Anthocyanins are a class of highly pigmented flavonoids distributed throughout nature. In addition to imparting red, purple and blue colour to many fruits and vegetables in our diet, anthocyanins have been associated with a variety of health benefits, including reducing risk factors of CVD in both human(Reference McKay and Blumberg1, Reference Ruel, Pomerleau and Couture2) and animal(Reference Tsuda, Horio and Kato3–Reference Mauray, Milenkovic and Besson6) models, protecting cognitive function in both human(Reference Krikorian, Shidler and Nash7) and animal(Reference Wang, Ho and Zhao8, Reference Joseph, Shukitt-Hale and Willis9) models and improving glucose metabolism(Reference Jayaprakasam, Olson and Schutzki10–Reference Takikawa, Inoue and Horio12). To maximise the benefit of anthocyanins in the diet, it is important to understand the bioavailability and metabolism of these compounds.
Previous studies have shown that many factors affect bioavailability. Several studies have shown that anthocyanins exhibit a complex dose response, with decreasing absorption efficiencies at increasing dose levels(Reference Kurilich, Clevidence and Britz13–Reference Charron, Kurilich and Clevidence15). Acylation results in a substantial decline in bioavailability(Reference Kurilich, Clevidence and Britz13–Reference Charron, Kurilich and Clevidence15). Anthocyanin backbone is also a key determinant of bioavailability, with pelargonidin-based anthocyanins being absorbed with much greater efficiencies than anthocyanins with other backbones(Reference Felgines, Texier and Besson16, Reference Carkeet, Clevidence and Novotny17). Metabolism is also profoundly affected by anthocyanin backbone, with pelargonidin-based compounds being primarily converted to metabolites, while a larger proportion of compounds with other backbones appearing intact after absorption(Reference Felgines, Texier and Besson16, Reference Carkeet, Clevidence and Novotny17).
While structure has been shown to have a profound impact on absorption and conversion to metabolites(Reference Charron, Clevidence and Britz14, Reference Felgines, Texier and Besson16, Reference Carkeet, Clevidence and Novotny17), very little is known about the effect of anthocyanin structure on anthocyanin pharmacokinetics. The objective of the present study was to determine the differences in pharmacokinetics (absorption, distribution, metabolism and excretion) of four different cyanidin-based anthocyanins. The effect of cooking on anthocyanin absorption was also investigated, since cooking has been shown to influence phytonutrient absorption(Reference Fielding, Rowley and Cooper18). Understanding of differences in absorption and metabolism rates of anthocyanins will be useful for development of intake recommendations for optimising health and reducing risk of chronic disease.
Experimental methods
Subjects, study design, diet and sample collection
A total of twelve non-smoking, healthy adults (six males and six females) from the Beltsville, MD, area participated in the present study. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Johns Hopkins University Bloomberg School of Public Health Committee on Human Research. Written informed consent was obtained from all subjects. Subject characteristics were 43 (sd 13) years old, 72 (sd 14) kg and BMI of 24 (sd 3·4) kg/m2.
The study was conducted as a three-treatment crossover with periods separated by 3-week breaks. Subjects were randomly assigned to one of the three groups (n 4), with each group receiving a different treatment order. Treatments were as following: 250 g purple carrot sticks served raw, 250 g purple carrot sticks served microwave-cooked and 500 g purple carrot sticks served microwave-cooked. Subjects consumed carrot treatments after a 12 h fast and after 2 d of an anthocyanin-free diet. Raw carrots were consumed with 6 g fat as dressing (soyabean and rapeseed oil) and cooked carrots were consumed with 6 g butter.
The purple carrots were washed, cut into sticks with the orange core discarded and mixed for batch uniformity. For cooked treatments, carrots were weighed before cooking and then one tablespoon or three tablespoons of water was added to 250 and 500 g doses. Cooked treatments were microwave-cooked (900 W microwave ovens; Sharp Electronics Corporation, Mahwah, NJ, USA) while covered for 12 or 20 min for the 250 and 500 g doses. For each treatment period, samples of cooked and raw carrots were frozen at − 80°C until extraction and analysis.
Blood was collected at 0, 0·5, 1, 1·5, 2, 2·5, 3, 3·5, 4, 5, 6, 7 and 8 h. Urine samples were collected at baseline and every 2 h until 16 h. From 16 to 24 h urine was pooled. During the collection period, subjects were provided an anthocyanin-free diet that included a snack, lunch and dinner provided at 2, 4 and 10 h, respectively, after carrot consumption. Caffeine consumption was prohibited on the dose day and for 1 d before dose. Vitamins and supplements were prohibited throughout the study.
