Consumption of oily fish is known to decrease the risk of CVD, primarily due to its n-3 fatty acid content(Reference Harris1). The inverse association between fish intake and the risk of CHD has been reported in various cohort studies(Reference Panagiotakos, Pitsavos and Zampelas2, Reference Iso, Kobayashi and Ishihara3). The mechanism by which n-3 fatty acids protect from CVD is related to the incorporation of EPA (20 : 5) and DHA (22 : 6) into membrane phospholipids. This inclusion produces changes in membrane properties(Reference Li, Wang and Tan4, Reference Calder5) with various beneficial effects: reduced activity of inflammatory cells and concentration of certain inflammation mediators(Reference Calder5), reduced arterial plaque fragility(Reference Thies, Garry and Yaqoob6), regulation of blood pressure(Reference Geleijnse, Giltay and Grobbee7) and improved endothelial function in hypercholesterolaemic subjects(Reference Goodfellow, Bellamy and Ramsey8). A daily intake of 3–4 g EPA+DHA has also been associated with a reduction of serum TAG(Reference Harris9), although other authors have observed that a lower n-3 fatty acid intake (1·5–3·5 g/d) also reduces serum TAG in hyperlipaemic patients(Reference Mori, Bao and Burke10). This TAG-lowering effect appears to be related to increased hepatic β-oxidation and decreased lipogenesis(Reference Harris and Bulchandani11).
Insulin resistance is considered an important risk factor for type 2 diabetes mellitus as well as an independent factor for CVD and the metabolic syndrome, even when glucose values are within the normal range(Reference Wallace and Matthews12).
Various articles have described the inverse association of a high consumption of PUFA, from vegetables and seeds, with the risk of developing type 2 diabetes mellitus or the impairment of glucose and insulin metabolism, compared to that of saturated fat from animal products(Reference Colditz, Manson and Stampfer13, Reference Feskens, Loeber and Kromhout14).
The effects of high consumption of n-3 PUFA on glucose metabolism have been widely studied(Reference Borkman, Chisholm and Furler15–Reference Delarue, LeFoll and Corporeau17), although the results with regard to insulin resistance are not conclusive. Most of the studies carried out on this issue have focused on supplementation with different doses of fish oils in healthy subjects or type 2 diabetes patients. Supplements of fish oils as high as 10 g/d have reported negative effects on blood glucose levels and insulin function in diabetic patients(Reference Borkman, Chisholm and Furler15).
It has been observed that insulin sensitivity cannot be restored in the liver of type 2 diabetes patients, and that once type 2 diabetes mellitus is present, the potential benefits of n-3 PUFA intake do not seem to be effective(Reference Vessby, Uusitupa and Hermansen16, Reference Delarue, LeFoll and Corporeau17).
In addition, recent studies carried out in the USA and Europe have reported increased insulin sensitivity, as well as decreased fasting glucose levels, in healthy subjects consuming fish oil(Reference Delarue, Couet and Cohen18). These studies have also associated the consumption of cod liver oil during pregnancy with protection from type 1 diabetes mellitus in the offspring(Reference Stene, Ulriksen and Magnus19).
Although many intervention studies have focused on the effects of n-3 PUFA consumption as fish oil or supplements on glucose and insulin metabolism, data related to fish consumption and its role in the regulation of insulin resistance are scarce(Reference Mori, Bao and Burke10).
Dietary advice for most of the population has recently been focused on reducing the intake of red meat and increasing fish consumption as a protective measure against developing CVD. The exception has been the advice to women at risk of iron deficiency, who have been encouraged to follow a diet rich in red meat.
On the other hand, it is known that iron overload leads to a number of metabolic disorders and the development of CVD, and reduction of iron stores improves glucose tolerance in type 2 diabetic patients(Reference Facchini and Saylor20). A negative association has been observed between iron stores and insulin sensitivity in healthy subjects consuming a red meat-based diet compared to a lacto-ovo vegetarian diet(Reference Hua, Stoohs and Facchini21).
Taking into account these premises, the present study aimed at comparing the effects of two diets, one rich in oily fish and the other in red meat, but similar in all other components, on lipid profile, oxidative status, glucose and insulin levels, and insulin sensitivity, in young, iron-deficient women.
Subjects and methods
The present study was designed and carried out following the CONSORT statement guidelines(Reference Moher, Schultz and Altman22).
