Due to extensive research done in the past three decades, it is well known that strenuous aerobic exercise can result in an increase of reactive oxygen and nitrogen species (RONS)(Reference Powers and Jackson1). However, there is now sufficient evidence that RONS are not only damaging agents, but also act as signalling molecules to initiate exercise adaptations(Reference Ji2) and to regulate muscle function(Reference Powers and Jackson1). In this context, most recent investigations indicated that high dosages of antioxidants can blunt cellular training adaptations(Reference Gomez-Cabrera, Domenech and Romagnoli3) and health-promoting effects(Reference Ristow, Zarse and Oberbach4). These findings have contributed important novel aspects to the ongoing debate whether supplementation with antioxidants is useful in terms of preventing or minimising oxidative stress and damage in response to strenuous exercise or detrimental by interfering with beneficial physiological effects of exercise training(Reference Vina, Gomez-Cabrera and Borras5). Until now, the question of antioxidant requirements (both regarding certain antioxidant micronutrients and regarding dosages of these antioxidants) for endurance athletes in general and the hypothetical need for additional antioxidant supplementation has not been discussed sufficiently(Reference Margaritis and Rousseau6). Among endurance athletes, Ironman/long-distance triathletes are an exceptional group to investigate due to their training and performance level, physiological characteristics(Reference Millet, Dreano and Bentley7) and nutritional challenges(Reference Jeukendrup, Jentjens and Moseley8), and urgently, to assess probable harmful effects of ultra-endurance sports in general. Some empirical and epidemiological data suggest that an extraordinarily high volume of exercise is associated with an increased risk of developing CVD(Reference Lee, Hsieh and Paffenbarger9) due to cumulative oxidative stress as one of the main potential mechanisms(Reference Knez, Coombes and Jenkins10). Therefore, it is of particular importance to determine antioxidant requirements for this continually growing population of athletes training for and competing in races with durations of several hours. Noteworthily, outcomes of studies that examined high-dosed antioxidant supplementation explicitly in competitors of long-distance triathlons or ultra-marathon runs are quite controversial, showing either beneficial(Reference Mastaloudis, Morrow and Hopkins11), adverse (i.e. pro-oxidant) effects(Reference Nieman, Henson and McAnulty12, Reference Knez, Jenkins and Coombes13) or no effects(Reference Nieman, Henson and McAnulty14) on the changes in the oxidative stress markers. Thus, instead of an interventional supply with antioxidants, it is reasonable to consider the nutritional conditions before, during and after such a competition. Therefore, when particularly addressing the question whether antioxidant requirements are modified in response to an acute ultra-endurance exercise, attention has to be paid to the antioxidant intake during such a competition and to the changes in the antioxidant status in response to this type of exercise. Furthermore, when drawing the focus on antioxidant requirements for this group of endurance athletes in general, it is important to take potential influencing factors, such as training-induced adaptations in the endogenous antioxidant system, interactions between endogenous antioxidant defences and nutritive antioxidants and their dietary pattern, into account.
The present data are part of a larger study that aimed to get a broader picture of exercise-induced skeletal muscle damage, inflammatory and immunological alterations(Reference Neubauer, König and Wagner15), myocardial stress(Reference König, Neubauer and Nics16), oxidative stress(Reference Neubauer, König and Kern17), genome stability(Reference Reichhold, Neubauer and Ehrlich18, Reference Reichhold, Neubauer and Hoelzl19) and the relationship of DNA damage with inflammatory responses(Reference Neubauer, Reichhold and Nersesyan20) in a large cohort of participants of an Ironman triathlon (n 42). Within the scope of the currently presented part of the present study, particular focus was drawn on the modifications in the plasma concentrations of endogenous low-molecular weight (i.e. uric acid, bilirubin and plasma protein) and nutritive antioxidants (i.e. α- and γ-tocopherol, carotenoids and vitamin C) after the Ironman race. Hence, the main objectives of this part of the present study are as follows: on the one hand, we aimed to particularise the relevance of the antioxidant responses to the previously reported indices of lipid peroxidation, protein oxidation and DNA stability in blood plasma and in circulating lymphocytes, respectively(Reference Neubauer, König and Kern17, Reference Reichhold, Neubauer and Hoelzl19). On the other hand, we intended to determine whether there are distinct time-points after an ultra-endurance exercise in which athletes are particularly vulnerable to oxidative damage. Importantly, we expected that the verification whether such hypothesised time-points are associated with alterations in the antioxidant status in plasma might provide indications for an increased requirement of nutritive antioxidants and a potential need for supplementation in certain phases after intense and very prolonged exercise. In the attempt to verify the time course of recovery, these responses were monitored until 19 d after the race, and thus longer than any other study in this context. Finally, it was important to examine oxidative stress and antioxidant responses under ‘authentic’ race conditions, i.e. without any nutritional intervention, but with careful assessment of the intake of antioxidant vitamins and carotenoids during the race and during the early recovery period.
