The precise cause of inflammatory bowel disease (IBD) is still unknown; however, genetically susceptible individuals seem to have a dysregulated mucosal immune response to enteric microbiota, which results in bowel inflammation( Reference Ordas, Eckmann and Talamini 1 ). Crohn's disease and ulcerative colitis are the two main components of IBD and the symptoms include abdominal pain, diarrhoea and poor ability to digest food, and about half of the severe cases require surgery in order to remove the affected bowl segment( Reference Tyagi, Kumar and Reddy 2 , Reference Singh, Singh and Singh 3 ). Anaemia is a common consequence of IBD, and recent studies have shown that the prevalence of anaemia in patients with IBD was found to be 70 % in children and 40 % in adults, with Fe deficiency being the most common cause, followed by anaemia of chronic disease( Reference Goodhand, Kamperidis and Rao 4 ). Fe-deficient anaemia has been reported to be present in patients with IBD independent of active disease; however, anaemia in chronic disease correlates better with disease activity( Reference Carter, Watts and Kosloski-Davidson 5 , Reference Lomer, Cook and Jan-Mohamed 6 ). The deficiency of Fe in IBD is due to a combination of poor intestinal absorption and blood loss. This so-called anaemia of chronic disease is seen in association with other inflammatory conditions, which is often refractory to oral Fe supplementation and may require treatment with parenteral Fe or erythropoietin (EPO)( Reference Stein, Hartmann and Dignass 7 ).
IL-10 is an important cytokine with potent anti-inflammatory activity and also a potent macrophage deactivator, blocking the induced synthesis of multiple inflammatory cytokines such as TNF-α, IL-1, interferon-γ, IL-6, IL-12, IL-17 and granulocyte-macrophage colony-stimulating factor( Reference Berg, Zhang and Weinstock 8 , Reference Ouyang, Rutz and Crellin 9 ). In IL-10-deficient mice, generated by gene targeting, most animals are growth retarded, anaemic and suffer from chronic colitis under specific pathogen-free conditions( Reference Kuhn, Lohler and Rennick 10 , Reference Kaser, Zeissig and Blumberg 11 ). Fe levels in the serum of IL-10 knockout (IL-10− / −) mice have been shown to reduce by 50 % compared with normal mice. Hence, Fe deficiency most probably contributes to anaemia( Reference Kuhn, Lohler and Rennick 10 ). Recent elucidation of the mechanisms that regulate Fe metabolism have shed light on anaemia in IBD, and have revealed an important role for hepcidin, an Fe-regulating hormone secreted by hepatocytes( Reference Hentze, Muckenthaler and Galy 12 , Reference Oustamanolakis, Koutroubakis and Messaritakis 13 ). Previous studies have also revealed that IL-6 increases hepcidin expression in vitro and in vivo, and plays an important role in the pathogenesis of anaemia of chronic disease including IBD( Reference Wang, Trebicka and Fu 14 ). The induction of hepcidin in response to either inflammatory stimuli or elevated serum Fe levels is dependent on signals provided by a subset of bone morphogenetic proteins (BMP)( Reference Wang, Trebicka and Fu 14 , Reference Babitt, Huang and Xia 15 ).
The successful application of anti-TNF-α antibody treatment signified a major breakthrough in the treatment of IBD, and resulted directly from convergent data in animal IBD models, indicating a role for TNF-α in chronic intestinal inflammation( Reference Maloy and Powrie 16 ). Despite the success of anti-TNF-α biological agents in IBD, approximately one-third of patients do not respond to anti-TNF-α treatment, and many others eventually lose responsiveness or become intolerant to these agents( Reference Maloy and Powrie 16 ). In addition, patients treated with anti-TNF-α show an increased incidence of severe infections and malignancies. Taken together, these results underscore the urgent need to develop new biological agents, particularly for patients who do not respond, lose responsiveness or cannot receive TNF-α blockers( Reference MacDonald, Biancheri and Sarra 17 ). CD52 is a cell-surface glycoprotein consisting of a short twelve amino acid peptide with a C-terminal glycosyl-phosphatidyl-inositol anchor, expressed on lymphocytes, monocytes and eosinophils( Reference Jones, Phuah and Cox 18 , Reference Rao, Sancho and Campos-Rivera 19 ). Alemtuzumab (Campath-1H), a humanised IgG1 monoclonal antibody (mAb), directly targets the cell surface CD52 and is effective in depleting lymphocytes by cytolytic effects in vivo ( Reference Jones, Phuah and Cox 18 ). Alemtuzumab is used clinically in the treatment of a wide range of autoimmune diseases, lymphoid malignancies and transplantation( Reference Qu, Li and Jiang 20 – Reference Scheinberg, Nunez and Weinstein 22 ). Our previous study showed that treatment with 20 μg of anti-mouse CD52 mAb once per week for 2 weeks improved the clinical and histological signs of colonic inflammation in IL-10− / − mice( Reference Zhu, Li and Yu 23 ). Presumably, CD52 mAb treatment could improve the nutritional status and Fe-deficient anaemia of IL-10− / − mice by attenuating colonic inflammation. The aim of the present study was to investigate the therapeutic effect of anti-mouse CD52 mAb on colitis and Fe-deficient anaemia in IL-10− / − mice with IBD.