Subjects
Thirty women between the ages of 18 and 30, who had originally been recruited to form part of a wider study on the prevention of iron deficiency through diet, volunteered to participate in this nutritional intervention study.
After being informed of the study conditions and having signed an informed consent, the women underwent blood tests to determine their biochemical parameters. They also completed questionnaires relating to lifestyle, chronic diseases and medical treatments.
Inclusion criteria required that the women be menstruating, with low iron stores, and lipid, glucose and insulin levels within the normal ranges. Exclusion criteria were smoking, anaemia, to suffer from any chronic gastric disease, be allergic to fish or be vegetarian.
Of the thirty women who were initially interested in participating in the trial five did not complete the study, for which reason the final number of participants was twenty-five. All presented low iron stores (mean ferritin levels < 20 ng/ml).
Study protocols were approved by the Ethics Committee of the Spanish National Research Council and the Clinical Research Ethics Committee of the Hospital Clinica Puerta de Hierro, both in Madrid, Spain.
Study design
The study consisted of a randomized crossover intervention involving two 8-week periods. The trial was held between the months of September and December. The twenty-five participants were randomly distributed into two groups of twelve and thirteen subjects, respectively.
Two diets, differing only in their red meat and oily fish content, were tested. Dietary instructions were given only in relation to meat, poultry, fish and egg intake. Volunteers were asked to maintain their usual dietary habits with regard to the rest of the food groups.
The ‘red meat diet’ contained five portions of red meat, one portion of lean fish, two portions of poultry and two eggs per week.
The ‘oily fish diet’ contained two portions of salmon, two cans of water-packed tuna (56 g each), one can of sardines in olive oil, one portion of lean fish, one portion of red meat, two portions of poultry and two eggs per week.
Dietary control and compliance
Each subject's dietary intake was regularly evaluated to control any possible changes in dietary patterns, including those related to the consumption of red meat or oily fish to detect possible dietary changes that could interfere with the study. Volunteers were asked to fill out a 24 h dietary recall with the menus they consumed every day, focusing on the ingredients. Once per month they completed a 72 h detailed intake report, specifying the types of food consumed and serving weights. Compliance was assured by the daily menu forms, as well as weekly interviews of volunteers by one member of the research team at the time of collection of the daily records. Dietary food and nutrient intakes, and energy provided by macronutrients were calculated by an informatic application using the Spanish Food Composition Database (DIAL, Alce Ingenieria, Spain). Compliance of the intervention was also assessed by personal telephone interviews throughout the whole intervention.
Anthropometric measures and blood pressure
Weight and height of each volunteer were measured, and BMI calculated, at 09.00 hours on day 0 (baseline) and on weeks 4 and 8 of each intervention period, after a 12 h fasting period. At the same time, blood pressure was measured by an automated manometer (Omron M3-intellisense; OMRON Healthcare Europe, Spain).
Blood sampling and biochemical assays
Volunteers reported to the laboratory facilities at baseline (day 0) and week 8 of each period. Blood samples were collected by venepuncture between 08.00 and 08.30 hours, after a 12 h fasting period. Serum and plasma were obtained after centrifugation at 1000 g for 30 min and stored at − 80°C.
Serum total cholesterol (Total-c), HDL-cholesterol (HDL-c) and TAG concentrations were determined by automated enzymatic methods (CHOD-PAP and GPO-PAP, Boehringer Mannheim, Germany; RA-XT autoanalyser, Technicon, USA). Serum LDL-cholesterol (LDL-c) concentration was calculated using the Friedewald formula(Reference Friedewald, Levy and Fredrickson23). Total-c/HDL-c and LDL-c/LDL-c ratios were calculated.
Total lipoperoxides (malondialdehyde and hydroxyalkenes) in blood were determined by quantitative colorimetry using a commercially available kit (Bioxytech LPO-586; Oxis Research, USA). Soluble intercellular adhesion molecule-1 (sICAM-1) and soluble vascular cell adhesion molecule-1 (sVCAM-1) serum concentrations were measured by the ELISA technique, using a commercially available kit (Quantikine Human sVCAM-1 and sICAM-1, Parameter; R&D Systems, USA).
Fasting serum glucose concentrations were analysed by an automatic analyser (RA 2000; Technicon, USA). Serum insulin levels were determined by means of an immunometric assay in an autoanalyser (Immulite® 2000 Insulin; Diagnostic Products Corporation-DPC, UK).
