Estimates of ovine pre-weaning mortality currently range from 10 to 30 % wordwide, with the neonatal period being the most vulnerable in this regard( Reference Sawalha, Conington and Brotherstone 1 ). Hypothermia, starvation and infectious disease have been identified as the major causes of mortality within the first 3 d of life( Reference Dwyer 2 ). With regard to infection, gastrointestinal nematodes (GIN) have been recognised as the most pervasive problem facing sheep production enterprises( Reference Good, Hanrahan and Crowley 3 ). Of these, Nematodirus battus has been identified as one of the most prevalent parasites affecting animals in cool, temperate climates; with lambs susceptible to infection from 5 weeks of age( Reference Denwood, Stear and Matthews 4 ). Subclinical infection with GIN results in significant production losses due to weight loss and reduced weight gain, particularly in young, naïve animals( Reference Good, Hanrahan and Crowley 3 ). The hypo-immunocompetent nature of the newborn ruminant increases its reliance on acquiring passive immunity in early life( Reference Brambell 5 , Reference Boland, Hayes and Sweeney 6 ). Therefore, adequate colostrum consumption is critical to provide nutrition and promote Ig transfer; thus augmenting the ensuing health and survival of the neonatal lamb( Reference Rose, Pearson and Cratchley 7 ). Ig are plasma proteins produced by lymphocytes in the mammalian bloodstream in response to foreign antigens. They are part of the innate immune system, which is the primary line of defense against pathogens and therefore are essential in the development of disease resistance( Reference Park and Jacobson 8 ). Previous work has found that young lambs are capable of mounting a specific antibody response to N. battus, which has been associated with the majority of adult worms being rejected by 21 d post infection( Reference Winter, Wright and Lee 9 ). As IgG constitutes approximately 92 % of Ig found in ovine colostrum( Reference Boland, Keane and Nowakowski 10 ), it the most prevalent Ig class and provides adequate immunity to the neonatal lamb during the initial weeks of independent life( Reference Mayer, Doleschall and Bender 11 ).
Previous work has outlined that excess I supplementation to ewes in late gestation alters the thyroid hormone status of the newborn lamb, which results in a failure to acquire passive immunity, evidenced by a reduced serum IgG concentration at 24 h postpartum( Reference Boland, Hayes and Sweeney 6 , Reference McGovern, Magee and Browne 12 ). This has been shown to occur independently of colostrum intake and colostrum IgG concentration( Reference Boland, Brophy and Callan 13 ) thus suggesting that there is an alteration in the functioning of the lambs’ intestinal absorptive process which occurs in utero. The primary site of IgG absorption in the newborn is the lower ileum( Reference Yvon, Levieux and Valluy 14 ); with a previous experiment identifying a link between excess maternal I supplementation and the failure of passive transfer (FPT) of IgG( Reference Boland, Brophy and Callan 13 ). These authors concluded that FPT was mediated through the thyroid hormone status of the animal and influenced the expression of Ig-related genes in the intestinal tract immediately postpartum( Reference McGovern, Magee and Browne 12 ).
Therefore, the objectives of this study were as follows: (1) to determine the effect of excess I supplementation to the pregnant ewe on the thyroid hormone status of both the ewe and her progeny at parturition; (2) to identify potential mechanisms responsible for FPT by examining the expression profiles of selected genes in the ileum, duodenum, thyroid and perirenal adipose tissue of the lamb at 24 h postpartum; and (3) to assess the effect of FPT on the growing lambs’ response to gastrointestinal infection.
Methods
All procedures involving ewes and lambs in this study were conducted under experimental licence from the Irish Medicines Board in accordance with the European Union (protection of animals used for scientific purposes) regulations 2012 (S.I. No. 543 of 2012). This study was conducted at University College Dublin, Lyons Research Farm, Newcastle, Co. Dublin.
Pre-experimental animal management
Ewes were confirmed as pregnant and twin-bearing to a synchronised oestrus (intervaginal progestagen pessaries; Chronogest and Folligon, Intervet Ireland Ltd) followed by an intramuscular injection of 500 IU (2·5 ml) pregnant mare serum gonadotropin (Chronogest and Folligon, Intervet Ireland Ltd), following a transabdominal ultrasound on day 77 post insemination (inseminated with fresh diluted semen, at a rate of 20 million spermatozoa per uterine horn, using laparoscopic artificial insemination (AI)). Before selection, the ewes were blocked on the basis of live weight (75·5 (se 2·29) kg) and balanced for body condition score (BCS) (2·96 (se 0·06)), age (3·4 (se 0·95) years), ewe breed and lamb-sire breed. BCS assessments were made by a trained technician and ewes were scored on a scale of 0–5( Reference Russel 15 ).
