 0·01) hepatic FAS activity than the control rabbits (Fig. 3). There were no differences in hepatic malic dehydrogenase activity or apo (apo A, apo B and apo E) levels among the groups (Fig. 3). The effects of dietary supplementation with NCG on serum metabolites related to hepatic lipogenesis in Rex rabbits are shown in Table 3. Serum cholesterol, HDL-cholesterol, and LDL-cholesterol levels, and the ratio of HDL-cholesterol to LDL-cholesterol were not significantly different among the treatment groups. The serum TAG concentration (P < 0·01) responded to increasing levels of NCG in a linear manner; the 0·12 % NCG group had a lower TAG concentration than both the control and the 0·04 % NCG groups.
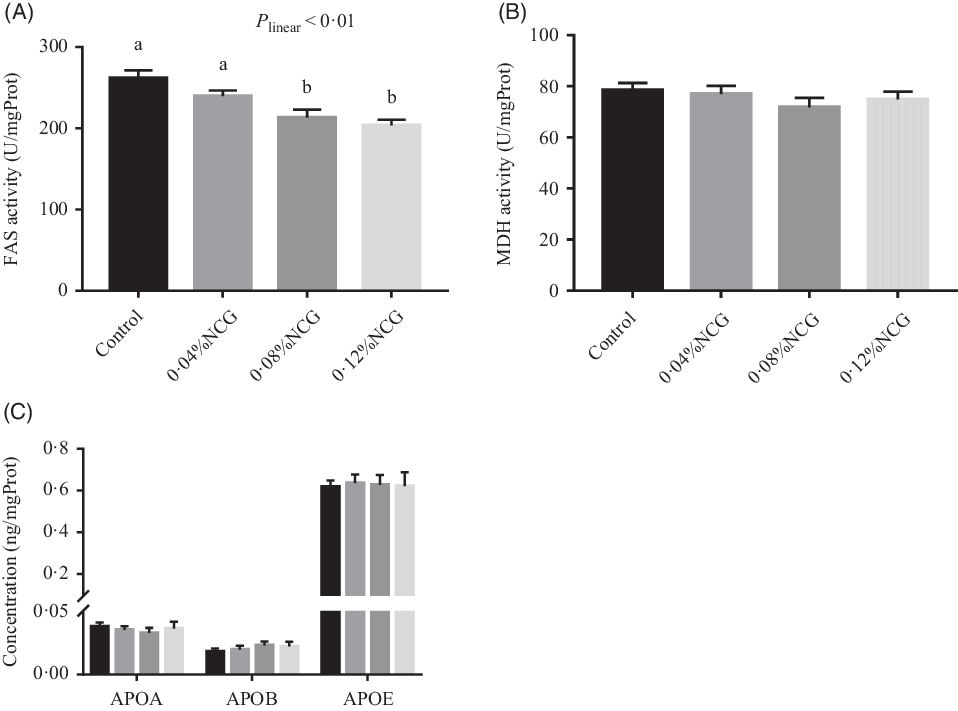
Fig. 3. Effects of dietary supplementation with N-carbamylglutamate (NCG) on lipogenic enzyme activity (A and B) and apo concentration (C) in the liver of Rex rabbits. Values are means with their standard errors of twelve rabbits. Control, rabbits fed a basal diet; 0·04, 0·08, and 0·12 % NCG, rabbits fed the basal diet supplemented with 0·04, 0·08, and 0·12 % NCG, respectively. a,b Mean values with unlike letters are significantly different (P < 0·05; linear effects of NCG levels). FAS, fatty acid synthetase; mgProt, mg protein; MDH, malic dehydrogenase; APOA, apo A; APOB, apo B; APOE, apo E. (C)  , Control;
, Control;  , 0·04 % NCG;
, 0·04 % NCG;  , 0·08 % NCG;
, 0·08 % NCG;  , 0·12 % NCG.
, 0·12 % NCG.
Table 3. Effects of dietary supplementation with N-carbamylglutamate (NCG) on serum metabolites related to hepatic lipogenesis in Rex rabbits
(Mean values with their standard errors – twelve rabbits per treatment)

H:L, ratio of HDL-cholesterol to LDL-cholesterol.
a,b,c Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Linear and quadratic effects of NCG levels.
