One of the well-recognised ageing theories indicates that generation of oxidative stress leads to cell and tissue damage and ultimately results in ageing and cell death(Reference Harman1). Chronic administration of d-galactose (DG) to BL/6J mice has been shown to induce changes which resemble accelerated ageing, such as formation of advanced glycation end products(Reference Song, Bao and Li2, Reference Hsieh, Wu and Hu3), neurological impairment(Reference Cui, Zuo and Zhang4, Reference Zhang, Li and Cui5), decreased serum antioxidative enzyme activities(Reference Cui, Zuo and Zhang4, Reference Zhang, Li and Cui5) and inflammation in the liver(Reference Hsieh, Wu and Hu3, Reference Zhang, Fan and Zheng6). Several antioxidants(Reference Hsieh, Wu and Hu3, Reference Lu, Zheng and Luo7, Reference Shu, Li and Yan8) and Chinese herbs(Reference Liu, Ho and Lai9) have been shown to attenuate the ageing damage in C57BL/6J mice treated with DG.
Fructo-oligosaccharide (FO), a well-defined prebiotic(Reference Gibson10), has been incorporated into drinks and desserts to improve bowel function in elderly persons(Reference Chen, Lu and Lin11). Recent clinical studies have initially shown that the consumption of a prebiotic mix(Reference Seidel, Boehm and Vogelsang12) and a FO supplement(Reference Yen, Kuo and Tseng13) beneficially reduces the blood indices of peroxidation status. However, the effect of FO in DG-induced hepatic oxidative damage has never been demonstrated.
The main goal of the present study was to assess the anti-ageing and hepatoprotective effects of FO in DG-administered Balb/cJ mice, by the determination of antioxidative enzyme activities and the morphology of the liver and the biochemical indices of liver function.
Materials and methods
Animals and diets
Male Balb/cJ mice (10 weeks old) were obtained from the BioLASCO Taiwan Company Limited (Taipei, Taiwan, ROC). After acclimatisation for 2 weeks, mice (n 40) were randomly divided into four groups (n 10): control (subcutaneous saline, basal diet), DG (subcutaneous 1·2 g d-galactose/kg body weight, basal diet), DG+FO (50 g active ingredients/kg basal diet) and DG+vitamin E (2 g α-tocopherol/kg basal diet, as an antioxidant positive control) and were killed after 52 d of treatment. The basal diet consisted of a ground rodent chow (Lab 5001; Purina Mills, St Louis, MO, USA) and sucrose that match the digestible sugar present in the FO syrup (Institute of Microbial Resources, Taichung, Taiwan, ROC)(Reference Chen, Lu and Lin11). The mixed powder diet was then re-formed into a small dough with deionised water in order to balance the liquid content among diets and to reduce spillage. The doses of supplements were used based on previous studies, indicating that supplementing 5 % (w/w) FO into a fibre-free diet beneficially modulated colonic microflora and reduced faecal toxicity(Reference Yeh, Lin and Chen14), while 0·2 % (w/w) α-tocopherol altered pro-oxidation status in rats(Reference Shin, Chang and Yang15). All animals were allowed to have free access to water and food during the study. Animal care followed the guidelines of the National Research Council(16) and was approved by the Institutional Animal Care and Use Committee in the Chung Shan Medical University. The mice were placed in metabolism cages during days 44–49, while fresh faeces were collected and frozen within 30 min of excretion. Faecal samples were lyophilised and kept at − 20°C for further analyses of microflora. The mice were anaesthetised with CO2 on day 52 after a 20 h fasting. Blood samples collected from the right atrium into heparinised tubes were centrifuged at 3000 g for 10 min and plasma samples were stored at − 20°C for the analysis of alanine aminotransferase activity. The mice were transcardially perfused with ice-cold normal saline (n 6) or neutral formalin (n 4) for 5 min or until the liver turned grey. Heparinised blood samples and livers, dissected from saline-perfused mice were frozen immediately for further analyses of enzyme activities, while those dissected from neutral formalin-perfused mice were fixed further with Bouin's solution overnight and then processed for histological routine.
Hepatic antioxidative enzymes
A portion of each liver was homogenised in 10 vol (v/w) of phosphate buffer (0·1 m, pH 7·4, containing 1 mm-EDTA). The homogenate was centrifuged at 10 000 g for 15 min at 4°C and the supernatant was immediately analysed. Superoxide dismutase activity was measured based on competition for superoxide radicals between superoxide dismutase and tetrazolium salt(Reference Flohé, Becker, Brigelius, Miquel, Quintanilha and Weber17). Glutathione peroxidase activity was measured indirectly by a coupled reaction with glutathione reductase that converted the oxidised glutathione to its reduced form with a concomitant oxidation of NADPH to NADP+(Reference Flohé and Gunzler18). The catalase activity was measured colorimetrically based on the transformation of methanol to formaldehyde using 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole(Reference Johansson and Håkan Borg19). Protein contents were analysed based on the Bradford method(Reference Bradford20), using a protein assay reagent (Life Science Research, Hercules, CA, USA). Enzyme activity was expressed as IU/mg protein.