Nutritional treatments
On day 113 of gestation, ewes were allocated to one of two nutritional treatment groups and transferred to individual, wooden-slatted pens measuring 1·1×1·4 m. The animals were allowed a 7-d adaption period before experimental feeding began on day 119 of gestation. All ewes were fed to meet 100 % of predicted metabolisable energy (ME) requirements for mature, gestating twin-bearing ewes( 16 , Reference Robinson, Rooke and McEvoy 17 ). Ewes received either a basal diet (C), which included mineral and I supplementation or they received C plus an additional daily allowance of 26·6 mg of I supplied in the form of calcium iodate (I, 42·9 mg/ewe per day; Devenish Nutrition). I inclusion levels were not confirmed analytically and are based on calculated formulation. This level of I equates to the level offered to ewes in a recently reported study( Reference Boland, Hayes and Sweeney 6 ) and is reflective of the level of intake achieved when ewes are offered free access mineral supplements indoors( Reference Crosby, Boland and Brophy 18 ).
Feeding
ME requirements, based on those outlined by the Agricultural and Food Research Council Technical Committee( 16 ) and including amendments( Reference Robinson, Rooke and McEvoy 17 ), were calculated individually for each ewe( Reference McGovern, Campion and Lott 19 ). Silage was offered at 08.00 hours each morning following the removal of the previous days’ silage refusals. The quantity of concentrate offered was calculated for each individual animal depending on the level of ME received from the silage and their individual ME allocation as per treatment group. Concentrates were offered daily at 09.00 hours or twice daily at 09.00 and 17.00 hours in equal allocations when daily allocation exceeded 500 g (fresh weight)/ewe. The iodine supplement was manually mixed into the concentrate on the day before feeding. Before mixing, the iodine allowance was first added to 20 g (fresh weight) of the concentrate that was used as a carrier for the supplement. Before the mineral inclusion, this carrier was dried using forced air circulation at 55°C for 72 h, following which it was ground through a 0·8 mm screen using a Christy and Norris hammer mill (Christy Turner Ltd). The daily concentrate allowance was then adjusted to take into account the carrier contribution. Ewes received an 18 % crude protein (CP) concentrate which comprised barley (40 %), soya hulls (29 %), whole-wheat distillers (25 %) and molasses (4 %), on a DM basis. In addition, the concentrate offered contained minerals (2 %) and cane molasses (2 %). The level of I in C was in excess of standard dietary requirements because of the inclusion of I in the mineral portion of C. The chemical composition of the feedstuff offered is outlined in Table 1. Representative samples of both silage and concentrate were taken each morning and frozen at –20°C for subsequent proximate analysis. Ewes had continuous access to clean, fresh drinking water at all times. Post lambing, all ewes were reverted to C and received grass silage ad libitum plus concentrates (1 kg), fed twice daily in equal allocations. At 3 d postpartum, all ewes were turned out onto a perennial rye-grass pasture and were rotationally grazed as a single group along with their lambs. At this stage, concentrate supplementation was discontinued.
Table 1 Chemical composition of silage and concentrate offered to ewes during late gestation

* Percentage of crude protein in the concentrate; 18 % crude protein.
Ewe measurements and lambing data
Ewes were assessed for BCS and weighed on an electronic scale (Prattley), with live weights recorded electronically (Tru-Test Group) on day 114 of gestation, 24 h, day 40 and 98 postpartum. A 10 ml blood sample was collected from all ewes on day 119 of gestation (trial start date) and 24 h postpartum via jugular venepuncture using heparinised vacutainers (ref: 367 526; Becton, Dickinson and Company). Blood samples were immediately placed on ice and centrifuged at 4°C and 1800 g for 15 min after which the plasma was pipetted into pour-off tubes and frozen at –20°C until further analysis.
All ewes lambed down in their individual pens where they remained with their lambs until 72 h postpartum. Within the 1st hour after parturition, the navel of each lamb was dipped in a 10 % I (Ritchey) solution, to aid in the control of erysipelas polyarthritis (joint-ill), following which time of birth, birth weight, sex and the level of assistance required by the ewe at lambing (referred to hereafter as lambing difficulty; LD) were recorded for each lamb. LD was assessed, by trained observers, using a score between 1 and 4 where 1 referred to a natural unassisted lambing and 4 referred to a caesarean section. The time of birth of the first lamb was used to calculate the gestation length (GL) of the ewe, with 12.00 hours on the day of AI taken as 0 h. At 24 h postpartum all lambs were ear-tagged with an electronic identification tag. All incidences of erysipelas polyarthritis (joint-ill) and Escherichia coli were recorded in lambs from birth until day 14 postpartum. E. coli was diagnosed in lambs that presented with a watery mouth, a distended abdomen and showed obvious signs of pain, lack of appetite and isolation from the dam.