Reagents
Cyanidin-3-galactoside and malvidin-3-galactoside were purchased from Indofine Chemical Company (Somerville, NJ, USA). Ethyl acetate and HPLC grade water and methanol were obtained from Thermo Fisher Scientific, Inc. (Pittsburgh, PA, USA). Formic acid and trifluoroacetic acid (TFA) were obtained from Sigma-Aldrich (St Louis, MO, USA).
Sample preparation for analysis
Carrots (60 g) were ground in a Waring blender (Torrington, CT, USA) with liquid N2 to form a powder. Powdered carrots (duplicate 3 g samples) were weighed into a test tube before adding 10 ml of 10 % methanolic formic acid. Samples were vortex-mixed for 1 min, sonicated for 10 min and then centrifuged at 2500 g for 10 min. The supernatant was decanted to a collection vial and samples were extracted three more times with 10 ml of 10 % methanolic formic acid. The combined extract was diluted 10 × with methanol–10 % aqueous formic acid (1:9) before injecting onto the liquid chromatography-MS.
Blood samples were collected into vacutainers containing EDTA and centrifuged at 2560 g for 10 min. Aqueous TFA (1·3 ml of 0·44 m) was added to plasma aliquots (2·2 ml) and urine was weighed and 10 ml aliquots were stored in vials containing 2 ml of 0·44 m-TFA. All samples were stored at − 80°C until analysis.
Before HPLC quantification of anthocyanins in plasma and urine, samples were thawed and malvidin-3-galactoside was added as an internal standard. Isolation and concentration of anthocyanins was performed using solid-phase extraction. SPE C-18 Sep-Pak cartridges (Waters Corporation, Milford, MA, USA) were conditioned with 5 ml methanol and then 5 ml of 0·44 m aqueous TFA before adding the sample. After adding the sample, water-soluble compounds were eluted with 5 ml of 0·44 m aqueous TFA, followed by elution of polar non-anthocyanin phenolics with 5 ml ethyl acetate before removing and collecting anthocyanins with 5 ml of 0·44 m methanolic TFA. Samples were dried under N2 and then reconstituted in methanol–10 % aqueous formic acid (1:9).
Liquid chromatography-MS conditions
The liquid chromatography-MS instrument was composed of an Agilent HP series 1100 G1946A mass selective detector with an electrospray ionisation source connected to a G1315A diode array detector, temperature-controlled column compartment (30°C), autosampler, automatic solvent degasser, binary pump, Zorbax SB-C18 column (5 μm, 250 × 4·6 mm) and guard cartridge (Agilent Technologies, Wilmington, DE, USA). The solvent system consisted of 10 % aqueous formic acid (solvent A) and methanol (solvent B). The gradient was linear from 5 % solvent B to 55 % solvent B over 20 min, then 100 % solvent B for 5 min to flush the column and back to 5 % solvent B for 10 min to re-equilibrate the column. The flow rate was 1 ml/min and injection volume was 50 μl. The mass selective detector source was positive electrospray ionisation with the spray chamber gas temperature set at 300°C, nebuliser pressure set at 60 psig, VCap at 3000 V and drying gas (N2) at 12 litres/min. Selected ion monitoring was used to identify individual anthocyanins and metabolites in the carrot, plasma and urine samples and to monitor potential anthocyanin glucuronide or sulphate metabolites. The G1315A diode array detector was set to collect signal at 530 nm. Limit of spectral detection for cyanidin-3-galactoside (Cy-3-gal; external standard for quantification) was 0·025 nmol/ml for a 50 μl injection. Data were collected using Agilent ChemStation software (Wilmington, DE, USA) and quantified from the G1315A diode array detector.
Calculations, statistics and compartmental modelling
The internal standard malvidin-3-galactoside was used to account for sample loss during extraction, which was on average 25 %. Molar concentrations of individual anthocyanins were calculated using an external standard curve of Cy-3-gal, since standards for the anthocyanins monitored were not available.