Hepatic TAG content
A portion of each liver was homogenised and extracted with 20 vol (v/w) of a chloroform–methanol mixture (2:1, v/v), according to Folch et al. (Reference Folch, Lees and Sloane Stanley21). Aliquots of lipid extracts were dried under vacuum and then dissolved in 95 % ethanol. Hepatic TAG concentrations were determined after enzymatic hydrolysis with lipases using a commercial kit (Randox Laboratories, San Francisco, CA, USA).
Plasma alanine aminotransferase activity
Plasma alanine aminotransferase activities were measured by catalysing the formation of pyruvate from alanine(Reference Horder, Rej and Bergmeyer22). Enzyme activity was expressed as μKat/l.
Quantification of faecal microflora by the fluorescence in situ hybridisation method
The genotypic probe Bif164 and a non-specific nucleic acid stain, 4′,6-diamidino-2-phenylindole, were used to quantify bifidobacteria and total bacteria, respectively(Reference Jansen, Wildeboer-Veloo and Tonk23). Probe fluorescence was detected with a Zeiss Axioskop2 microscope (Carl Zeiss, Jena, Germany), as described previously(Reference Chen, Cheng and Liu24).
Histological evaluation
Liver tissues were fixed in Bouin's solution overnight and then processed for histological routine. Paraffin sections (4 μm) were mounted on microscope slices and stained with haematoxylin and eosin. Histological evaluation was done under 200 × magnification.
Statistical analyses
Data were presented as means with their standard errors and analysed using SPSS version 14 (SPSS, Inc., Chicago, IL, USA). Parametric data and log-transformed bacteria counts were analysed by one-way ANOVA, followed by the least significant difference test. Differences were considered significant at P < 0·05.
Results
Body and liver weights
There were no significant differences in body weight and weight gain among groups (data not shown). Relative liver weight (percentage of body weight) was similar among groups, 4·7 (se 0·6) , 4·7 (se 0·5) , 4·6 (se 0·4) and 4·7 (se 0·6) % for the control, DG, DG+FO and DG+vitamin E groups, respectively.
Antioxidative enzyme activities and TAG content in the liver
Superoxide dismutase and glutathione peroxidase activities were significantly suppressed by DG treatment by approximately 32·3 % (P < 0·001 v. control) and approximately 29·7 % (P = 0·016 v. control), respectively (Table 1). Both FO (P < 0·001 v. DG) and vitamin E (P < 0·001 v. DG) reversed the DG-induced decrease in superoxide dismutase activity, and tended to ameliorate (P = 0·49 for DG+FO v. control; P = 0·11 for DG+vitamin E v. control) the DG-induced decrease in glutathione peroxidase activity. Catalase activity was non-significantly altered by DG (P>0·05 v. control). However, catalase activity in the DG+vitamin E group was raised by approximately 20·0 % (P = 0·049 v. DG). Hepatic TAG concentration was 234·4 (se 5·6), 238·6 (se 3·4) and 243·8 (se 5·6) μmol/g liver in the control, DG+FO and DG+vitamin E groups, respectively, all of which were significantly lower than that shown in the DG group, 252·6 (se 3·4) μmol/g liver (P < 0·05, respectively).
Table 1 Hepatic antioxidative enzyme activities in d-galactose-treated Balb/cJ mice
(Mean values with their standard errors, n 6)
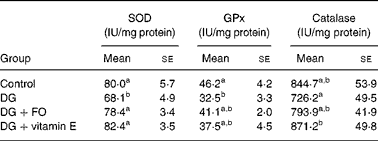
SOD, superoxide dismutase; GPx, glutathione peroxidase; DG, d-galactose; FO, fructo-oligosaccharide.
a,b Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
Plasma alanine aminotransferase activity
The plasma alanine aminotransferase level (μKat/l) was 0·20 (se 0·03), 0·32 (se 0·05) (P = 0·016 v. control), 0·22 (se 0·02) and 0·17 (se 0·02) in the control, DG, DG+FO and DG+vitamin E groups, respectively. The DG-induced change in alanine aminotransferase activity was normalised by FO (P>0·05 v. control) and vitamin E (P>0·05 v. control), respectively.
Histopathological observation
The liver histological study was conducted to determine the protective effect of FO on DG-induced injury. The hepatocytes in the DG group were filled with lipids in the absence of the nucleus (Fig. 1(b)). However, this histological alteration was not observed in the control (Fig. 1(a)) group, and was ameliorated in the presence of FO (Fig. 1(c)) and vitamin E (Fig. 1(d)).