Experimental methods
Study design and subjects
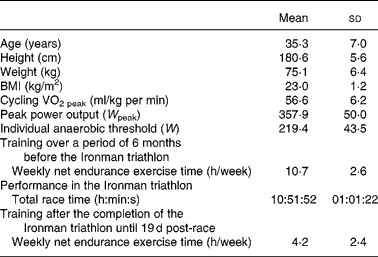
The design of the study has been described previously(Reference Neubauer, König and Wagner15, Reference Neubauer, König and Kern17). Briefly, it comprised forty-eight non-professional, well-trained male triathletes, who participated in the 2006 Ironman Austria. The study was conducted according the guidelines of the Declaration of Helsinki, and the study was approved by the Ethics Committee of the Medical University of Vienna. All the study participants were informed about the purpose and the risks of the investigation before they provided written informed consent. Forty-two of the subjects completed the study, and were included in the statistical analysis. The physiological characteristics of the study participants (assessed using a cycle ergometer 3 weeks before the Ironman triathlon) in addition to their training history are summarised in Table 1 (for more detailed information, see Neubauer et al. (Reference Neubauer, König and Wagner15) and Neubauer et al. (Reference Neubauer, König and Kern17)). All the participants were physically fit, non-smokers, within a normal range of BMI, and free of acute and chronic illnesses. They were asked to provide extensive information on their medical and health status, their training schedule and their nutritional habits including a supplementation and an FFQ. The subjects did not take any medication 6 weeks before the Ironman race until the end of the study. Crucially, as an inclusion criterion for participating in the study, they were instructed to avoid antioxidant supplementation at larger doses, i.e. more than 100 % of RDA(21) (in addition to their normal dietary antioxidant intake) throughout this period (except during the race, see below). Consequently, they did not consume more than 60 mg of vitamin C, 10 mg of α-tocopherol, 4 mg of β-carotene (which equates the upper level of the region-specific recommendation range(22) since there is no RDA value available), 70 μg of Se, 15 mg of Zn or any other antioxidant in the form of supplements. Compliance was controlled by self-reported documentation of the athletes as well as by measurement of the plasma concentrations of most of these antioxidants. Only during the Ironman race, the dietary intake (including antioxidant-fortified beverages and food) was ad libitum, but the quantities of the intake of nutrients were recorded both by the athletes themselves via a 24 h dietary recall (see below) and by the helpers who monitored their food intake at each of the supply stations of the race. The Ironman triathlon (consisting of 3·8 km swimming, 180 km cycling and 42·2 km running) took place in Klagenfurt, Austria, on 16 July 2006 on a warm (27·2°C) and dry day(Reference Neubauer, König and Wagner15, Reference Neubauer, König and Kern17). Blood samples were collected 2 d pre-race, immediately post-race (within 20 min), and 1, 5 and 19 d post-race. Except the Ironman race itself, the study participants abstained from intense exercise 48 h before each blood sampling, and they had fasted overnight before the 2 d pre-race blood sampling, and 5 and 19 d post-race blood sampling. After the Ironman race until the end of the study, the subjects underwent only moderate (‘recovery’) training (Table 1).
Table 1 Main characteristics of the subjects and their performance in the Ironman triathlon
(Mean values and standard deviations)
Assessment of the intake of antioxidant vitamins and carotenoids during the race and within the first 24 h post-race
Before each blood sampling, the athletes were required to provide 24 h dietary recalls. The main focus was on the intake of antioxidant vitamins and carotenoids during the race and within the first 24 h post-race. Analyses were performed based on the German/Austrian Food database BLS II (BGVV, Berlin Germany and ÖLS, Vienna, Austria). Special food and beverage items used by the athletes (e.g. carbohydrate bars and sports drinks) were added to the database.
Plasma concentrations of nutritive antioxidants and antioxidative biochemical substances
Plasma concentrations of α- and γ-tocopherol, β-carotene, lutein/zeaxanthin, cryptoxanthin and lycopene were measured with HPLC (Ultimate 3000; Dionex Corporation, Vienna, Austria). Plasma vitamin C was analysed photometrically. Plasma concentrations of total protein, total bilirubin and uric acid were measured using an automatic analyser (Vitro DT 60 II module; Ortho-clinical Diagnostics, Neckargemünd, Germany).
Plasma concentrations of lipid peroxidation and protein oxidation markers
As reported in a previous publication(Reference Neubauer, König and Kern17) and described before(Reference Ramel, Wagner and Elmadfa23), both malondialdehyde and conjugated dienes were measured with HPLC (pump, detector and integrator; Hitachi, Tokyo, Japan and column; Merck, Darmstadt, Germany). Oxidised LDL concentrations and advanced oxidation protein products (AOPP) were determined using an ELISA kit (Mercodia AB, Uppsala, Sweden) and a colorimetric assay kit (Immundiagnostik AG, Bensheim, Germany), respectively(Reference Neubauer, König and Kern17).