Materials and methods
Animals
C3H/HeJBir IL-10− / − (C3H.IL-10− / −) and wild-type mice (12 weeks old at the beginning of the study) were obtained from the Jackson Laboratory. Mice were bred and maintained in a specific pathogen-free condition at the Model Animal Research Center of Nanjing University (Nanjing, China). C3H.IL-10− / − mice were used because the severity of colitis-related characteristics is most severe on C3H/HeJBir for IL-10− / − models with defined genetic backgrounds. A previous experiment demonstrated that 100 % of mice would develop chronic colitis with retarded growth under specific pathogen-free conditions( Reference Berg, Davidson and Kuhn 24 ). Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Animal Care and Use Committee of the Model Animal Research Center, Nanjing University (Nanjing, China).
Drug administration protocol
C3H.IL-10− / − and wild-type mice were divided into the wild-type group, the control group and the treatment group, each group containing eight mice, with three to five mice housed in one cage. The treatment group received the anti-CD52 mAb (20 μg diluted in PBS; MBL) treatment once per week for 2 weeks, while the wild-type and control mice received the same volume of vehicle (PBS). At 4 weeks after the final drug administration, the therapeutic effects of CD52 mAb were evaluated.
Histology
After mice were euthanised, proximal colons were fixed in 10 % buffered neutral formalin and embedded in paraffin. Thereafter, 6 μm-thick sections were stained with haematoxylin and eosin. Taking into account the number of lesions as well as the severity of the disease, two independent pathologists blinded to the study design gave an inflammation score to samples. Each proximal colon segment was scored from 0 to 4 on the following well-established criteria( Reference Singh, Singh and Singh 3 ): grade 0, no change from normal tissue; grade 1, one or few multifocal mononuclear cell infiltrates in the lamina propria; grade 2, intestinal lesions involved with several multifocal cellular infiltrates in the lamina propria; grade 3, lesions involved moderate inflammation and epithelial hyperplasia; grade 4, inflammation involved most of the colon sections. The summation of scores per mouse provided a total colonic disease score.
Analysis of haematology and iron concentration
At the endpoint of the experimental measures, mice were anaesthetised with an intraperitoneal injection of 150 mg/kg of ketamine and 8 mg/kg of xylazine. Blood samples were collected from the cannulated postcava with a portion mixed with the anticoagulant EDTA for the measurements of Hb concentration and haematocrit, and the remaining untreated blood processed for the measurements of serum Fe concentrations, which were measured as described previously( Reference Babitt, Huang and Xia 15 ). Briefly, blood was collected in microtainer serum separator tubes (BD Biosciences), and serum was isolated according to the manufacturer's instructions. Serum Fe and unsaturated Fe-binding capacity were measured by colorimetric assay using the Fe/unsaturated Fe-binding capacity kit (Thermo Electron Corporation). The total Fe-binding capacity was calculated as the sum of serum Fe and unsaturated Fe-binding capacity measurements, and transferrin saturation percentage was calculated as serum Fe/total Fe-binding capacity × 100. Immediately after the harvest, the spleen was sectioned and weighed. The quantitative measurement of splenic Fe stores was performed as described previously( Reference Babitt, Huang and Xia 15 ). Results are reported as μg Fe/g wet weight tissue.