Fig. 1 Liver histology in Balb/cJ mice treated for 52 d with (a) vehicle control (saline, subcutaneous (s.c.)), (b) d-galactose (1·2 g/kg, s.c.), (c) d-galactose (1·2 g/kg, s.c.)+fructo-oligosaccharide (5 %, w/w) or (d) d-galactose+vitamin E (0·2 %, w/w). Livers were dissected after systematic perfusion with neutral formalin (n 4) for 5 min and then fixed in Bouin's solution overnight. Tissues were processed for histological routine and stained with haematoxylin and eosin (original magnification, 200 × ). Scale bar represents 50 μm.
Faecal microflora
The faecal bifidobacteria concentration was the lowest in the DG group, which was significantly increased by FO (P = 0·001 v. DG) and vitamin E (P = 0·022 v. DG), respectively (Table 2). The faecal total bacteria counts were similar among groups. FO significantly (P = 0·032 v. DG) increased the relative proportions (percentage of total bacteria) of faecal bifidobacteria to 33·0 (se 2·3 ) % compared with that in the DG group, 21·0 (se 2·0 ) %.
Table 2 Faecal total bacteria and Bifidobacterium counts of d-galactose-treated Balb/cJ mice
(Mean values with their standard errors, n 10)
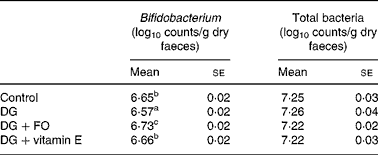
FO, fructo-oligosaccharide; DG, d-galactose.
a,b,c Mean values with unlike superscript letters within a column were significantly different (P < 0·05; ANOVA followed by the least significant difference test).
Discussion
This is the first study to show that FO, besides its prebiotic effect on colonic microflora(Reference Yen, Kuo and Tseng13, Reference Yeh, Lin and Chen14), exerted systematic effects on antioxidative enzyme activities and TAG synthesis in the liver that were altered by chronic DG administration in Balb/cJ mice.
d-Galactose is normally metabolised by d-galactokinase and galactose-1-phosphate uridyltransferase(Reference Kaplan and Pesce25). An overdose of DG leads to the accumulation of galactitol, which in turn leads to osmotic stress and the generation of reactive oxygen species(Reference Wang26). In the present study, we found that FO, similar to the antioxidant vitamin E, prevented the decrease in hepatic superoxide dismutase activity and potentially modulated glutathione peroxidase activity, suggesting that the utilisation of FO in the large intestine exerted systematic antioxidative effects. Although mechanisms remained unclear, in vitro studies have shown that lactic acid bacteria per se (Reference Lin and Chang27, Reference Lin and Yen28) and the fermentations of FO by several strains of bifidobacteria(Reference Wang, Lai and Chen29) exert free radical-eliminating effects. Therefore, FO may reduce hepatic oxidative stress partially through its fermentation product by colonic lactic acid bacteria. An increased faecal bifidobacteria concentration observed in the DG+FO group could further enhance the antioxidative ability of FO.
Incorporation of FO into a high-carbohydrate diet has been shown to suppress the activity of lipogenic enzymes in rats(Reference Delzenne and Kok30). The present study further indicated that FO reduced fatty liver in DG-treated mice, which may be mediated by propionate that is shown to down-regulate liver lipogenesis(Reference Delzenne and Williams31). Vitamin E diminished hepatic TAG accumulation in the present study, which, on the other hand, may be mediated by its potential enhancing effect on PPAR(Reference Azzi, Gysin and Kempná32) that is shown to regulate fatty acid oxidation(Reference Reddy and Rao33).
FO is easily incorporated into drinks. The elderly with poor oral function can obtain sufficient dietary fibre by taking this type of dietary supplement. The level of FO offered in the present study is equivalent to 25 g/d for adults whose daily dry food intake is 500 g. An adequate intake for total fibre in foods is set as 25 and 38 g/d for young women and men, respectively(34). Therefore, the dose of FO supplement used in the present study is applicable to adults, including the elderly.
In conclusion, the present study suggests that FO, besides being a prebiotic fibre, could prevent oxidative stress and fatty liver that occur during ageing.
Acknowledgements
The present study was partially funded by the National Science Council of Taiwan, NSC 96-2320-B-031-MY3. H.-L. C. designed and carried out the study, and wrote the manuscript. C.-H. W. collated and analysed the data and co-wrote the manuscript. Y.-W. K. carried out the study. C.-H. T. conducted the histological analysis. The technical assistance for the tissue slides from Mr Yang, Lien-Chuan, and the sponsor of FO by the Institute of Microbial Resources (Taichung, Taiwan, ROC) were greatly appreciated. All authors read and approved the findings of the study. There are no conflicts of interest.