Total antioxidant capacity of plasma
As reported previously(Reference Neubauer, König and Kern17, Reference Reichhold, Neubauer and Hoelzl19), three approaches were applied to assess the total antioxidant capacity of plasma: the Trolox equivalent antioxidant capacity (TEAC), the ferric reducing ability of plasma (FRAP) and the oxygen radical absorbance capacity (ORAC) assays. The first two assays are used to measure the ability of a substance to transfer one electron to reduce compounds such as radicals or metals, while the ORAC assay is based on the ability of a substance to quench free radicals by hydrogen donation(Reference Knasmuller, Nersesyan and Misik24). Briefly, the TEAC assay is used to measure the ability of antioxidants in the plasma to delay the oxidation of the 2,2-azinobis (3-ethylbenzthiazoline-6-sulphonic acid) radical. The assay was performed as described previously(Reference Tomasch, Wagner and Elmadfa25). The FRAP assay was conducted according to the protocol of Benzie & Strain(Reference Benzie and Strain26) with modifications developed by the same authors(Reference Benzie and Strain27). The protocol reflects the capacity of a substance to reduce the ferric tripyridyltriazine (Fe3+-tripyridyl triazine) to the ferrous and intense blue-coloured (Fe2+-) tripyridyl triazine complex. Finally, the ORAC assay was carried out as described by Ou et al. (Reference Ou, Hampsch-Woodill and Prior28) and Huang et al. (Reference Huang, Ou and Hampsch-Woodill29). This assay is based on the antioxidant inhibition of peroxyl radicals, which are generated by plasma antioxidants 2,2′-azobis(2-amidinopropane)-dihydrochloride.
DNA damage in lymphocytes
As described by Reichhold et al. (Reference Reichhold, Neubauer and Hoelzl19) and Wagner et al. (Reference Wagner, Reichhold and Knasmuller30), the single-cell gel electrophoresis (comet) assay including the use of lesion-specific enzymes endonuclease III and formamidopyrimidine glycosylase was applied to assess single- and double-strand breaks (determined as the percentage of DNA in the tail), and oxidised purines and pyrimidines, respectively. Twenty-eight subjects were randomly selected for the single-cell gel electrophoresis assay, and lymphocytes were harvested from the blood samples by centrifugation (for details, see Reichhold et al. (Reference Reichhold, Neubauer and Hoelzl19)). The single-cell gel electrophoresis assays were conducted according to the methods described by Tice et al. (Reference Tice, Agurell and Anderson31), and oxidative DNA base damage was assessed on the basis of the protocols of Collins et al. (Reference Collins, Duthie and Dobson32).
Haematological profile
The haematological profile was assessed using a MS4 Hematology 3-Part-Differential-Analyzer (Melet Schloesing Laboratories, Maria Enzersdorf, Austria). Exercise-induced percentage changes in plasma volume were calculated(Reference Dill and Costill33) until 5 d post-race to assess potential alterations (i.e. an initial contraction followed by a potential expansion) of plasma volume, which may persist for 3–5 d after the cessation of demanding exercise(Reference Shaskey and Green34). All the results were adjusted for these changes, except for the plasma antioxidant capacity, and ratios of oxidised LDL–LDL and AOPP–total protein. For the determination of these markers, we used the data uncorrected for changes in plasma volume to consider their actual concentration to which the body responds. Correspondingly, ‘real’ plasma concentrations of antioxidants (i.e. without adjustment for plasma volume changes) were used when potential associations with the plasma antioxidant capacity were examined. In the present study, the adjustment for plasma volume changes had no significant impact on the statistical results.
Data analysis
All statistical analyses were performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). Details of the data analysis have been presented previously(Reference Neubauer, König and Wagner15, Reference Neubauer, König and Kern17, Reference Gleeson, Robertson and Maughan35). The main effect of time was obtained using the repeated-measures ANOVA. To examine significant relationships, Pearson's (for normally distributed data) or Spearman's (for non-normally distributed data) correlation was used. Furthermore, the subjects were divided into four percentile groups by the plasma concentrations and intakes of the antioxidant vitamins and β-carotene. One-factorial ANOVA and post hoc analyses with Scheffé's test were applied to assess whether differences in the changes of oxidative stress markers, total antioxidant capacity and DNA damage in lymphocytes were associated with the percentile distribution. The significance level was set at a P < 0·05, and is reported as P < 0·05, < 0·01 and < 0·001. Significance levels are quoted two sided.
Results
Race results
The study participants completed the Ironman triathlon in 10 (sd 1) h 52 (sd 1) min. Of forty-eight subjects, three subjects dropped out of the Ironman race due to self-reported fatigue symptoms, while three other subjects could not participate in one or more blood samplings.
Intake of vitamin C, α-tocopherol and β-carotene during the Ironman race and within the first 24 h post-race
The average intake of vitamin C, α-tocopherol and β-carotene was 393 (sd 219), 113 (sd 59) and 9·1 (sd 17·9) mg during the Ironman race, and 244 (sd 248), 10·1 (sd 5·7) and 2·7 (sd 2·3) mg within the first 24 h after the race, respectively. Data pertaining to the consumption of these antioxidants are summarised in Table 2.