Flow cytometry analysis
Blood was also collected for flow cytometry analysis. Erythrocytes were lysed with lysis buffer (Sigma) for 10 min at room temperature. Cell suspensions were washed twice in Roswell Park Memorial Institute (RPMI)-1640 (Sigma), and isolated cells were thoroughly suspended in each tube in 500 μl RPMI-1640. For immunofluorescent staining, cells were counted and approximately one million cells transferred to each flow test-tube. These cells were stained with fluorescein isothiocyanate-conjugated anti-CD4 (RM4-5; BD Biosciences), phycoerythrin-conjugated anti-CD45 (30-F11; BD Biosciences) or an appropriate negative control. Then, the stained cells were incubated at room temperature for 30 min in the dark. The cells were washed twice with 2 ml RPMI-1640 at room temperature and suspended in 500 μl RPMI-1640, and the cells were analysed by flow cytometry (BD FACS Calibur).
Analysis of serum erythropoietin
Blood samples were collected from the cannulated postcava with a portion mixed with the anticoagulant EDTA for measurements of EPO, which was performed with a Quantikine Mouse Erythropoietin Kit (R&D Systems).
Analysis of hepatic hepcidin gene expression
After mice were killed, the liver was excised and total RNA prepared using TRIzol reagent as directed by the manufacturer (Invitrogen). Quantitative RT-PCR was carried out as described previously(
Reference Li, Yu and Zhu
25
), with primers specific for hepcidin. Relative expression was calculated using the
![]() $$2^{ - \Delta \Delta C _{t}} $$
method after normalising to glyceraldehyde 3-phosphate dehydrogenase or actin. The primers used to amplify hepcidin mRNA were 5′CCATCTGCATCTTCTGCTGT3′ and 5′AGAGAGGTCAGGATGTGGCT3′.
$$2^{ - \Delta \Delta C _{t}} $$
method after normalising to glyceraldehyde 3-phosphate dehydrogenase or actin. The primers used to amplify hepcidin mRNA were 5′CCATCTGCATCTTCTGCTGT3′ and 5′AGAGAGGTCAGGATGTGGCT3′.
Western blotting analysis of the phosphorylated form of Smad1/5/8 and total Smad1
Liver tissue samples were homogenised in radioimmunoprecipitation assay buffer containing a cocktail of protease and phosphatase inhibitors, separated by PAGE and transferred to a polyvinylidene difluoride membrane by electroblotting at 4°C. The transferred membranes were then blocked for 2 h at room temperature with 5 % non-fat dried milk in Tris-buffered saline with 0·1 % Tween-20 before incubating with anti-mouse antibodies against phospho-Smad1/5/8, total Smad1 or actin (Cell Signaling Technology) at 1:500 dilution in blocking buffer at 4°C overnight. After washing with Tris-buffered saline with 0·1 % Tween-20, the membranes were incubated with a peroxidase-conjugated secondary antibody, diluted at 1:10 000 in blocking buffer for 1 h at room temperature. Detection was performed by incubating the membranes with ECL Plus (AMRESCO) and exposed to X-ray films. Band intensities were quantified after background subtraction and used to calculate the changes in the relative amounts of the corresponding proteins. Quantification of the blots was achieved densitometrically using Quantity One 1-D analysis software (Bio-Rad).
Statistical analysis
Statistical analyses were performed using SPSS software version 17.0 (SPSS, Inc.). Data are expressed as means with their standard errors. One-way ANOVA was used for multiple comparisons, and results were considered statistically significant if P values were < 0·05.
Results
CD52 monoclonal antibody treatment reduced weight loss typically associated with colonic inflammation in IL-10−/− mice
We first examined the effect of CD52 mAb on body weight loss in IL-10− / − mice. Both the control and CD52 mAb-treated mice resulted in a significantly lower percentage of body weight compared with wild-type mice, as shown in Fig. 1. The vehicle-treated IL-10− / − mice developed spontaneous colitis, as shown by a weight loss of about 92 (sem 5·1) % of the initial body weight. In contrast, IL-10− / − mice treated with CD52 mAb showed significant improvements in the body weight.

Fig. 1 Therapeutic effect of CD52 monoclonal antibody on body weight in IL-10− / − mice 4 weeks after the final drug administration. Values are means (n 8 per group), with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01.