The WinSAAM software package (version 3.0.7; Kennett Square, PA, USA) was used to develop a compartmental model of anthocyanin kinetics. The mathematical prediction of plasma and urine anthocyanin contents for each anthocyanin was compared to measured data points and model parameters were adjusted until model prediction was in good accord with observed data. A least-squares procedure was then used to minimise the difference between model prediction and observed data.
Model parameter values across treatments were compared using a one-way repeated-measures ANOVA, and pair-wise comparisons were carried out with a Tukey's test. P values < 0·05 were considered statistically different. Statistics were performed using SigmaStat software (version 3.0; Aspire Software International, Ashburn, VA, USA).
Results and discussion
Five anthocyanins forms were present in the carrot treatments: cyanidin-3-(xylose-glucose-galactoside), cyanidin-3-(xylose-galactoside), cyanidin-3-(xylose-sinapoyl-glucose-galactoside), cyanidin-3-(xylose-feruloyl-glucose-galactoside) and cyanidin-3-(xylose-coumuroyl-glucose-galactoside). Total anthocyanin content of treatments ranged from 357 to 714 μmol (Table 1). Content of individual anthocyanins ranged from 6 to 430 μmol. All anthocyanin forms except cyanidin-3-(xylose-coumuroyl-glucose-galactoside) were detected in both plasma and urine (Fig. 1).
Table 1 Anthocyanin contents (μmol) of treatments
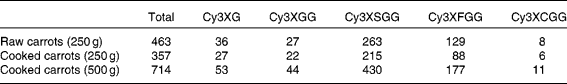
Cy3XG, cyanidin-3-(xylose-galactoside); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside); Cy3XFGG, cyanidin-3-(xylose-feruloyl-glucose-galactoside); Cy3XCGG, cyanidin-3-(xylose-coumuroyl-glucose-galactoside).

Fig. 1 Plasma anthocyanin concentration (a) and cumulative urinary anthocyanin content (b) after volunteers consumed 250 g raw purple carrots. Values represent means with their standard errors. Cy3XG, cyanidin-3-(xylose-galactoside) (![]() ); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside) (
); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside) (![]() ); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside) (
); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside) (![]() ); Cy3XFGG, cyanidin-3-(xylose-feruloyl-glucose-galactoside) (
); Cy3XFGG, cyanidin-3-(xylose-feruloyl-glucose-galactoside) (![]() ).
).
A twelve-compartment model (Fig. 2), composed of four sub-models (one for each anthocyanin form) of three compartments each, was used to analyse the kinetics of the individual anthocyanins in purple carrots. Each sub-model consisted of an upper gastrointestinal (GI) compartment, a plasma compartment and a urine compartment. Movement of anthocyanins through the system was described by first-order linear equations. The parameters Kj,i represent fractional transfer coefficients, describing the fraction of material in compartment i that is transferred to compartment j per unit time (with subscript K0, i representing movement out of the system). Upper GI passage and absorption were calculated as separate parameters, and a multiplicative function of the two described the movement from the GI tract into plasma. Fractional sd on all model parameters after least-squares minimisation of residuals were less than 10 % (fractional sd of up to 60 % are considered acceptable to conclude that actual parameter values have been identified(Reference Wastney, Patterson and Linares19)). The model allowed determination of absorption efficiencies, rates and half-lives for upper GI transfer into plasma and rates and half-lives for plasma elimination of anthocyanins into urine. Values for absorption efficiencies (mass absorbed divided by mass of dose expressed as percentage) for individual treatments were calculated separately, while values for rates and half-lives of upper GI transfer into plasma and for plasma elimination of anthocyanins into urine were calculated from pooled data because they did not show differences by treatment.

Fig. 2 Compartmental model of anthocyanin metabolism. Ki,j represents the fractional transfer coefficient from compartment j to compartment i. For irreversible exit from the system, the fractional transfer coefficient is referred to as K0,j. UGI, upper gastrointestinal tract; Cy3XG, cyanidin-3-(xylose-galactoside); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside); Cy3XFGG, cyanidin-3-(xylose-feruloyl-glucose-galactoside).
Cooking did not have an impact on bioavailability of anthocyanins (Table 2). In contrast, a decrease in absorption efficiency of individual anthocyanins was observed with the larger dose. When the data were pooled to calculate absorption of total anthocyanins(Reference Kurilich, Clevidence and Britz13), the absorption efficiencies for the larger and smaller doses were not significantly different; presumably the difference was lost due to the increased variability resulting from pooling the data for individual anthocyanins (Table 3).