Hand milking
At parturition sixteen ewes per treatment group were randomly selected and an udder cover placed on them, until 24 h postpartum in order to prevent the lambs from suckling. Ewes were hand milked at 1, 10 and 18 h postpartum as previously described( Reference Crosby, Boland and Brophy 18 ). In brief, each ewe received an intramuscular injection of 10 IU of oxytocin (Oxytocin-S; Intervet Ltd) immediately before each milking to ensure total milk let-down( Reference Doney, Peart and Smith 20 ). Ewes were milked out completely by hand and the total volume was recorded after each milking. From this, the total volume produced up to 18 h postpartum was calculated. For the determination of total solid (TS) and CP content, a colostrum sample (25 ml) was taken at the 1 h milking and stored at –20°C until required.
Following each milking, the lambs were fed measured quantities of colostrum from their dam via a stomach tube. Depending on the yield of colostrum, lambs received the maximum amount of colostrum available, within the range of 20–50 ml colostrum per kg birth weight. The quantity of colostrum fed to each lamb was recorded.
Lamb blood sample collection
At 1 h postpartum, and before stomach tubing where appropriate, a 10 ml plasma blood sample was collected via jugular venepuncture in heparinised vacutainers (BD; Ref: 367 880) from all lambs. The blood samples were immediately placed on ice and centrifuged at 4°C at 1800 g for 15 min, after which, the plasma was pipetted into pour-off tubes and stored at –20°C until further analysis. At 24 h postpartum (and before euthanasia where appropriate) and fortnightly thereafter until day 70, a blood sample was collected via jugular venepuncture in non-heparinised vacutainers (BD; Ref: 368 975) from all lambs. Blood samples were stored at room temperature for 1 h before storage at 4°C where they remained for 24 h. Samples were subsequently centrifuged at 4°C and 1800 g for 15 min to obtain the serum, which was then frozen at –20°C until further analysis.
Organ collection and small intestinal morphology
At 24 h postpartum, a subgroup of twenty-four lambs (n 12/treatment group) were euthanised with Euthatal (pentobarbitone sodium BP; Merial Animal Health Limited) at a rate of 1 ml/kg body weight. This resulted in eighteen ewes per treatment rearing twin lambs and twelve ewes per treatment rearing single lambs (defined hereafter as rearing rank: twin=2 and single=1). Before euthanasia, the rectal temperature of each animal was recorded. Following euthanasia the liver, spleen, kidneys, perirenal adipose tissue, heart, lungs, thyroid and digestive tract were dissected and weighed. After excision, the abomasum was emptied along the mesentery and the contents collected in a 50 ml sterile container (Sarstedt Ltd). The pH of the abomasal contents was then assessed using an Orion 3-Star pH meter (Thermo Fisher Scientific Inc.).
Intestinal tissue from the duodenum (approximately 10 cm from the abomasum) and ileum (approximately 10 cm from the ileo-caecal junction) was aseptically isolated and fixed in 10 % phosphate buffered formalin for villus height (VH) and crypt depth (CD) measurements. Additional sections of the duodenum and ileum were excised, emptied by dissecting along the mesentery and rinsed using PBS (Oxoid; Thermo Fisher Scientific Inc.). Approximately 1–2 g of duodenum, ileum, thyroid and perirenal adipose tissue were collected and placed in 10 ml tubes (Sarstedt Ltd) containing 5 ml of RNAlater™ (Applied Biosystems Ltd), where they were stored for 24 h. The RNALater™ was then removed and the tissue samples were stored at –80°C.
Lamb weight and factory data collection
All lambs were weighed fortnightly from days 14–70 postpartum and once every 28 d thereafter until slaughter. Lambs were weighed on an electronic scale and live weight recorded electronically. Lambs were slaughtered once they reached 45 kg live weight. Any lambs drafted before or after this weight was reached had their days-to-slaughter corrected for live weight before statistical analysis. At slaughter, carcass weights were recorded and subsequently used to calculate kill-out percentage (KO %).