Next, we investigated the protective effects of CD52 mAb treatment on colitis severity. Compared with wild-type mice, IL-10− / − mice that received vehicle treatment showed more infiltrations of mononuclear and polymorphonuclear cells in colonic mucosa, and the mean histological scores were also significantly higher than those in wild-type mice. Mice that received CD52 mAb treatment showed a significant reduction in colonic inflammation, reduced leucocyte infiltration, lower mean inflammation scores, and a partially restored glandular and goblet cell architecture when compared with mice in the control group (Fig. 2).
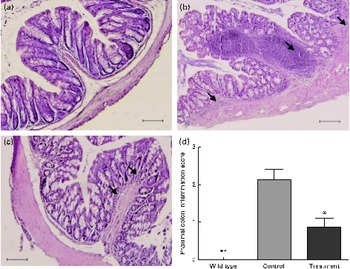
Fig. 2 Histological sections of the proximal colon from the wild type and two groups of IL-10− / − mice 4 weeks after the final drug administration. (a) Colon of a wild-type mouse, (b) mice with PBS treatment showed significant lymphocyte infiltration and distortion of glands, while (c) CD52 monoclonal antibody-treated mice showed markedly decreased infiltration of inflammatory cells. (d) The histological inflammation scores of these three groups in the proximal colon are presented. Representative sections from three separate experiments (200 × magnification) are shown. Values are means (n 8 per group), with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01. → , Infiltration of inflammatory cells. (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
CD52 monoclonal antibody treatment reduced the number of CD4+ blood T lymphocytes in IL-10−/− mice
It has been shown that CD4+ T-helper type 1 and T-helper type 17 cells mainly mediate chronic inflammation in the colon of IL-10− / − mice. Next, we delineated the effect of CD52 mAb on depleting T lymphocytes (Fig. 3). The leucocytes that infiltrated the blood were characterised by flow cytometry analysis. After the development of chronic colitis, although an increased percentage of CD4+ and CD4+CD45+ T cells was found in the blood of vehicle-treated control mice when compared with the wild-type mice, no statistical difference was observed between the two groups (P>0·05; Fig. 3). However, in the blood of CD52 mAb-treated mice, a significant decrease in the percentage of CD4+ and CD4+CD45+ T cells was observed compared with the vehicle-treated IL-10− / − mice (P< 0·01; Fig. 3). Taken together, these data indicated that CD52 mAb reduces activated T-cell populations in the blood.

Fig. 3 Effect of CD52 monoclonal antibody (mAb) on depleting T lymphocytes in IL-10− / − mice 4 weeks after the final drug administration. The numbers in the upper right quadrant indicate the total percentage of CD4+CD45+ T cells in blood lymphocytes (a) and the numbers in the rectangular gate indicate the percentage of CD4+ T cells in blood (b). SSC-H, side scatter-height. The percentage of CD4+CD45+ T cells in blood lymphocytes and CD4+ T cells in blood was calculated and depicted (c and d). Values are means (n 8 per group), with their standard errors represented by vertical bars. ** Mean values were significantly different from those of the control group (P< 0·01). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn).
CD52 monoclonal antibody treatment ameliorated anaemia by improving the iron status in IL-10−/− mice
As shown in Fig. 4, development of colonic inflammation resulted in significant anaemia in the IL-10− / − model of IBD. Compared with the wild-type group, Hb concentration and the percentage of haematocrit were significantly decreased in IL-10− / − mice (P< 0·01). After treatment with CD52 mAb, blood Hb values and haematocrit were significantly increased compared with the control group treated with vehicle alone (P< 0·05). Although CD52 mAb treatment increased the percentage of haematocrit, there was still a significant reduction compared with the wild-type mice (P< 0·05; Fig. 4(b)). However, no differences were observed between the wild-type and treated mice in relation to Hb concentration (Fig. 4(a)).

Fig. 4 Therapeutic effect of CD52 monoclonal antibody on (a) Hb concentration and (b) haematocrit in IL-10− / − mice 4 weeks after the final drug administration. Values are means (n 8 per group), with their standard errors represented by vertical bars. ** Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01.
We next investigated whether CD52 mAb treatment could regulate the level of serum Fe. As shown in Fig. 5, serum Fe levels and transferrin saturation were significantly decreased in the IL-10− / − model of IBD compared with the wild-type mice (P< 0·01). After CD52 mAb treatment, serum Fe levels and transferrin saturation increased considerably (P< 0·05), which were not statistically different from the wild-type mice (Fig. 5(a) and (b)). We also measured splenic Fe stores. As expected, mice in the control group showed a lower splenic Fe content when compared with those in the wild-type group (P< 0·01). However, in the CD52 mAb-treated IL-10− / − mice, there was a significant increase in the concentrations of Fe (P< 0·01; Fig. 5(c)).