The effects of dietary supplementation with NCG on NO production and NOS activity in Rex rabbits are shown in Fig. 4. The serum NO concentration increased in a linear manner (P = 0·02) in response to dietary NCG supplementation at 0·08 and 0·12 %, while serum inducible NOS and total NOS activities increased in a linear manner (P = 0·05 and P = 0·01, respectively) in response to dietary NCG supplementation at 0·08 %.

Fig. 4. Effects of dietary supplementation with N-carbamylglutamate (NCG) on nitric oxide production and nitric oxide synthases (NOS) activity in Rex rabbits. Values are means with their standard errors of twelve rabbits. Control, rabbits fed a basal diet; 0·04, 0·08, and 0·12 % NCG, rabbits fed the basal diet supplemented with 0·04, 0·08, and 0·12 % NCG, respectively. a,b Mean values with unlike letters are significantly different (P < 0·05; linear effects of NCG levels). iNOS, inducible nitric oxide synthase; TNOS, total nitric oxide synthase.
In this study, we also found that the serum concentration of GH was significantly (P = 0·04; Fig. 5) higher in the 0·08 and 0·12 % NCG treatment groups compared with the control group, but did not differ significantly from each other. In addition, serum IGF-1 levels increased in a linear manner (P = 0·04), with the maximum response observed in the 0·12 % NCG group. There were no differences in serum insulin concentrations among the four treatments.

Fig. 5. Effects of dietary supplementation with N-carbamylglutamate (NCG) on hormone levels in Rex rabbits. Values are means with their standard errors of twelve rabbits. Control, rabbits fed a basal diet; 0·04, 0·08, and 0·12 % NCG, rabbits fed the basal diet supplemented with 0·04, 0·08, and 0·12 % NCG, respectively. a,b Mean values with unlike letters are significantly different (P < 0·05; linear effects of NCG levels). GH, growth hormone; IGF-1, insulin-like growth factor 1.
Table 4 shows correlations between hepatic lipogenesis parameters and body fat deposition and the concentration of serum NO, GH, and IGF-1 in rabbits after NCG treatment. There was a significant positive correlation between the TAG content and perirenal fat percentage (P < 0·01) in addition to the FAS activity and perirenal fat percentage (P < 0·05), and a negative correlation between the TAG and NO contents (P < 0·05) in addition to the FAS activity and IGF-1 content (P < 0·05).
Table 4. Correlations between hepatic lipogenesis parameters and body fat deposition and the concentrations of serum nitric oxide, growth hormone (GH) and insulin-like growth factor 1 (IGF-1) in rabbits after N-carbamylglutamate (NCG) treatment
(Pearson’s correlation coefficients)

FAS, fatty acid synthetase.
* Significant correlation between two indices (P < 0·05; two-tailed).
The results of assays to assess hepatic health indicators as a further measure of the response of rabbits to the nutritional regimens tested are depicted in Table 5. Our results showed that dietary NCG supplementation had no effect on the serum alanine transaminase, aspartate transaminase, and cholinesterase activities or the liver index, expressed as the percentage of the BW. The concentration of serum ammonia decreased in a linear manner (P = 0·02) response to increasing levels of NCG, and the lowest serum ammonia concentration was observed in the 0·08 % NCG group.
Table 5. Effects of dietary supplementation with N-carbamylglutamate (NCG) on hepatic health indicators in Rex rabbits
(Mean values with their standard errors – twelve rabbits per treatment)

ALT, alanine transaminase; AST, aspartate transaminase; CHE, cholinesterase.
a,b Mean values within a row with unlike superscript letters are significantly different (P < 0·05).
* Linear and quadratic effects of NCG levels.