Laboratory analysis
Proximate analysis
The DM content (g/kg) of the concentrate and grass silage was determined by drying the samples at 55°C for 72 h in a ventilated oven with forced-air circulation. N content (g/kg DM) of the silage and concentrate was determined using a LECO FP528 instrument (Leco Instruments Ltd) according to the method of Dumas( 21 ). The N concentration of the concentrate and grass silage was multiplied by 6·25 to determine CP concentrations( Reference Kjeldahl 22 ). The neutral detergent fibre (assayed with and without a heat stable amylase for concentrate and grass silage samples, respectively, and without sodium sulphite for both feeds; expressed inclusive of residual ash), acid detergent fibre (expressed inclusive of residual ash) and acid detergent lignin (expressed inclusive of residual ash) concentrations (g/kg DM) of the feedstuff were determined( Reference Van Soest, Robertson and Lewis 23 ) using an ANKOM200 Fibre Analyzer (ANKOM Technology). Ash concentrations (g/kg DM) of the feedstuff were determined by complete combustion in a muffle furnace at 550°C for 4 h. The gross energy concentration (MJ/kg DM) of the silage and concentrate was determined using bomb calorimetry (Parr 1281 bomb calorimeter; Parr Instrument Company)( Reference Sauvant, Perez and Tran 24 ), whereas the ME concentration (MJ/kg DM) was calculated using the following equation( Reference Sauvant, Perez and Tran 24 ):
where CFo is crude fibre in the organic matter and CPo is CP in the organic matter.
Diethyl ether extract concentrations (g/kg DM) of the feedstuff were determined using light petroleum ether and Soxtec instrumentation (Tecator). The concentration of starch in the concentrate was determined using the Megazyme Total Starch Assay Procedure( 21 ). Mineral analysis of silage and concentrate without the addition of the I supplement was carried out by Sciantec Analytical Services Ltd and is presented in Table 2.
Table 2 Major and trace element composition of silage and concentrate offered to ewes during late gestationFootnote *
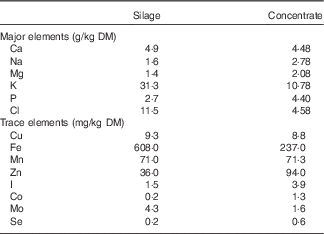
* Mineral analysis of silage and concentrate, without the addition of the I supplement, was carried out by Sciantec Analytical Services Ltd.
Blood sample analysis
Total serum Ig concentration (g/l) was determined using the zinc sulphate turbidity test( Reference McEwan, Fisher and Selman 25 ). These results were then reduced by a factor of 0·09 to provide the IgG-only content of the serum( Reference Larson, Ward and Frederiksen 26 ). The efficiency of IgG absorption within the first 24 h postpartum was further calculated. It was assumed that in lambs, as in calves, 0·075 of birth weight is equivalent to blood plasma volume( Reference Quigley, Hammer and Russel 27 ). Therefore, the following equation was used to calculate the IgG absorption efficiency:
 $$\eqalign { & {\rm IgG}\;{\rm absorption\,{\equals}}\, \cr & \left( {{{{\rm Birth}\;{\rm weight {\times}0} {\!}\cdot {\!}{\rm 075{\times}Serum}\;{\rm IgG}\;{\rm concentration}} \over {{\rm Total}\;{\rm IgG}\;{\rm fed}}}} \right){\rm {\times}100}{\rm .}$$
$$\eqalign { & {\rm IgG}\;{\rm absorption\,{\equals}}\, \cr & \left( {{{{\rm Birth}\;{\rm weight {\times}0} {\!}\cdot {\!}{\rm 075{\times}Serum}\;{\rm IgG}\;{\rm concentration}} \over {{\rm Total}\;{\rm IgG}\;{\rm fed}}}} \right){\rm {\times}100}{\rm .}$$
Plasma total tri-iodothyronine (T3) and total thyroxine (T4) concentrations were determined using commercial RIA kits (T3: KIP1631 or T4: KIP1641; DIAsource ImmunoAssays S.A.) where all samples were measured on the same assay in duplicate. The intra-assay CV were 3·85 and 4·96 % for the T3 and T4 assays, respectively.
Colostrum analysis
The N content (g/kg DM) of the colostrum was determined using a LECO FP528 instrument according to the method of Dumas( 21 ). The N concentration in the colostrum was multiplied by 6·38 to determine CP concentrations( Reference Kjeldahl 22 ). The TS (g/l) content of the colostrum was determined using a direct forced-air method where 3 ml of colostrum and/or milk were placed in an oven for 4 h at 100°C( Reference Van Soest, Robertson and Lewis 23 ).
The colostrum samples were thawed at room temperature the day before laboratory analysis. IgG concentration of the samples was determined by the ELISA method (Ovine IgG ELISA Kit, Cat. No. 7620; Alpha Diagnostic International). Samples were diluted 1:1 000 000 before being assayed in duplicate, with an inter-assay CV of 5 %. The concentration of IgG in the samples was calculated from a standard reference curve containing known concentrations of IgG. Any sample that resulted in an IgG concentration that fell outside the range of the standard reference curve was retested after further dilution according to the test recommendations.