Table 2 Total energy intake, intake of macronutrients, antioxidant vitamins and β-carotene during and within the first 24 h after the Ironman race
(Mean values and standard deviations)

Plasma concentrations of nutritive antioxidants
The time courses of plasma concentrations of vitamin C, α-tocopherol, γ-tocopherol and carotenoids in response to the Ironman race are summarised in Table 3. An immediate significant increase in response to the triathlon was observed in both vitamin C and α-tocopherol (+54 and +18 %, respectively; for both P < 0·01). One day post-race, vitamin C concentrations decreased to values that were similar to the pre-race values, whereas other antioxidant nutrient concentrations dropped significantly to below the pre-race concentrations: γ-tocopherol ( − 25 %; P < 0·01), lutein/zeaxanthin ( − 15 %; P < 0·05), cryptoxanthin ( − 8 %; P < 0·05) and β-carotene ( − 15 %; P < 0·01). The decrease in α-tocopherol 1 d post-race ( − 4 %) did not reach statistical significance. Most nutritive antioxidant concentrations were similar to the pre-race values, except for vitamin C, 5 and 19 d post-race. Vitamin C level increased to above the pre-race levels 5 d post-race (+21 %; P < 0·01), and remained elevated 19 d post-race (+24 %; P < 0·01).
Table 3 Plasma concentrations of endogenous and nutritive antioxidants
(Mean values and standard deviations)

Mean values were significantly different from the pre-race value: * P < 0·05, ** P < 0·01, *** P < 0·001.
Plasma concentrations of endogenous antioxidative compounds
Plasma levels of uric acid significantly (P < 0·001) increased immediately after the triathlon (+49 %). Subsequently, the uric acid concentrations gradually declined, but remained significantly (P < 0·001) elevated at all the time-points (P = 0·001 for 19 d post-race) v. the pre-race values(Reference Neubauer, König and Kern17). Plasma total bilirubin was significantly (P < 0·001) higher immediately after the race (+39 %) than pre-race, increased further 1 d post-race (+65 %), and significantly (P < 0·001) declined below the pre-race concentrations 5 d post-race. Plasma total protein increased immediately after the Ironman race (+4 %; P < 0·01), whereas there was a significant decrease to below the pre-race values thereafter.
Total antioxidant capacity of plasma
The time course of the total plasma antioxidant capacity, assessed by TEAC, FRAP and ORAC assays, is shown in Fig. 1. Similar time courses were observed for all these assays. As described previously(Reference Neubauer, König and Kern17, Reference Reichhold, Neubauer and Hoelzl19), TEAC, FRAP and ORAC values increased significantly (P < 0·001) immediately after the Ironman race (+48, +35 and +18 %, respectively). The values of all the assays remained significantly higher than the pre-race values until 1 d after the race (+25, +24 and +11 %, respectively; P < 0·001 for TEAC and FRAP, and P < 0·05 for ORAC). Five days after the race, TEAC, FRAP and ORAC values had declined back to the baseline levels, and 19 d post-race, they remained similar to the pre-race values.

Fig. 1 Time course of the total plasma antioxidant capacity as assessed by the Trolox equivalent antioxidant capacity (TEAC), the ferric reducing ability of plasma (FRAP) and the oxygen radical absorbance capacity (ORAC) assays 2 d pre-race (PRE), immediately post-race, and 1, 5 and 19 d post-race (POST). Values are means and standard deviations are represented by vertical bars. Mean values were significantly different from the pre-race values: * P < 0·05, *** P < 0·001.
Plasma concentrations of lipid peroxidation and protein oxidation markers
For more detailed information on oxidative stress responses, the reader is referred to a previously published article(Reference Neubauer, König and Kern17). Briefly, except for oxidised LDL and the oxidised LDL–LDL ratio, all oxidative stress markers significantly increased in response to the Ironman triathlon. While conjugated dienes, AOPP and the ratio of AOPP to total protein peaked immediately post-race (+91, +25 and +20 %, respectively; P < 0·01 for conjugated dienes and AOPP, and P < 0·01 for AOPP–total protein ratio), the increase of malondialdehyde reached statistical significance 1 d post-race (+9 %; P < 0·01) (after correcting the values for the increase in plasma volume at this time-point). One day post-race, with the exception of oxidised LDL (which decreased) and oxidised LDL–LDL ratio (which tended to remain elevated), all oxidative stress markers were above the pre-race levels. Five days post-race, all the oxidative stress markers returned to the pre-race values, remaining at these concentrations 19 d post-race.
DNA damage in lymphocytes
As reported by Reichhold et al. (Reference Reichhold, Neubauer and Hoelzl19), a decrease of DNA strand breaks was observed immediately after the race. One day post-race, DNA migration due to strand breaks increased (P < 0·01), then returned to the pre-race levels 5 d post-race, and decreased further to below the initial levels 19 d post-race (P < 0·01). Compared with the pre-race situation, no significant changes were observed in the levels of endonuclease III- and formamidopyrimidine glycosylase-sensitive sites throughout the entire monitoring period.