Fig. 5 Therapeutic effect of CD52 monoclonal antibody (mAb) on the iron status in IL-10− / − mice 4 weeks after the final drug administration. Levels of serum iron (a), transferrin saturation (b) and splenic iron stores (c) in wild-type mice, control mice or CD52 mAb-treated mice. Values are means (n 8 per group), with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01.
CD52 monoclonal antibody treatment reduced the levels of serum erythropoietin in IL-10−/− mice
EPO is a glycoprotein hormone produced primarily by the kidney in the regulation of erythrocyte production, exerting its haematopoietic effects by stimulating the proliferation of committed erythroid progenitor cells and their development into mature erythrocytes. EPO functions as the primary mediators of a general protective response to tissue hypoxia by acting to maintain adequate tissue oxygenation through the adjustments of circulating red cell mass by using a hormonal feedback control system involving the kidney and the bone marrow( Reference Cuzzocrea, Mazzon and Di 26 , Reference Katsanos, Tatsioni and Natsi 27 ). As expected, compared with the wild-type group, the decreases in blood Hb concentration and haematocrit were accompanied by the significant increases in the plasma levels of EPO in the control group (P< 0·01), while CD52 mAb treatment considerably reduced the EPO values (P< 0·01), which were still significantly lower than those for wild-type mice (P< 0·05; Fig. 6).

Fig. 6 Therapeutic effect of CD52 monoclonal antibody on the serum levels of erythropoietin in IL-10− / − mice 4 weeks after the final drug administration. Values are means (n 8 per group), with their standard errors represented by vertical bars. ** Mean values were significantly different from those of the control group (P< 0·01).
CD52 monoclonal antibody treatment reduced the levels of liver hepcidin in IL-10−/− mice
As shown in Fig. 7, the IL-10− / − mice in the control group expressed significantly higher levels of hepcidin in the liver than the wild-type mice (P< 0·01), while the relative expression of liver hepcidin mRNA successfully decreased after CD52 mAb treatment (P< 0·05), which did not differ statistically from that of the wild-type mice.

Fig. 7 Therapeutic effect of CD52 monoclonal antibody on the relative expression of liver hepcidin mRNA in IL-10− / − mice 4 weeks after the final drug administration. Values are means (n 8 per group), with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01.
CD52 monoclonal antibody treatment reduced the expression of total Smad1 protein in IL-10−/− mice
We carried out additional experiments with the IL-10− / − model of IBD to further elucidate the mechanism of the down-regulation of hepcidin. As shown in Fig. 8, we found that the hepatic expression of total Smad1 protein, one of the receptor-associated Smads that transduces signals from the BMP receptor complex, was significantly increased in the control mice compared with the wild-type mice (P< 0·01), while the relative expression of total Smad1 protein was decreased with CD52 mAb treatment (P< 0·05). However, the phosphorylated form of Smad1/5/8 appeared to occur normally, as indicated by a trend towards a decrease in the ratio of phosphorylated Smad1/5/8 to actin with CD52 mAb treatment.

Fig. 8 Therapeutic effect of CD52 monoclonal antibody (mAb) on total Smad1/actin and phosphorylated (p) form of Smad1/5/8 in IL-10− / − mice 4 weeks after the final drug administration. (a) Representative Western blotting of liver lysates for pSmad1/5/8, total Smad1 and actin in wild-type mice, control mice or CD52 mAb-treated mice. Each lane corresponds to an individual mouse. Quantification of the band intensities of (b) pSmad/actin and (c) total Smad1/actin. Values are expressed in arbitrary units. Values are means (n 4 per group), with their standard errors represented by vertical bars. Mean values were significantly different from those of the control group: * P< 0·05, ** P< 0·01.