Discussion
NCG is a metabolically stable analogue of N-acetylglutamate, which has been proven to increase the intestinal endogenous synthesis of arginine and arginine level in circulating blood(Reference Wu, Knabe and Kim9). In the present study, we consistently found that the serum concentration of arginine was higher in rabbits in the 0·08 and 0·12 % NCG groups. This increase was accompanied by an increase in the concentration of proline, a precursor for intestinal arginine synthesis(Reference Wu28). Additionally, NCG treatment had no effect on the serum lysine level in rabbits, suggesting that NCG supplementation did not cause lysine-arginine antagonism. Inconsistent with a previous report showing that NCG stimulated intestinal arginine synthesis via activating CPS-1 and P5CS in piglets(Reference Wu, Knabe and Kim9), dietary supplementation with NCG in the present study had a significant effect on the activity of only intestinal CPS-I in rabbits. To confirm this result, the gene expression levels of CPS-I and P5CS were further determined. Unfortunately, dietary supplementation with NCG had no significant effect on the mRNA expression levels of these enzymes. Nevertheless, our results suggested that dietary NCG regulates intestinal CPS-I at the posttranscriptional level in rabbits. Another interesting finding of this study is that 0·04 % NCG supplementation was not sufficient to stimulate the endogenous synthesis of arginine, although dietary 0·04 % NCG increased CPS-I activity and its substrate (proline) concentration for arginine synthesis in vivo. Furthermore, the significant decrease in the concentration of an essential amino acid (phenylalanine) in response to dietary NCG supplementation at 0·12 % was rather astonishing. However, knowledge of the influence of NCG on phenylalanine metabolism appears limited, and we have been unable to find any other study with which to confirm this result. The increased serum cysteine concentration may imply that dietary NCG supplementation has beneficial effects on antioxidant capacity in animals because cysteine, as one of the amino acids involved in glutathione synthesis, is involved in the body’s antioxidant defence(Reference Nina, Ana and Mihaela29). A recent study showed that dietary NCG supplementation enhanced antioxidant status against oxidative stress in rats(Reference Cao, Xiao and Liu11).
The most novel and significant result of the present study is that the perirenal and subcutaneous fat contents expressed as the percentage of BW were significantly lower in the 0·08 and 0·12 % NCG groups compared with the control group. Moreover, the addition of NCG did not appear to significantly affect hepatic health indicators, except for the ammonia concentration, which was obviously decreased by 0·08 and 0·12 % in NCG-treated rabbits compared with the control rabbits. Similarly, dietary NCG supplementation was shown to decrease the plasma ammonia concentration in Holstein heifers(Reference Wang, Azarfar and Wang30) and piglets(Reference Yang, Fu and Kong31). The accumulation of non-protein N in the form of ammonia is the most likely cause of liver damage(Reference Silanikove and Tiomkin24). Decreased ammonia accumulation in the blood may be due to the increased concentration of arginine, because arginine plays a crucial role in ammonia detoxification via the hepatic urea cycle when CPS-I is activated(Reference Oba, Baldwin and Owens32). Our results may indicate that dietary NCG supplementation is effective in reducing unfavourable fat deposition in rabbits with no negative effects on hepatic health. In the present study, weight gain and final BW did not differ between control and NCG-treated rabbits. Thus, decreased fat deposition in NCG-treated rabbits might be accompanied by greater tissue protein accretion. Previous studies proved that NCG supplementation increased muscle protein synthesis in piglets(Reference Frank, Escobar and Nguyen33). Inconsistent with our results, a study conducted by Wu et al.(Reference Wu, Knabe and Kim9) did not find a difference in fat content between NCG-supplemented and control piglets. However, Wu et al.(Reference Wu, Knabe and Kim9) supplemented the diet of piglets from 4 to 14 d of age with NCG, whereas we supplemented the diet of rabbits from 3 to 5 months of age with NCG. Notably, young animals exhibit a rapid growth rate, but this high growth rate is not linked to excessive fat deposition during early development(Reference Tzeng and Becker34). Therefore, the conflicting results on the effects of NCG on fat deposition in these two studies may be attributed to the timing of NCG supplementation.