Associations with plasma concentrations of nutritive and endogenous antioxidants, intake of antioxidant vitamins and carotenoids and plasma antioxidant capacity
Moderate to strong positive correlations were observed between the pre- and post-race changes in uric acid on the one side, and between ORAC (r 0·36; P < 0·05), TEAC (r 0·54; P < 0·001) and FRAP (r 0·73; and P < 0·001) on the other side. Furthermore, correlations were found between pre- and post-race changes in TEAC and in vitamin C (r 0·33; P < 0·05), pre- and post-race changes in FRAP and in total bilirubin (r 0·42; P < 0·01), and ORAC levels and total plasma protein concentrations pre-race (r 0·44; P < 0·01), post-race (r 0·46; P < 0·01) and 5 d post-race (r 0·39; P < 0·05). The dose–effect relationship logarithmic regression of the vitamin C intake during the race v. the post-race plasma concentration is shown in Fig. 2.

Fig. 2 Logarithmic regression of the change in the plasma vitamin C concentration from pre-race to post-race v. the vitamin C intake during the Ironman race (y = − 12·91+8·33 × log(x); r 2 0·31; P = 0·248). For details, see Discussion.
Associations with nutritive and endogenous antioxidants, oxidative stress markers and DNA damage
Significant positive correlations were observed between DNA migration due to endonuclease III-sensitive sites and the ORAC values immediately post-race (r − 0·54; P < 0·01) and also 1 d post-race (r − 0·65; P < 0·05) (Fig. 3)(Reference Neubauer, Reichhold and Nersesyan20). Furthermore, no associations were found between antioxidant responses on the one side, and between lipid peroxidation and protein oxidation markers on the other side.

Fig. 3 Inverse correlation between the total plasma antioxidant capacity as assessed by the oxygen radical absorbance capacity (ORAC) assay and oxidative DNA damage as detected by the use of the lesion-specific enzyme endonuclease (ENDO) III immediately after the Ironman triathlon (r − 0.54; P < 0·001).
Results based on the group distributions into percentiles by plasma concentrations and intakes of the antioxidant vitamins and β-carotene and effects of supplementation
No associations with oxidative stress markers, total antioxidant capacity and endpoints of DNA damage were observed on the basis of percentile distribution by the plasma concentrations and intakes of vitamin C, α-tocopherol and β-carotene. Furthermore, there was no significant difference either in the pre-race values or in the post-race values of any antioxidant variable between the study participants who were supplementing with antioxidants (in accordance with our instructions and inclusion criteria) and those who were not supplementing with antioxidants.
Discussion
One of the key findings of the present study is the indication that an enhanced plasma antioxidant capacity in response to the Ironman triathlon prevented oxidative DNA damage in circulating lymphocytes. Although the ad libitum intake of antioxidants during the race might have exceeded the acute demands in exogenously derived antioxidants, it might have affected plasma concentrations and, consequently, preserved antioxidant responses to a certain degree. The observed decrease in the plasma concentrations of carotenoids and γ-tocopherol 1 d post-race points to an additional stress generated by a low intake of these antioxidant nutrients and phytochemicals during and within the first 24 h after the race and an increased need for nutritive antioxidants in the early recovery phase after an acute bout of ultra-endurance exercise. Furthermore, the present results underline the importance of an adequate habitual intake of nutritive antioxidants to maintain a normal physiological antioxidant status in reference to the current recommendations(22).
Changes of endogenous and nutritive antioxidants in response to the Ironman race, and the contribution of these antioxidants to changes in the plasma antioxidant capacity
As suggested in the literature(Reference Knasmuller, Nersesyan and Misik24, Reference Prior and Cao36), a ‘battery’ of different measurements was applied to adequately assess the total antioxidant capacity in plasma. Actually no single test fully reflects the overall antioxidant capacity of plasma due to the different methodological principles of and associated limitations with these assays(Reference Knasmuller, Nersesyan and Misik24, Reference Prior and Cao36, Reference Nikolaidis and Jamurtas37). However, the time courses of the values obtained in the TEAC, FRAP and ORAC assays were similar over the entire whole monitoring period (Fig. 1), suggesting a biologically significant finding. The plasma antioxidant capacity increased significantly following the Ironman triathlon, which reflects an acute response to oxidative stress(Reference Prior and Cao36, Reference Liu, Bergholm and Makimattila38), and remained elevated 1 d post-race(Reference Neubauer, König and Kern17, Reference Reichhold, Neubauer and Hoelzl19). While TEAC was found to be still increased 4 d after a marathon(Reference Liu, Bergholm and Makimattila38), levels of the TEAC, FRAP and ORAC had returned to the pre-race values 5 d post-race along with oxidative stress markers in the present study. Noteworthily, we observed associations between the activities of the erythrocyte glutathione peroxidase and TEAC levels throughout the monitoring period, which may indicate a synergistic interaction between erythrocytes and the plasma antioxidant capacity(Reference Neubauer, König and Kern17). There is emerging evidence that the efficient erythrocytes' antioxidant machinery, due to the erythrocytes' mobility and high cell density in the blood, protects not only their own membrane and local environment, but also contributes to the maintenance of circulatory antioxidant levels and the oxidant-scavenging ability throughout the circulation(Reference Nikolaidis and Jamurtas37, Reference Buehler and Alayash39). Furthermore, both TEAC and glutathione peroxidase activities were positively related with a variety of physiological training-related determinants(Reference Neubauer, König and Kern17). Nevertheless, on the basis of these results, it is unclear whether or to which extent the training adaptations of endogenous antioxidant mechanisms (in particular, antioxidant enzymes) had an impact on the plasma antioxidant capacity.