Insulin sensitivity was estimated using the Homeostasis Model Assessment (HOMA) and the Quantitative Insulin Sensitivity Check Index (QUICKI), which were calculated using the following formulas:

To compare the effect of both dietary treatments, changes between baseline values and the values at the end of each period (8 weeks) were calculated as follows:
Statistics
The SPSS 15.0 statistical package for Windows (SPSS Inc., Chicago, IL, USA) was used to study the data. Sample stratified randomization was made with Excel (Microsoft Office 2003; Microsoft Inc., Redmond, WA, USA).
Data are presented as means and standard deviations. Percentage change data were arcsin transformed when necessary. Data were analysed with a univariant ANOVA to study the influence of dietary treatment (fixed effects), the group to which volunteers were assigned and the order in which they started the intervention study (random effects). Once significant differences due to group and order were discarded, the data were analysed by a mixed model and multivariate ANOVA (fixed factor: type of diet). Values of P ≤ 0·05 were considered to be statistically significant.
Results
Baseline parameters of the groups starting with either the red meat or the oily fish diet are shown in Table 1. No significant differences between them were observed at the beginning of the nutritional intervention.
Table 1 Baseline biochemical parameters of volunteers in the group starting with the red meat diet and the group starting with the oily fish diet
(Mean values and standard deviations)

HOMA, Homeostasis Model Assessment; QUICKI, Quantitative Insulin Sensitivity Check Index; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, vascular cell adhesion molecule-1.
Compliance was confirmed by the analysis of dietary reports. The two diets varied significantly only with regard to meat and fish consumption (Table 2). Intake of the food groups including cereals, legumes, vegetables, fruits, dairy products, eggs, sugars and oils was similar in both diets. In relation to nutrient intake (Table 3), no significant differences between diets were observed in total energy consumed or energy from macronutrients. The only significant differences between diets were those derived from oily fish consumption. With the oily fish diet, intake of n-3 PUFA, mainly in the form of EPA (20 : 5) and DHA (22 : 6), was significantly higher than with the red meat diet. Consequently, the PUFA/SFA ratio was also significantly higher while the n-6/n-3 PUFA ratio was 50 % lower than with the red meat diet. In contrast, intake of oleic acid, which contributes to more than 90 % of MUFA, was significantly higher during the red meat diet. SFA intake did not significantly differ between the diets.
Table 2 Food group intake of the volunteers during the oily fish or red meat diets (four portions per week)*
(Mean values and standard deviations)

* For details of subjects and procedures, see the Subjects and methods section and Table 1.
Table 3 Macronutrient and micronutrient intake of the volunteers during the oily fish or red meat diets (four portions per week)*
(Mean values and standard deviations)

* For details of subjects and procedures, see the Subjects and methods section and Table 1.
Body weight, BMI (22·1 (sd 2·2) kg/m2) and blood pressure remained constant during the entire intervention study.
Total-c and LDL-c levels decreased equally with both diets (Table 4), while HDL-c levels increased with both diets, the change being significantly higher with the oily fish diet than with the red meat one. Total-c/HDL-c and LDL-c/HDL-c ratios tended to decrease to the same extent with both diets. A non-significant tendency for TAG levels to increase with the red meat diet and to decrease with the oily fish diet was observed.
Table 4 Change (%) in serum lipids, lipoperoxides and adhesion molecules, of the volunteers during the oily fish or red meat diets (four portions per week)*
(Mean values and standard deviations)
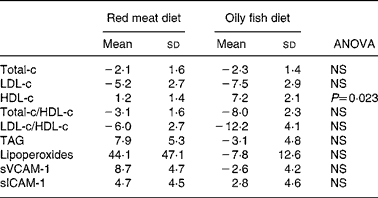
HDL-c, HDL-cholesterol; LDL-c, LDL-cholesterol; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, vascular cell adhesion molecule-1; Total-c, total cholesterol.
* For details of subjects and procedures, see the Subjects and methods section and Table 1.
Oxidation and inflammation parameters (lipoperoxides, sVCAM-1 and sICAM-1) displayed no significant differences between diets (Table 4), although lipoperoxide concentration displayed a marked tendency to increase with red meat diet.
Glucose levels displayed a slight tendency to decrease during the intervention, although the influence of diet was not significant (Fig. 1). Significant differences were seen between insulin levels, which were nearly 20 % lower with the oily fish diet than with the red meat diet. As a result, HOMA and QUICKI indexes also improved significantly with the oily fish compared to the red meat diet.