Table 2 Absorption efficiency (%) of anthocyanins for different carrot treatments
(Mean values with their standard errors)

Cy3XG, cyanidin-3-(xylose-galactoside); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside); Cy3XFGG, cyanidin-3-(xylose-feryloyl-glucose-galactoside).
a,b Mean values were statistically different between anthocyanin forms (P < 0·05).
c,d Mean values were statistically different between treatments (P < 0·05).
Table 3 Kinetic parameters of individual anthocyanin-based compounds
(Mean values with their standard errors)
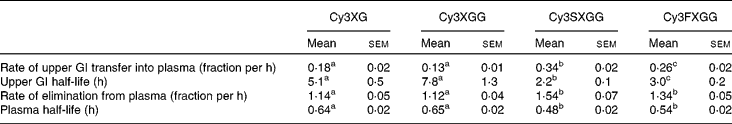
Cy3XG, cyanidin-3-(xylose-galactoside); Cy3XGG, cyanidin-3-(xylose-glucose-galactoside); Cy3XSGG, cyanidin-3-(xylose-sinapoyl-glucose-galactoside); Cy3XFGG, cyanidin-3-(xylose-feryloyl-glucose-galactoside); GI, gastrointestinal.
a,b Mean values were statistically different between anthocyanin forms (P < 0·05).
Absorption efficiency of individual anthocyanins ranged from 0·12 to 0·25 % for non-acylated anthocyanins and from 0·0079 to 0·019 % for acylated anthocyanins. For each treatment, absorption efficiencies of the two acylated anthocyanins did not differ from each other and absorption efficiencies of the two non-acylated anthocyanins did not differ from each other, but non-acylated anthocyanins were absorbed with greater efficiencies than acylated anthocyanins. This may be partially related to the lower affinity of acylated anthocyanins for transporter bilitranslocase. Bilitranslocase is an organic anion carrier found in tissues, including liver(Reference Baldini, Passamonti and Lunazzi20), gastric musoca(Reference Battiston, Macagno and Passamonti21) and kidney(Reference Vanzo, Terdoslavich and Brandoni22). A previously published study of the interaction between anthocyanins and bilitranslocase suggests that mono- and di-glucosylated anthocyanins were good ligands for bilitranslocase, whereas the acylated anthocyanins that were tested appeared not to be(Reference Passamonti, Vrhovsek and Mattivi23) good ligands for bilitranslocase.
Based on plasma appearance, rates of passage through the upper GI tract showed half-lives ranging from 2·2 to 8 h. This may seem longer than expected, since the peak plasma concentration was observed within the first few hours, and since previous studies have also shown very early peak plasma times for anthocyanins, as reviewed by McGhie & Walton(Reference McGhie and Walton24). However, these anthocyanins were delivered embedded in the carrot matrix, thus the GI transit time reflects time for digestion and release of anthocyanins from the carrot tissue as well as absorption. Early peak plasma times, therefore, would reflect easily accessible anthocyanins and digestion time would be extended as anthocyanins are released from the plant matrix. The acylated anthocyanins exhibited shorter half-lives for GI absorption than the non-acylated. This may reflect the absorbability of non-acylated anthocyanins along a larger proportion of the GI tract. In other words, if acylated anthocyanins are absorbed across a shorter length of the GI tract, then the effective half-life of transit into plasma through the absorbing section of the GI tract would be reduced. Cyanidin-3-(xylose-sinapoyl-glucose-galactoside had the shortest upper GI transfer time, followed by cyanidin-3-(xylose-feruloyl-glucose-galactoside), then the two non-acylated anthocyanins, for which upper GI transfer time was not different.
Elimination of different anthocyanins from the plasma was also dependent on structure. Non-acylated compounds were eliminated more slowly than acylated compounds. Elimination rates of the two non-acylated compounds did not differ from each other and those of the two acylated compounds did not differ from each other. As with GI absorption, differences in interactions with transporters at the tissue level (such as bilitranslocase which is found in liver(Reference Baldini, Passamonti and Lunazzi20), kidney(Reference Battiston, Macagno and Passamonti21) and vascular epithelium(Reference Maestro, Terdoslavich and Vanzo25)) may explain the differences in the observed plasma kinetics of the different anthocyanin forms.