Duodenal and ileal morphology
The preserved intestinal segments were prepared using standard paraffin-embedding techniques. Cross-sections of 5 µm thickness of each sample were stained with haematoxylin–eosin( Reference Pierce, Sweeney and Brophy 28 ). VH and CD were measured on the stained sections of the ileal tissue and only VH measurements were taken from the duodenal tissue, using a light microscope (100× objective) fitted with an image analyser (Image-Pro Plus 9.1; Media Cybernetics). Measurements of twenty well-orientated intact villi and crypts were measured for each animal. VH was measured from the crypt–villus junction to the tip of the villus and CD was measured from the crypt–villus junction to the base.
Extraction and assessment of RNA integrity
Total RNA was isolated from the ileum, duodenum, thyroid and perirenal adipose tissues using TRI reagent (Molecular Research Center) according to the manufacturer’s instructions, followed by further purification with the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich). In brief, approximately 25 mg of tissue was homogenised in 1·0 ml of Trizol using a steel bead (Qiagen Ltd) and the Qiagen TissueLyser II (2×120 s maximum speed). Chloroform (200 µl) was then added to each sample, and following centrifugation (12 000 g , 15 min), the aqueous phase was transferred directly onto an RNeasy column (Qiagen Ltd). The RNA was further purified as per the manufacturer’s instructions and included an on-column DNase step (Sigma-Aldrich).
The total RNA was quantified using a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific Inc.) and the purity was assessed by determining the ratio of the absorbance at 260 and 280 nm. All total RNA samples with acceptable 260:280 nm ratios were used for further analysis. Total RNA was analysed in an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.) using RNA 6000 Nano LabChips (Caliper Technologies Corp.). The RNA Integrity Number for the ileum, duodenum, thyroid and perirenal adipose tissues were 9·1 (sem 0·06); 9·0 (sem 0·08); 9·2 (sem 0·15) and 8·7 (sem 0·19), respectively.
Complementary DNA synthesis and quantitative real-time PCR
Total RNA from each sample (1 µg) was reverse transcribed using the high capacity complementary DNA (cDNA) reverse transcription kit (Applied Biosystems Ltd) according to the manufacturer’s instructions. The purified cDNA was diluted in RNase- and DNase-free water to a volume of 250 µl and stored at –20°C. Oligonucleotide primers were designed with Primer ExpressTM Software, version 2.0 (Applied Biosystems) and synthesised by MWG-Biotech. All assays were assessed for specificity using dissociation analysis. Assays with efficiencies between 90 and 110 % were deemed acceptable. Target genes quantified in the ileum and duodenum were: Fc fragment of the IgG receptor transporter, α (FCGRT), Fc receptor, IgA, IgM high affinity (FCAMR), polymeric Ig receptor (PIGR), β-2-microglobulin (B2M), cellular myelocytomatosis oncogene, TNFα, upstream stimulator factor 1 (USF1), upstream stimulator factor 2 (USF2), thyroid hormone receptor-α (THRA), thyroid hormone receptor-β (THRB), the ras-related proteins (RAB) RAB11a and RAB25 and the mucin genes (MUC) MUC1 and MUC3A; whereas THRA, THRB and uncoupling protein 1 (UCP1) were quantified in the thyroid and perirenal adipose tissues of lambs. All primer sequences for the target and reference genes were as presented in a previous study( Reference McGovern, Magee and Browne 12 ).
The quantitative PCR (qPCR) assays were performed in a total volume of 20 µl, containing 2×Fast SYBR PCR Master Mix (Life Technologies Ltd), 1 µl of a forward and reverse primer mix (300 nm each) and 5 µl cDNA. qPCR was carried out in duplicate, on the 7500 ABI Prism Sequence Detection System (Applied Biosystems Ltd). Thermocycling conditions were as follows: 95°C for 10 min, followed by 95°C for 15 s, and 60°C for 1 min for forty cycles.
A total of six potential reference genes: β-actin, glyceraldehydes-3-phosphate dehydrogenase (GAPDH), glucose-6-phosphate dehydrogenase, hypoxanthine phosphoribosyltransferase 1 (HPRT1), ribosomal protein L19 and succinate dehydrogenase (SUCD) were analysed using the geNorm algorithm on the qbase+ package (Biogazelle)( Reference McEwan, Fisher and Selman 25 ). GAPDH and SUCD were the most stably expressed in the ileum, duodenum and thyroid, and were subsequently used to normalise the gene expression data in these tissues; while HPRT1 and SUCD were used to normalise the gene expression data in the perirenal adipose tissue. The mean C t values of duplicates of each sample were used by the qBASE plus algorithm to generate the normalised relative quantities for each gene.