Our findings suggest that several mechanisms contributed to the increase of the antioxidant capacity of plasma in response to the Ironman triathlon. First, in line with the previous findings(Reference Mastaloudis, Morrow and Hopkins11, Reference Liu, Bergholm and Makimattila38), obtained associations between changes in uric acid and those in the TEAC, FRAP and the ORAC assays(Reference Neubauer, König and Kern17, Reference Reichhold, Neubauer and Hoelzl19) indicate that uric acid may be responsible for the rise in the total plasma antioxidant capacity to a considerable extent. Plasma concentrations of the potent hydrophilic antioxidant uric acid are known to increase during intense exercise, with it being produced from increased purine metabolism(Reference Liu, Bergholm and Makimattila38) and probably also because of impaired renal clearance(Reference Mastaloudis, Morrow and Hopkins11). Interestingly, TEAC, FRAP and uric acid increased with the athletes' performance in the Ironman race(Reference Neubauer, König and Kern17). However, as discussed previously(Reference Neubauer, König and Kern17), this phenomenon is probably reflective of a higher degree of exhaustion in those athletes with a higher training- and performance-status rather than with a specific training adaptation. Secondly, further correlations were observed between changes in FRAP and total bilirubin (that probably increased as a result of enhanced haemolysis(Reference Suzuki, Peake and Nosaka40)) and between pre- and post-race levels of ORAC and total plasma protein. The latter observation is in agreement with the results of a previous study that indicated the contribution of protein-associated thiol (sulphydryl) groups to plasma antioxidant capacity after a marathon(Reference Liu, Bergholm and Makimattila38). Thirdly, the moderate correlation of the exercise-induced increase of vitamin C with the change of TEAC might point to a small, but significant effect of vitamin C on the total antioxidant capacity. Correspondingly to a study in ultra-marathon runners(Reference Benzie and Strain26), the immediate increase in vitamin C probably can be attributed to both mobilisation of ascorbic acid from the adrenal glands(Reference Gleeson, Robertson and Maughan35) and/or leucocytes(Reference Viguie, Frei and Shigenaga41) and consumption during the race along with the ingestion of specified and vitamin-fortified beverages and food (e.g. sports drinks and carbohydrate bars). The high variation in the intake of vitamin C and, even more, of β-carotene can probably be explained by the different products used by the study participants. The lacking association between α-tocopherol and the total antioxidant capacity could be explained by the overestimation of hydrophilic substances with the applied assays(Reference Knasmuller, Nersesyan and Misik24).
Potential explanations for the lacking dose effects between intake of antioxidative vitamins or β-carotene during the race and their post-race plasma concentrations
No dose effect between the intake and plasma concentrations of antioxidative vitamins or β-carotene was observed based on the correlations or the group distribution into percentiles by the intake. However, based on these findings, one cannot rule out that the relatively high intake of vitamin C and α-tocopherol during the race may have affected plasma concentrations (at least partly) and, consequently, might have preserved antioxidant responses to some extent. With regard to vitamin C, the fact that the logarithmic regression of the intake during the race and the change in the plasma concentration pre-race to post-race was not significant can be taken as an indication that the average intake of 393 mg might have exceeded the needs and/or absorption capacity (Fig. 2). The optimal bioavailability of vitamin C is known to be reached at an intake of 200 mg/d(Reference Rousseau, Hininger and Palazzetti42). Therefore, the consumption during the race, which was nearly double-fold above this threshold (within a mean period of 10·7 h), could have influenced its plasma concentration, but probably, did not lead to a further increase(Reference Margaritis and Rousseau6). Concerning α-tocopherol, its increase in plasma levels immediately post-race is probably associated with exercise-induced changes in the lipoprotein metabolism, and might rather reflect a shift from tissue stores to plasma circulation than a response to the intake(Reference Mastaloudis, Morrow and Hopkins11, Reference Mastaloudis, Leonard and Traber43). An alternative or complementary explanation for the lack of obvious effects of the intake of antioxidative vitamins and carotenoids on its post-race plasma concentrations might be their metabolisation rapidly after appearance in the circulation (by scavenging RONS).