Discussion
IL-10 affects the growth and differentiation of many haemopoietic cells in vitro, and most IL-10− / − mice are growth retarded and anaemic, which can be explained by disturbed nutrition absorption as a result of chronic intestinal inflammation( Reference Kuhn, Lohler and Rennick 10 , Reference Babitt, Huang and Xia 15 ). Although the exact pathogenesis of IBD has not been fully understood, a resistance of T-cell apoptosis in the lamina propria is suspected, resulting in inappropriate cell activation and proliferation accompanied with an exaggerated immune response and the release of pro-inflammatory cytokines( Reference Ordas, Eckmann and Talamini 1 , Reference Zhu, Li and Yu 23 , Reference Baumgart and Sandborn 28 ). IL-10− / − mice develop T-cell-dependent spontaneous colonic inflammation that is characterised by both T-helper type 1 and T-helper type 17 immune responses( Reference Liu, Tonkonogy and Sartor 29 ). In the present study, we used anti-mouse CD52 mAb to deplete lymphocytes by cytolytic effects in IL-10− / − mice. CD52 mAb treatment significantly decreased the percentage of CD4+ and CD4+CD45+ T cells in the blood and the infiltration of inflammatory cells in the colon compared with vehicle treatment in IL-10− / − mice. We also found that CD52 mAb treatment ameliorated the inflammation score and disease severity, resulting in significant improvements in the body weight through increased intestinal absorption.
Kuhn et al. ( Reference Kuhn, Lohler and Rennick 10 ) demonstrated that Fe levels in the serum of IL-10− / − mice were reduced by 50 % compared with normal animals, and Fe stores were found to be depleted in the spleen. Furthermore, alterations of haemopoietic tissues in IL-10− / − mice under specific pathogen-free conditions were similar to those under conventional breeding conditions( Reference Kuhn, Lohler and Rennick 10 ). Several parameters such as serum Fe levels, Fe-binding capacity and transferrin saturation were used to evaluate anaemia( Reference Babitt, Huang and Xia 15 ). Both Fe-deficient anaemia and anaemia of chronic disease, the two main types of anaemia found in patients with IBD, are characterised by the decreases in serum Fe concentration and transferrin saturation. In the present study, haematocrit and blood Hb concentration decreased substantially in IL-10− / − mice, suggesting significant anaemia, and we found that the levels of serum Fe, transferrin saturation and splenic Fe stores were significantly decreased. Anaemia in IL-10− / − mice is similar to the findings reported in human patients with IBD, where Fe-deficient anaemia is the most prevalent diagnosis( Reference Goodhand, Kamperidis and Rao 4 ). The present findings are in line with Carter et al. ( Reference Carter, Watts and Kosloski-Davidson 5 ) showing that transferrin saturation and splenic Fe concentration were significantly decreased in the T-cell transfer model of chronic colitis, and Gasche et al. ( Reference Gasche, Reinisch and Lochs 30 ) demonstrated that serum Fe levels and transferrin saturation were significantly lower in anaemic patients with Crohn's disease. Mice treated with CD52 mAb significantly improved the Fe status and anaemia. These haematological and Fe concentration modifications may result, at least in part, from an increase in intestinal absorption because of reduced colonic inflammation with CD52 mAb treatment.
The anaemia of IBD probably results from decreased erythropoiesis, secondary to increased levels of pro-inflammatory cytokines and reactive oxygen metabolites, and is characterised by impaired Fe utilisation, lower EPO production than needed and a low response of bone marrow erythroid progenitor cells to EPO( Reference Katsanos, Tatsioni and Natsi 27 ). In the present study, we determined the levels of EPO and observed substantial increases in IL-10− / − mice receiving PBS treatment, while CD52 mAb treatment significantly reduced the levels of EPO, which was still significantly lower than that for the wild-type group. The increases in the levels of EPO are indicative of significant hypoxia in mice with colitis, probably due in large part to the decreases in haematocrit. The increases in the levels of EPO may depend on the presence of active disease in patients with IBD and in mice with colitis. However, the increases in the levels of EPO observed in the present study were not sufficient to prevent the overall loss of erythrocytes. Furthermore, it should be noted that EPO, in addition to its role in erythrocyte production, can also provide anti-inflammatory effects in IBD. Recent studies have characterised EPO as a potent anti-inflammatory cytokine in experimental colitis by inhibiting NF-κB-inducible immune pathways( Reference Nairz, Schroll and Moschen 31 ). However, the expression of anti-inflammatory mediators such as transforming growth factor-β, IL-10, IL-27, IL-35 and adiponectin is not regulated by EPO treatment at least in the mouse models investigated so far, implying that EPO is a direct anti-inflammatory mediator( Reference Nairz, Sonnweber and Schroll 32 ). The depletion of activated T cells by CD52 mAb treatment, resulting in a reduced production of the anti-inflammatory cytokine, may contribute to the mechanism of decreased levels of EPO in the present investigation. The interconnections between EPO activity and immune modulation still warrant further notification.