Determination of faecal egg count
Faecal samples were collected per rectum from all lambs once fortnightly from days 42–70 postpartum. All samples were placed on ice until their return to the laboratory where they were stored at 4°C before assessment. Faecal egg count (FEC) were determined using the modified McMaster method( 29 , Reference Kelly, Good and Hanrahan 30 ). FEC was distinguished separately as ‘N. battus’ (FECN) and ‘other trichostrongyles’ (FECOT) with the number of eggs/g (epg) enumerated. Before statistical analysis, FEC values were transformed using the natural logarithm (loge(X+25)) to stabilise the variance.
Statistical analysis
In this study there are two treatment groups; C and I. The difference (μ1–μ2) is expressed as a percentage of μ1: that is, (μ1–μ2)/μ1×100 and denoted by d. The CV is by definition σ/μ1×100. According to the University College Dublin Animal Research Ethics Committee guidelines the formula is written as n 2×(CV/d)2×(1·96+0·842)2, that is, n 15·7×(CV/d)2. Lamb performance in terms of growth rate is the most important outcome variable in this study. Comparisons with the control were based on a significance level of 5 % and a power of 80 %. From previous work CV=18 % and d=10 %(19). Taking this into account the sample size per group is n 15·7×(13·5/10)2=50·9. In each treatment, twelve lambs were euthanised, and a natural mortality of 4 % was assumed. Therefore, in order to retain fifty-one lambs per treatment for growth rate studies, thirty-two twin-bearing ewes per treatment were required.
Data were analysed as a completely randomised block design using the mixed model procedure (PROC MIXED) in SAS( 31 ). The binary data, namely lamb health, was analysed using PROC GENMOD. The individual ewe was considered the experimental unit for all parameters analysed. Descriptive statistics were performed to identify continuous and categorical variables and their distributions analysed to fit the assumptions of normality using the UNIVARIATE procedure. The statistical model used for all parameters included the fixed effect of treatment, whereas the ewe data was adjusted for live weight at the beginning of the experiment by covariance. Date of birth was fitted as a covariate for all lamb parameters analysed. A repeated measure analysis was performed on ewe DM intake, live weight, BCS and colostrum production data, and lamb live weight and FEC data. In all, six covariance structures were compared: compound symmetry, heterogeneous compound symmetry, autoregressive order 1, heterogeneous autoregressive order 1, toeplitz and heterogeneous toeplitz; whereas the covariance structure that yielded the lowest Bayesian information criterion was used in the model. The fixed effects of time (as the repeated constant), treatment, sex, rearing rank, the two-way interaction of ewe nutritional treatment×time and rearing rank×time were included in the model. A total of four ewes (n 2/treatment) gave birth to one lamb plus one mummified fetus and these were excluded from the final analysis. Therefore, the final number of ewes used for analysis in each treatment (n) were thirty and thirty for the C and I treatments, respectively. There was no interaction between ewe nutritional treatment×rearing rank (P>0·25); therefore, it was removed from the model before the final analysis of lamb weight and growth rate data. Rearing rank wasn’t a significant factor influencing FECN or FECOT (P>0·05) hence it was removed from the final model before analysis. Lamb birth weight was included as a covariate in the analysis of lamb growth rate data. A Tukey’s adjustment was applied where multiple observations were compared in the final analysis. All data presented in the tables and figures are expressed as least-square means with their standard errors. The probability value, which denotes statistical significance, was P<0·05, while values tending towards significance were 0·05<P<0·10.
Results
Ewe DM, crude protein and metabolisable energy intake, live weight and body condition score
Dietary treatment had no effect on the DM intake (1·29 v. 1·29 (sem 0·01) kg/d), CP (190 v. 189 (sem 2·72) g/d) or the ME intake (13·26 v. 13·20 (sem 0·13) ME/ewe per d) of ewes throughout the feeding period (P>0·05). I supplementation had no effect on the live weight, BCS or BCS change of the ewes (P>0·05) when assessed throughout the experimental period (data not shown).
Lambing parameters
I supplementation had no effect on ewe GL, LD or the combined litter weight of lambs at birth, with means ranging from 9·55 to 9·66 (sem 0·126) kg, for the C and I progeny, respectively (P>0·05). I supplementation did not affect the incidence of joint-ill or E.coli infection from birth until day 14 postpartum (P 0·235; data not shown).
Colostrum yield, IgG concentration and intake
Treatment had no effect on colostrum yield or IgG concentration at 1, 10 and 18 h postpartum or on the total yield to 18 h (P>0·05). There was no effect of I supplementation on the TS or CP concentrations in the colostrum (P>0·05; data not shown).