Antioxidant vitamin and carotenoid status
It is notable that the plasma concentrations of antioxidant vitamins and carotenoids of the participants were in a normal physiological range and, with the exception of α-tocopherol (as discussed below), adequate in reference to the current recommended values(22). Importantly, the subjects' habitual antioxidant supplementation was restricted to low dosages (according to the inclusion criteria), and no significant differences in any antioxidant parameter were observed between those who were supplemented with antioxidants and those who were not. Hence, the present results support the assumption that the requirements for vitamin C and β-carotene in competitive endurance athletes, to a great if not to the full extent, can be achieved by a diversified and well-balanced diet(Reference Rousseau, Hininger and Palazzetti42). Furthermore, the athletes' regular intake of fruits and vegetables apparently was sufficient to maintain the plasma concentrations of vitamin C and β-carotene above those levels which are considered as beneficial in preventing cancer and CVD throughout the study period(22). Endurance athletes seem to meet the general recommendations for these antioxidants probably also due to their increased daily energy expenditure and food intake, provided that the nutrient density is adequate(Reference Rousseau, Hininger and Palazzetti42), which is also supported by our FFQ data (unpublished results). Exemplarily, the mean pre-race plasma concentration of vitamin C (66·6 (sd 13·0) μmol/l) was beyond the level that has been observed to be sufficient to saturate immunocompetent cells (>50 μmol/l)(22, Reference Levine, Conry-Cantilena and Wang44). On the other hand, although in a normal physiological range(22), plasma α-tocopherol concentrations of the subjects were moderate, and in agreement with a cross-sectional study in well-trained athletes(Reference Rousseau, Hininger and Palazzetti42), high inter-individual variation among the subjects were found. However, when interpreting α-tocopherol status in response to intense training and/or acute demanding endurance exercise, one has to bear in mind that α-tocopherol plasma concentrations may mainly reflect mobilisation from tissue stores to plasma circulation(Reference Margaritis and Rousseau6, Reference Rousseau, Hininger and Palazzetti42). Furthermore, despite an often low vitamin E intake(Reference Rousseau, Hininger and Palazzetti42), well-trained athletes might not necessarily be at risk for a marginal vitamin E status, since certain mechanisms responsible for the incorporation of plasma α-tocopherol into muscle cells seem to be enhanced due to training(Reference Margaritis and Rousseau6). In view of the recent findings that high-dosed supplementation with vitamin E resulted in pro-oxidant effects after an Ironman triathlon(Reference Nieman, Henson and McAnulty12), endurance athletes should be encouraged to abstain from supra-physiological doses of vitamin E, but be encouraged to increase their dietary vitamin E intake. The latter requires a substantial increase in the consumption of foods that are rich in fats, such as nuts, margarine and certain oils(Reference Rousseau, Hininger and Palazzetti42). This is supported by our (unpublished) FFQ data showing that the plasma α-tocopherol concentration was highest (29·6 (sd 6·6) μmol/l) in the subject group that reported daily intake of nuts.
While evidence of the benefits of antioxidant supplementation in athletes in general is still scarce and inconsistent(Reference Reichhold, Neubauer and Ehrlich18, Reference Knasmuller, Nersesyan and Misik24), the optimal bioavailability and combined action of multiple phytochemical and antioxidant compounds derived from fruits and vegetables cannot be replaced by supplementation(22, Reference Prior and Cao36, Reference Rousseau, Hininger and Palazzetti42). Finally, in the light of the postulation that RONS play important physiological roles as signalling molecules (e.g. by stimulating training adaptations in the endogenous antioxidant defences)(Reference Ji2), supra-physiological doses of antioxidants may even blunt beneficial exercise-induced cellular adaptations by excessive elimination of RONS(Reference Gomez-Cabrera, Domenech and Romagnoli3, Reference Ristow, Zarse and Oberbach4).
Early recovery responses of the antioxidant vitamins and carotenoids in the plasma
The observation that plasma concentrations of carotenoids and tocopherols decreased to below the pre-race values 1 d post-race significantly, except for α-tocopherol (P>0·05) (Table 3), may indicate an increased need for antioxidant nutrients in the early recovery period after an acute bout of ultra-endurance exercise. However, contrary to the low intake of β-carotene and the moderate intake of α-tocopherol, the consumption of vitamin C within the first 24 h post-race (partly provided by vitamin-fortified foods and drinks) was sufficient to prevent a decrease to below the baseline plasma vitamin C levels. Similarly, Palazzetti et al. (Reference Palazzetti, Rousseau and Richard45) reported a drop in serum concentrations of antioxidant vitamins after 4 weeks of overloaded training, which was compensated by the intake of an antioxidant mixture containing vitamin C and α-tocopherol at doses that can be provided by a diversified and well-balanced diet. The authors suggested that the antioxidant supplementation at a physiological level sufficiently helped to preserve the antioxidant system during heavy training in subjects with initially low antioxidant intakes(Reference Palazzetti, Rousseau and Richard45). Taken together, these results indicate that the intake of antioxidant nutrients along with a diet rich in phytochemicals after such a major competition has to be considered carefully.
Effect of antioxidant responses on oxidative stress markers and DNA damage
Another main aim of the present study was the clarification of the question whether the observed strong antioxidant responses in plasma have an influence on the markers of oxidative damage of blood lipids and blood cell compounds(Reference Neubauer, König and Kern17) and endpoints of DNA stability in circulating lymphocytes(Reference Reichhold, Neubauer and Hoelzl19). Notably, plasma and blood cells are of particular importance in regulating and reflecting systemic redox status changes during and after exercise(Reference Nikolaidis and Jamurtas37). As reported previously, a transient increase of DNA strand breaks 1 d post-race was detected(Reference Reichhold, Neubauer and Hoelzl19), but no indications of oxidatively damaged DNA immediately or 1 d post-race (as indicated by the use of lesion-specific enzymes in the single-cell gel electrophoresis assay) were observed. Importantly, negative correlations were observed between ORAC and endonuclease III-sensitive sites immediately (Fig. 3) and 1 d after the Ironman race(Reference Neubauer, Reichhold and Nersesyan20). Concomitant with a recent animal study(Reference Avula, Muthukumar and Zaman46), where an enhanced serum antioxidative capacity prevented lymphocyte apoptosis, the present findings suggest a protective role of the increased plasma antioxidant capacity in DNA stability.