Many studies have shown that anthocyanins are absorbed and excreted intact. Possible metabolites include glucuronidated, sulphated and/or methylated conjugates(Reference Charron, Clevidence and Britz14, Reference Carkeet, Clevidence and Novotny17, Reference Wu, Cao and Prior26–Reference Wu, Pittman and Prior29), and different anthocyanins are metabolised to different extents(Reference Wu, Pittman and Prior27). These metabolites may be formed by enzymes such as UDP-glucuronosyltransferase, UDP-glucose dehydrogenase or catechol-O-methyltransferase, and these biotransformations may take place in the small intestine, liver or kidney. For cyanidin-based anthocyanins, as studied in the present experiment, many reports have suggested that the intact forms are the primary forms found in blood and urine(Reference Charron, Clevidence and Britz14, Reference Wu, Cao and Prior26, Reference Matsumoto, Inaba and Kishi30, Reference Matsumoto, Ito and Yonekura31). For the present experiment, we looked for but did not detect glucuronidated or sulphated metabolites in either blood or urine, suggesting that these anthocyanins were primarily absorbed and excreted intact. The ability to obtain good agreement between model prediction and experimental data with a mathematical model that was based only on intact forms is in accord with minimal metabolism of these complex anthocyanin forms. Protocatechuic acid is a possible degradation product of cyanidin-based anthocyanins(Reference Tsuda, Horio and Osawa32–Reference Woodward, Kroon and Cassidy35). We did not monitor potential formation of phenolic acids. Protocatechuic acid can be formed from the cyanidin aglycone when exposed to nearly neutral pH(Reference Kay, Kroon and Cassidy34, Reference Woodward, Kroon and Cassidy35), possibly in blood, in the colon or during sample processing. The importance of this pathway in metabolism of anthocyanins is unclear(Reference Matsumoto, Ito and Yonekura31, Reference Prior and Wu36).
The physiological behaviour of the complex cyanidin derivatives in purple carrots was similar to that observed for simpler cyanidin forms. Both chokeberry and elderberry contain primarily simple cyanidin forms and thus offer a useful comparison. Previous studies with chokeberry and elderberry delivered doses as low as 147 mg and as high as 3·57 g(Reference Cao, Muccitelli and Sanchez-Moreno37–Reference Wiczkowski, Romaszko and Piskula43); the doses used in the present study (321–643 mg total anthocyanins) fell in the centre of this range. We observed a maximum plasma total anthocyanin concentration of 5–5·8 nmol/l, compared to 97 nmol/l for elderberry extract containing 720 mg cyanidin-based anthocyanins(Reference Milbury, Cao and Prior38), 96 nmol/l for chokeberry extract containing 721 mg cyanidin-based anthocyanins(Reference Kay, Mazza and Holub41) or 32 nmol/l for chokeberry juice delivering cyanidin-based anthocyanins at 0·8 mg/kg body weight(Reference Wiczkowski, Romaszko and Piskula43). These comparisons, though limited, suggest that the more complex anthocyanins, especially the acylated anthocyanins, have more limited absorption than the simpler cyanidin forms. Plasma half-lives reported from other studies(Reference Matsumoto, Ito and Yonekura31, Reference Frank, Janssen and Netzet42, Reference Wiczkowski, Romaszko and Piskula43) tended to be longer (on the order of 2 h) than those reported here because previous half-lives were calculated simply from plasma decay curves, which are influenced not only by pathways of anthocyanin exit from plasma but also by simultaneous entry of anthocyanins into plasma. If one does not account for these multiple processes when calculating half-lives, the estimation of half-life is inaccurate (and lengthened when entry into plasma is neglected). Mathematical modelling accounts for the different processes occurring simultaneously, thus delivers more accurate values for half-lives. Our plasma half-life values ranged from 29 to 38 min for the different anthocyanin forms.
The present study reports for the first time the rates of absorption and elimination for anthocyanins. Differences in rates of metabolism highlight where differences in metabolic pathways and mechanisms probably exist. In addition, information about rates of absorption and elimination of nutrients and phytonutrients provide critical pieces of information for development of intake recommendations.
Acknowledgements
Author contributions were as follows: J. A. N., B. A. C. and A. C. K. designed and conducted the study; A. C. K. performed the laboratory analysis; and J. A. N. conducted the mathematical modelling, interpreted data, and wrote the manuscript. This work was supported by the USDA Agricultural Research Service and an USDA CSREES IFAFS grant 2000-4258. There are no conflicts of interest to report.