Dietary treatment had no effect on the intake of colostrum or IgG when measured at 1, 10 and 18 h postpartum or on an absolute basis to 18 h (P>0·05; data not shown). Similarly, there was no effect of dietary treatment on the volume of colostrum or on the quantity of IgG fed, per kg of lamb birth weight, to lambs in both the C and I treatment groups. However, excess I supplementation of the ewe reduced the absorption efficiency of colostral IgG within the first 18 h postpartum (P<0·0001; data not shown).
Blood metabolites
Thyroid hormone concentration
Supplementation of the ewe with excess I in late gestation tended to increase the concentration of total T3 (P 0·07; Table 3) and increased the concentration of total T4 at 24 h postpartum (P<0·05). There was no effect of dietary treatment on the concentration of total T4 in the lamb serum at either 1 or 24 h postpartum (P>0·05). However, lambs born to I-supplemented ewes had a lower serum concentration of total T3 at 1 h (P<0·05). This effect tended to remain until 24 h postpartum (P=0·09).
Table 3 The effect of prepartum dam nutritional treatment on ewe (n 30/treatment) and lamb (n 60/treatment) total tri-iodothyronine (T3) and thyroxine (T4) (nmol/l) concentrations
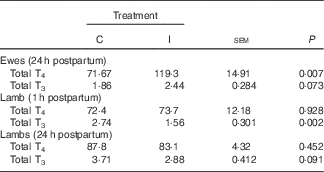
C, basal diet, no added supplement; I, ewes supplemented with 26·6 mg of I added as calcium iodate.
Lamb serum IgG
The effect of dietary treatment on the serum IgG concentration of the progeny is presented in Fig. 1. Progeny of I ewes had lower concentrations of serum IgG from 24 h to day 28 postpartum (P<0·01); however, this had reversed on day 70 postpartum (P<0·05) with the progeny of I ewes having higher concentrations of serum IgG when compared with the progeny of C ewes.

Fig. 1 The effect of prepartum dam nutritional treatment on the serum IgG concentration of the lamb postpartum. Treatments: C, basal diet, no added supplement (![]() ); I, C plus 26·6 mg of iodine added as calcium iodate (
); I, C plus 26·6 mg of iodine added as calcium iodate (![]() ). Values are least-square means (n 60 lambs per treatment for 24 h postpartum and n 48 lambs per treatment thereafter), with their standard errors. *Significant difference between C and I (P<0·01). For a colour figure, see the online version of the paper.
). Values are least-square means (n 60 lambs per treatment for 24 h postpartum and n 48 lambs per treatment thereafter), with their standard errors. *Significant difference between C and I (P<0·01). For a colour figure, see the online version of the paper.
Organ weights and small intestinal morphology
Excess I supplementation to the ewe had no effect on the rectal temperature or abomasal pH of the progeny at 24 h postpartum (P>0·05; data not shown). There was no effect of excess I supplementation to the ewe on the weight of the fetal digestive tract, liver, lungs, thyroid and kidneys when assessed on an absolute basis, at 24 h postpartum (P>0·05). The above results remained non-significant after correction for fetal birth weight (data not shown). Dietary treatment affected the weight of the spleen (P=0·05), which was 19 % lighter, and tended to affect the weight of the perirenal adipose tissue (P=0·06), which was 12·8 % lighter in the progeny of I ewes; however, these effects were not present after correction for fetal birth weight (P>0·05). After correction for birth weight, the progeny of C ewes tended to have a lighter heart weight (P=0·09; data not shown).
The effect of excess I supplementation on VH in the duodenum and ileum, and CD and VH:CD ratio in the ileum of lambs at 24 h postpartum are shown in Fig. 2 and 3. Histological examination of the duodenum indicated there was no effect of dietary treatment on the VH at 24 h postpartum (P>0·05). Progeny of the I ewes had a reduced VH and VH:CD ratio (P<0·01), despite the lack of difference in ileum CD (P>0·05) at 24 h postpartum.

Fig. 2 The effect of prepartum dam nutritional treatment on the villus height (µm) in the duodenum and the ileum of the lamb at 24 h postpartum. Treatments: C, basal diet, no added supplement (![]() ); I, C plus 26·6 mg of iodine added as calcium iodate (
); I, C plus 26·6 mg of iodine added as calcium iodate (![]() ). Values are least-square means (n 12 lambs per treatment), with their standard errors represented by vertical bars. *Significant difference between C and I for villus height in the ileum (P<0·01). For a colour figure, see the online version of the paper.
). Values are least-square means (n 12 lambs per treatment), with their standard errors represented by vertical bars. *Significant difference between C and I for villus height in the ileum (P<0·01). For a colour figure, see the online version of the paper.