Hepcidin, an Fe-regulating hormone secreted by hepatocytes, controls the amount of Fe entering the circulation by binding and down-regulating ferroportin, which is a plasma membrane transporter that pumps Fe out of phagocytes and duodenal enterocytes, the sites where Fe is recycled from aged erythrocytes and absorbed from the diet, respectively( Reference Hentze, Muckenthaler and Galy 12 , Reference Nemeth, Tuttle and Powelson 33 ). In the normal condition, the expression of hepcidin is regulated transcriptionally in response to changing serum Fe levels. Elevated serum Fe levels promotes hepcidin expression, leading to the down-regulation of ferroportin and the decreased entry of Fe into the circulation. Conversely, low serum Fe levels lead to reduced hepcidin expression, elevated ferroportin expression and increased movement of Fe into the circulation( Reference Zhang, Senecal and Ghosh 34 , Reference Qiao, Sugianto and Fung 35 ). Recent studies have shown that hepcidin expression is increased during multiple forms of intestinal inflammation in mice, including the initial phase of dextran sulfate sodium colitis, IL-10− / − colitis and T-cell transfer colitis, with BMP 2, 4, 6 and IL-6 playing a positive regulatory role in this process( Reference Wang, Harrington and Trebicka 36 – Reference Zhao, Zhang and Enns 38 ). In addition, the neutralisation of BMP 6 and IL-6 significantly reduced hepcidin levels in mice with colitis( Reference Wang, Trebicka and Fu 14 , Reference Pietrangelo, Dierssen and Valli 39 , Reference Song, Tomosugi and Kawabata 40 ). Andriopoulos et al. ( Reference Andriopoulos, Corradini and Xia 41 ) reported that BMP 6 induces hepcidin expression by activating Smad1/5/8, which is in line with another study showing that haemojuvelin, a glycosyl-phosphatidyl-inositol-linked membrane-associated protein, binds to BMP and enhances their effectiveness to activate the BMP–SMAD signalling pathway to stimulate hepcidin transcription in hepatocytes( Reference Babitt, Huang and Wrighting 42 ). Additionally, Shanmugam et al. ( Reference Shanmugam, Ellenbogen and Trebicka 37 ) also showed that Smad1 protein played an important role in hepcidin expression in two distinct types of innate colitis. The present study demonstrated that significantly higher levels of hepcidin and total Smad1 protein (possibly together with Smad5 and Smad8) in the liver were observed in IL-10− / − mice. In addition, treatment with CD52 mAb substantially reduced their expression. The present findings indicated that elevated hepcidin expression may contribute to dysregulated Fe metabolism and inflammation in IL-10− / − mice.
In conclusion, CD52 mAb treatment may be beneficial in the treatment of patients with IBD. In the present experimental model, we first showed that anti-CD52 therapy ameliorated anaemia by reducing colonic inflammation. These findings may open novel horizons in the treatment of patients with IBD by the resetting of immunological homeostasis in the gut by the depletion of activated T cells in the gut mucosa. However, there are still many unknown aspects that need to be identified to make us understand the complex immunity and ecosystem of the intestine after CD52 mAb treatment.
Acknowledgements
The present study was partly supported by the Model Animal Research Center, Nanjing University (Nanjing, China).
This study was supported in part by funding from the National Ministry of Health for the Digestive Disease (grant no. 201002020) and the National Science Foundation of China (grant no. 81200263, 30972881 and 81170365). The National Ministry of Health for the Digestive Disease and the National Science Foundation of China had no role in the design, analysis or writing of this article.
H. W., J. D. and L. Zuo carried out the majority of the biochemical analysis and wrote the manuscript. W. Zhu designed the experiment. W. Zhang, J. Liu and N. L. contributed to the supervision of the work and the drafting of the manuscript. J. Li, Y. L., L. G., J. Z., L. Zhang, W. Zhang and J. G. contributed to the technical support, scientific advice and manuscript revision.
The authors have no conflicts of interest.