The development of adipose tissue is thought to be related to the ability to undergo lipogenesis(Reference Muñoz, Estany and Tor35). Because the liver is the major site of lipogenesis in growing rabbits(Reference Liu, Fu and Li36), the activities of enzymes related to lipogenesis were measured in this study. We found that the FAS activity was significantly lower in rabbits fed a diet containing 0·08 or 0·12 % NCG compared with those fed the control diet. FAS is an essential enzyme in the final step of the lipogenic pathway(Reference Wu, Fang and Guo7). Serum cholesterol, TAG, LDL-cholesterol, and HDL-cholesterol are produced by hepatic lipogenesis(Reference Álvaro, Paucar and Satué37). The inhibition of hepatic FAS activity reduces the availability of fatty acids for esterification into TAG and storage in adipose tissue. As expected, serum TAG levels were significantly lower in the 0·08 and 0·12 % NCG groups compared with the control group. Intriguingly, the serum TAG concentration and hepatic FAS activity were significantly positively correlated with perirenal fat content but not subcutaneous fat content in rabbits after NCG treatment. NCG supplementation seemed to preferentially reduce fat accumulation in the perirenal fat depot rather than in the subcutaneous fat depot by inhibiting hepatic lipogenesis. Apo are major components of LDL and VLDL particles, which transport TAG from the liver and hence transport endogenous fat to the blood circulation(Reference Yin, Qi and Huo38). In the present study, the hepatic apo contents (apo A, apo B and apo E) was not altered by NCG supplementation of the rabbits’ diet, indicating that NCG treatment only inhibited hepatic lipogenesis but did not affect the process of TAG transport from the liver to extrahepatic tissues.
To explore the possible mechanisms of NCG regulation of lipogenesis, the effects of NCG supplementation on the production of NO and hormones were further measured. There is growing evidence that arginine reduces both blood TAG levels and body fat partly through the production of NO(Reference Tan, Yin and Liu2,Reference Jobgen, Fried and Fu20) . Consistent with the increase in the serum arginine concentration observed in the present study, the addition of 0·08 or 0·12 % NCG stimulated NO production by promoting NOS activity. Although the serum NO level was not significantly correlated with hepatic lipogenic enzyme activity, it showed a strong negative correlation with the lipogenic product (i.e. TAG) concentration in rabbits after NCG treatment. Lipogenesis in hepatocytes is also dependent on hormone (e.g. GH and insulin) status(Reference Baker and Gibbons39). In the present study, GH levels increased in NCG-treated rabbits. However, we did not find a significant correlation between serum GH level and hepatic lipogenic ability. Therefore, the relationship between GH and hepatic lipogenesis in the NCG-treated rabbits remains unclear and warrants further investigation. Notably, NCG supplementation increased the serum concentration of IGF-1, compared with the control group. Circulating IGF-I is synthesised by stimulation of GH and works together with GH to support metabolic function in adults(Reference Vijayakumar, Novosyadlyy and Wu40). In the present study, we also found a strong negative correlation between the serum IGF-1 level and hepatic lipogenic enzyme activity after NCG treatment. Collectively, our data in rabbits indicated that dietary supplementation with NCG might reduce hepatic lipogenesis in rabbits through modulating the arginine-NO pathway and the synergistic activity of GH and IGF-1.
In conclusion, NCG could serve as a dietary supplement to reduce unfavourable fat deposition in rabbits since it appeared to have no negative effects on growth performance and hepatic health. Although the exact mechanism by which NCG at high doses decreases circulating phenylalanine remains unclear, the amount of NCG used to supplement the diet needs to be considered to prevent essential amino acids deficiency. NCG supplementation preferentially reduced fat accumulation in the perirenal fat depot rather than the subcutaneous fat depot by inhibiting hepatic lipogenesis. In addition, the arginine-NO pathway, as well as the synergistic activity of GH and IGF-1, might be involved in the NCG-mediated regulation of hepatic lipogenesis in rabbits. Because fat deposition is determined by the balance between lipogenesis and lipolysis/fatty acid oxidation inside the body, the effect of dietary NCG addition on lipolysis should be further studied.
Acknowledgements
This work was supported by Ningbo key agricultural projects (2014C10012), Ningbo key projects to enrich the people through science and technology (2015C10041), and the agricultural technology extension funds of Zhejiang University.
Q. X. conducted a literature review and analysed the data. Q. S., X. L., and W. L. conducted the animal trial and the laboratory work. X. D. and X. Z. co-wrote manuscript and approved the final version. Y. W. was the principle investigator. He designed the experiment and oversaw the development of the study.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000860