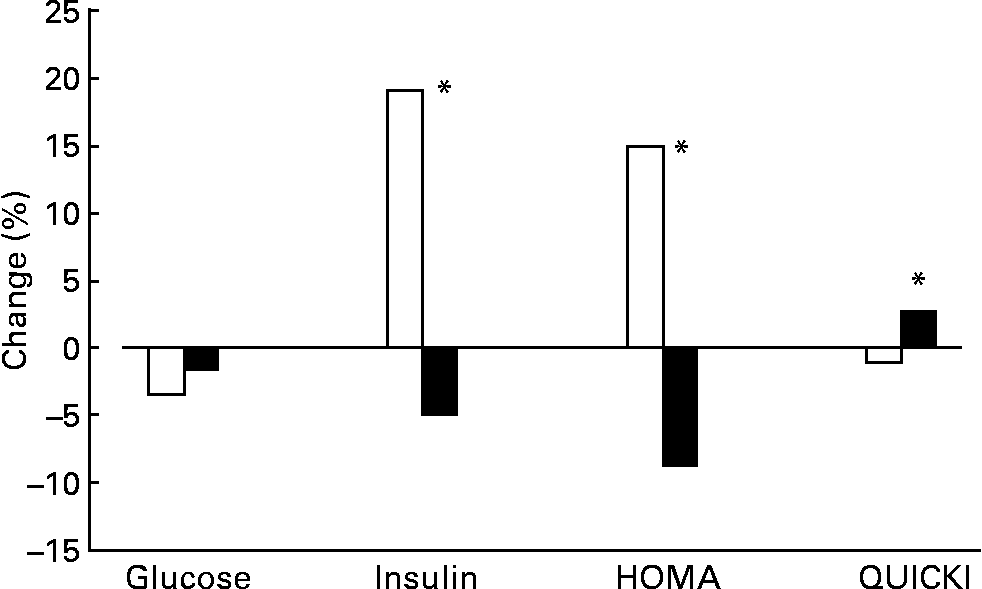
Fig. 1 Change (%) of glucose and insulin levels, as well as Homeostasis Model Assessment (HOMA) index and Quantitative Insulin Sensitivity Check Index (QUICKI) between baseline and the end of each dietary period with oily fish (■) or red meat (□) diets (four portions per week). Glucose changes between dietary treatments were not significantly different. Insulin, HOMA and QUICKI changes were significantly different between dietary treatments: *P = 0·030, 0·050 and 0·036, respectively.
Discussion
The results of the present study show that a high consumption of oily fish, rich in n-3 PUFA, increases insulin sensitivity compared to a diet rich in red meat in young iron-deficient women. Furthermore, the present study demonstrates that it is feasible to carry out a dietary intervention increasing oily fish intake within the framework of a varied diet and normal portion sizes.
The present study is part of a wider investigation about the effects of oily fish consumption on iron deficiency after our group previously observed that adding salmon to a phytate-rich meal increases iron absorption(Reference Navas-Carretero, Pérez-Granados and Sarriá24). In the present study, no significant differences were observed in iron status parameters between the two experimental diets(Reference Navas-Carretero, Sarriá, Pérez-Granados, Kalambaheti and Charoenkiatkul25). However, increasing oily fish consumption may induce changes in cardiovascular risk parameters and there are no data in the scientific literature on cardiovascular risk of iron-deficient women. This is the first time to our knowledge that these findings have been reported in young women with low iron stores.
The red meat diet reflects contemporary dietary habits in Spain(26) while the oily fish diet coincides with the current Spanish guidelines(Reference Aranceta and Serra-Majem27). PUFA intake during the oily fish diet (6·5 % of total energy intake) was between Spanish and British recommendations(Reference Aranceta and Serra-Majem27, 28), and n-3 PUFA intake did not exceed the ‘generally recognized as safe’ (GRAS) levels marked by the Food and Drug Administration(29). In order to prevent diet-related chronic diseases, one of the population nutrient intake goals published by the WHO is for n-3 PUFA to represent 1–2 % of the total energy intake(30). The corresponding Spanish dietary guidelines establish that 0·5–1 % of total energy should come from linolenic acid and 0·2–0·5 % from EPA and DHA, within a n-6/n-3 ratio of 4/1 to 10/1(Reference Ros31). The present study demonstrated that it was not possible to achieve the goals for EPA and DHA intake with a diet containing five portions of red meat per week. In contrast, the oily fish diet provided tolerable upper intake levels for these two essential n-3 PUFA. On the other hand, both diets provided the recommended intake of α-linolenic acid, as all participants consumed similar amounts of vegetables and legumes.