Except the correlations between the changes in vitamin C and TEAC, no further associations between the plasma levels of nutritive antioxidants, on the one side, and alterations in the plasma antioxidant capacity, oxidative stress markers or endpoints of DNA stability, on the other side, were observed. As a possible explanation, it is feasible to suggest that the acute oxidative stress response to an ultra-endurance exercise may exceed the capacity of exogenously consumed antioxidants. Otherwise, the lack of significant effects of especially nutritive antioxidants on exercise-induced increases of lipid peroxidation and protein oxidation markers(Reference Neubauer, König and Kern17) could be due to the fact that plasma concentrations of antioxidant nutrients of all the subjects were in a normal physiological range(22). Another important point to keep in mind is that our data are in reference to a sample cohort of young and healthy athletes with an adequate training status(Reference Neubauer, König and Kern17), which is associated with an adaptive improvement of endogenous antioxidant and repair systems(Reference Ji2, Reference Vina, Gomez-Cabrera and Borras5). It is possible that significant effects (e.g. differences in the magnitude of oxidative stress markers) may be obtained when comparing younger v. older, poorly trained v. well-trained athletes or athletes with a lower v. a higher antioxidant status. As reported previously(Reference Neubauer, König and Kern17), we found that even minor differences in the training status among the same group of athletes resulted in different changes in lipid peroxidation markers in response to the Ironman race, indicating that better training levels might confer enhanced protection against oxidative stress. However, on the contrary, individual differences in the plasma levels of antioxidant nutrients might have been too small to detect similar findings related to the nutritive antioxidant status. Furthermore, in this context, it is important to note the general difficulty in determining correlations among markers of DNA damage, biomarkers of oxidative stress and antioxidant parameters(Reference Dotan, Lichtenberg and Pinchuk47).
Methodological considerations and limitations
Despite our efforts to address the main factors influencing the antioxidant status and requirements, we are aware of the major difficulties to tightly control all of them in a field-based study, e.g. to completely forbid supplementation for athletes. However, the study participants were precisely instructed to restrict from using antioxidant supplementation higher than 100 % RDA (see Experimental methods). RDA (not the current dietary recommended intakes) levels as established by the US Food and Nutrition Board of the Institute of Medicine(21) were used for the following reasons: on the one hand, antioxidant dosages up to 100 % of RDA represent physiological doses. On the other hand, these RDA levels are used for labelling food and supplementation products (based on the Austrian food law), which facilitated the compliance of the study participants. As described, no effects of antioxidant supplementation were observed. Furthermore, in awareness of the obvious difficulties associated with the self-reported dietary intake data during such a competition (e.g. the athletes' ability to recall information thereafter), the food intake at each of the supply stations of the race was carefully monitored by supporters to verify the nutritional information gained from the 24 h dietary recalls.
Implications for the antioxidant intake in ultra-endurance exercise
The general picture that emerges from the data of the present study is that training- and acute exercise-induced adaptations in protective mechanisms including endogenous antioxidant defences counteracted severe oxidative damage of blood lipids, blood cell compounds(Reference Neubauer, König and Kern17) and DNA in circulating lymphocytes(Reference Reichhold, Neubauer and Ehrlich18–Reference Neubauer, Reichhold and Nersesyan20) in response to an ultra-endurance exercise. Importantly, the increase in the total plasma antioxidant capacity played a major role, and a protective effect was apparent with regard to DNA stability. Endogenous mechanisms seem to be more important in the acute exercise-induced changes of the plasma antioxidant capacity than the impact of exogenously derived antioxidants. However, the present results indicate that the antioxidant intake during and in the early recovery period after intense and very prolonged exercise requires specific attention. Furthermore, the present results illustrate that requirements of nutritive antioxidants to maintain an adequate physiological antioxidant status in ultra-endurance athletes (in reference to recommendations), to a large, if not to the full extent, can be achieved by consumption of a balanced and well-diversified diet.
Acknowledgements
The present data are part of a research project that was funded by the Austrian Science Fund (FWF). The authors' contributions are as follows: O. N. and K.-H. W. contributed to the study design and realisation of the study; K.-H. W. and S. K. were involved in the supervision of the work; S. R., L. N., C. H., J. V., B. S. and O. N. conducted the experimental work; O. N. and S. R. performed the statistical analysis; O. N. wrote the manuscript. Furthermore, the authors would like to thank Anna Chalopek and Norbert Kern for their assistance in the data collection and chemical analysis, and all the study participants for their efforts. None of the authors has a conflict of interest to declare.