The present findings that oily fish consumption increased HDL-c and tended to reduce serum TAG coincide with those of other authors(Reference Mori, Bao and Burke10, Reference Finnegan, Minihane and Leigh-Firbank32–Reference Rice34). We attribute the absence of significant differences in TAG levels between dietary treatment groups to the fact that all volunteers presented the lipid profiles and TAG concentrations of healthy individuals. Data from the literature show that the most important changes observed in TAG levels after the dietary treatments were either due to a related disease of an individual volunteer(Reference Mori, Bao and Burke10, Reference Finnegan, Minihane and Leigh-Firbank32) or to n-3 PUFA taken in the form of dietary supplements or fish oils(Reference Kris-Etherton, Harris and Appel33, Reference Rice34).
Variations in Total-c and LDL-c have not always been observed in other studies using n-3 fatty acids(Reference Finnegan, Minihane and Leigh-Firbank32, Reference Rice34). Some authors even report increases in LDL-c levels after consumption of doses of n-3 PUFA above 1·7 g/d, most likely in subjects presenting hypertriacylglyceridaemia(Reference Finnegan, Minihane and Leigh-Firbank32, Reference Kris-Etherton, Harris and Appel33) as well as an elevated susceptibility of LDL particles to oxidation(Reference Finnegan, Minihane and Leigh-Firbank32, Reference Pedersen, Petersen and Major-Pedersen35, Reference Lapointe, Couillard and Lemieux36). Other authors have stated that the consumption of low amounts of n-3 PUFA (0·9, 0·6 or 0·3 g/d n-3 in the form of fish oil) for a long period does not significantly influence the susceptibility of LDL to oxidative modifications(Reference Higgins, Carroll and McCarthy37). Supplementation with n-3 PUFA, as compared with n-6 PUFA or MUFA, has been reported to reduce lag time, indicating enhanced LDL oxidizability, and also the oxidation rate, suggesting decreased oxidizability of LDL particles(Reference Turini, Crozier and Donnet-Hughes38, Reference Lee and Wander39).
In the present study, the level of oxidation products (lipoperoxides) did not increase after an oily fish diet that provided an average of 2·75 g dietary n-3 PUFA/d, indicating that an intake of nearly 3 g n-3 PUFA from oily fish does not affect oxidation in the study participants. It is well known that excess tissue iron is associated with a high oxidative stress in iron overload conditions(Reference Facchini and Saylor20), while these women presented low iron stores, which may have exerted a protection against oxidation in the oily fish diet.
The absence of effects of the oily fish diet on soluble adhesion molecules (sVCAM-1 and sICAM-1) is consistent with the findings of Miles et al. (Reference Miles, Thies and Wallace40), who observed no changes in these parameters in young and elderly individuals after fish oil consumption. Values of sVCAM-1 and sICAM-1 in the women of the present study were similar to those observed by our group in healthy post-menopausal females(Reference Schoppen, Pérez-Granados and Carbajal41).
As SFA intake from the two diets was similar, the high (nearly 50 g/d) oleic acid (18 : 1) intake, constituting approximately 25 % of the energy consumed, of individuals on the red meat diet may partly explain the favourable results with regard to Total-c and LDL-c in this diet. Oleic acid exerts a modulating effect on lipid metabolism and reduces cardiovascular risk(Reference Covas42). Therefore, by providing extra oleic acid the red meat diet seems to have compensated for the fact that its n-3 PUFA content was lower than that of the oily fish diet. The fact that oleic acid is the main component of olive oil, which is a characteristic component of the Mediterranean diet and was thus consumed by all participants, may also have influenced the study results. Moreover, participation in the study in and of itself may have played a role in improving the dietary habits, and thus the nutritional status, of the volunteers.
As for the results in relation to insulin sensitivity, the present study confirms that a moderate consumption of oily fish beneficially affects insulin action by reducing insulin levels while maintaining pre-existing glucose concentration values, while excessive intake of fish and n-3 PUFA (up to 10 g/d) have shown deterioration of glucose control in subjects suffering from type 2 diabetes mellitus(Reference Martín de Santa Olalla, Sánchez-Muniz and Vaquero43).
In accordance with previous findings(Reference Mori, Bao and Burke10, Reference Martín de Santa Olalla, Sánchez-Muniz and Vaquero43–Reference Ramel, Martinez and Kiely47), we attribute the positive effects of the oily fish diet to its EPA and DHA content, which is ten times higher than that of the red meat diet, while α-linolenic acid intake was similar in both periods. It has been assessed that membrane enrichment with PUFA improves glucose uptake which is related to an increase in the levels of insulin-responsive GLUT1 and GLUT4 in the plasma membrane(Reference Nugent, Prins and Whitehead48). The ingestion of both n-6 and n-3 fatty acids has been found to suppress hepatic lipogenesis, reduce the hepatic output of TAG, enhance ketogenesis, and induce fatty acid oxidation in both the liver and the skeletal muscle(Reference Martín de Santa Olalla, Sánchez-Muniz and Vaquero43, Reference Nugent, Prins and Whitehead48).
With regard to the effect of oleic acid on insulin sensitivity, bibliographic data indicate that it is more beneficial than SFA(Reference Paniagua, Gallego de la Sacristana and Sánchez49) although the effects of MUFA on insulin resistance have never been compared with those of PUFA(Reference Mori, Bao and Burke10, Reference Vessby, Uusitupa and Hermansen16, Reference Vessby, Tengblad and Lithell50). The present results indicate that the oily fish diet provides 1·5–2 g oleic acid/d less than the red meat diet but seems to improve insulin sensitivity by replacing it with n-3 fatty acids. The present results are also in agreement with a previous report indicating that middle-aged meat eaters present a lower insulin sensitivity than lacto-ovo vegetarians(Reference Hua, Stoohs and Facchini21).
To the best of our knowledge, this is the first nutritional intervention study with oily fish to report decreased insulin resistance in young iron-deficient women. This is in agreement with previous reports(Reference Mori, Bao and Burke10, Reference Martín de Santa Olalla, Sánchez-Muniz and Vaquero43–Reference Ramel, Martinez and Kiely47), suggesting that regular oily fish consumption exerts a beneficial effect in men and women. However, n-3 PUFA capsules were more effective than salmon in one study carried out in overweight and obese adults who received controlled energy-restricted diets(Reference Ramel, Martinez and Kiely47), and a daily fish meal had no effect in overweight subjects unless it was incorporated into a weight-loss regimen(Reference Mori, Bao and Burke10, Reference Abete, Parra and Crujeiras46).
While the beneficial effects of PUFA on insulin sensitivity and glucose levels are well documented(Reference Feskens, Loeber and Kromhout14, Reference Griffin, Sanders and Davies45, Reference Vessby, Tengblad and Lithell50, Reference Storlien, Jenkins and Chisholm51), most of the studies carried out to date have used supplements or fish oils to include high doses of PUFA in the diet. A remarkable fact of the present study is the inclusion of a whole food as part of a healthy diet, which is an important approach in present nutrition research. Even if an isolated food component is shown to be bioactive, the whole food acts synergistically to influence the risk of chronic diseases(Reference Jacobs and Steffen52). From a public health point of view, the results obtained are relevant, giving additional support to the recommendations concerning oily fish consumption. Moreover, the present results emphasize that changes in insulin sensitivity must be shifted from the traditional glucocentric towards a lipocentric point of view(Reference Mittra, Bansal and Bhatnagar53).
Conclusion
Changing dietary habits by increasing oily fish consumption and reducing red meat intake may protect against the development of type 2 diabetes mellitus in young women. Further studies using oily fish diets in subjects at high risk of type 2 diabetes mellitus or the metabolic syndrome should be carried out. Finally, iron-deficient women, who are advised to increase red meat intake, should be aware of the beneficial effects on insulin sensitivity of consuming oily fish within a high iron bioavailability diet.
Acknowledgements
This study was supported by the Spanish Ministry of Science and Innovation (projects AGL 2002-04411-C02-01 ALI and AGL2006-09519 ALI). S. N.-C. was the holder of a Comunidad de Madrid-European Social Fund FPI fellowship. S. S. had a research contract from the Spanish Ministry of Science and Innovation (Juan de la Cierva). S. N.-C. and A. M. P.-G. carried out the intervention study and laboratory analysis. M. P. V. was the principal investigator of the project. The article was co-written by all authors. Sardines and tuna cans were provided by Calvo SA (Spain). The authors would like to thank the volunteers, Laura Barrios for statistical support and Dr Angeles Carbajal for her dietary advice. None of the authors had any conflict of interest.