Fig. 3 The effect of prepartum dam nutritional treatment on the crypt depth (CD) and the villus height (VH):CD ratio in the ileum of the lamb at 24 h postpartum. Treatments: C, basal diet, no added supplement (![]() ); I, C plus 26·6 mg of iodine added as calcium iodate (
); I, C plus 26·6 mg of iodine added as calcium iodate (![]() ). Values are least-square means (n 12 lambs per treatment), with their standard errors represented by vertical bars. *Significant difference between C and I for VH:CD ratio in the ileum (P<0·01). For a colour figure, see the online version of the paper.
). Values are least-square means (n 12 lambs per treatment), with their standard errors represented by vertical bars. *Significant difference between C and I for VH:CD ratio in the ileum (P<0·01). For a colour figure, see the online version of the paper.
Gene expression data
Excess I supplementation to the ewe down-regulated the expression of B2M, and THRB in the ileum of the lamb at 24 h postpartum; while the expression of FCAMR and PIGR were up-regulated (P<0·05; Table 4). Supplementation of the ewe with excess I had no effect on the expression of FCGRT, THRA, USF1, USF2, RAB11a, TNF and MUC3a in the ileum of lambs at 24 h postpartum (P>0·05). Maternal dietary treatment tended to affect the mRNA expression of albumin (ALB) in the ileum (P=0·07), with lower levels expressed in the progeny of I-supplemented ewes. In the duodenum, there was no effect of maternal supplementation with excess I on the expression of FCAMR, FCGRT, PIGR, THRA, THRB, USF1, USF2, RAB11a and TNF in lambs at 24 h postpartum (P>0·05). The progeny of I-supplemented ewes had an increased expression of RAB25, whereas the expression of MUC1 was down-regulated in the duodenum (P<0·05). There was no effect of dietary treatment on the mRNA expression of THRA and THRB in the thyroid tissue of lambs; however, the expression of THRB was down-regulated in the perirenal adipose tissue of I progeny (P<0·05; Table 5). In addition, there was no effect of excess I supplementation to the ewe on the expression of UCP1 in the perirenal adipose tissue of lambs in the present study (P>0·05).
Table 4 The effect of prepartum dam nutritional treatment on the normalised relative quantity of Ig-associated genes; TNFα and mucins in the ileum and duodenum of lambs at 24 h postpartum (n 12 lambs per treatment)

C, basal diet, no added supplement, I, ewes supplemented with 26·6 mg of iodine added as calcium iodate; THRA, thyroid hormone receptor-α; THRB, thyroid hormone receptor-β; FCGRT, Fc fragment of the IgG receptor transporter, α; FCAMR, Fc receptor, IgA, IgM high affinity; PIGR, polymeric Ig receptor; B2M, β-2-microglobulin; ALB, albumin; USF1, upstream stimulator factor 1; USF2, upstream stimulator factor 2; RAB, ras-related protein; MUC, mucin genes.
Table 5 The effect of prepartum dam nutritional treatment on the normalised relative quantity of Ig-associated genes in the thyroid and perirenal adipose tissue of lambs at 24 h postpartum (n 12 lambs per treatment)
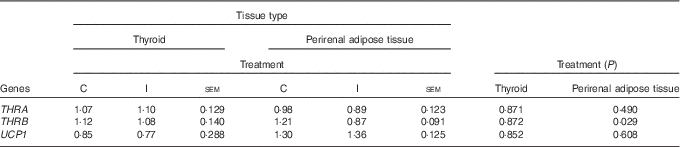
C, basal diet, no added supplement; I, ewes supplemented with 26·6 mg of I added as calcium iodate; THRA, thyroid hormone receptor-α; THRB, thyroid hormone receptor-β; UCP1, uncoupling protein 1.
Lamb performance
Live weight
There was no effect of excess I supplementation to the ewe on the live weight of the progeny from birth to day 98 postpartum (weaning; P>0·05; Fig. 4.). There was an effect of rearing rank (P=0·001) from day 14 until weaning at day 98 postpartum; where single reared lambs remained heavier than those reared as twins. However, there was no interaction between ewe dietary treatment and rearing rank (P>0·05) for lamb live weight.

Fig. 4 The effect of prepartum dam nutritional treatment on lamb live weight postpartum. Treatments: C, basal diet, no added supplement (![]() ); I, C plus 26·6 mg of iodine added as calcium iodate (
); I, C plus 26·6 mg of iodine added as calcium iodate (![]() ). Values are least-square means (n 60 lambs per treatment for ‘day 0’ (birth weight) and n 48 lambs per treatment thereafter), with their standard errors. For a colour figure, see the online version of the paper.
). Values are least-square means (n 60 lambs per treatment for ‘day 0’ (birth weight) and n 48 lambs per treatment thereafter), with their standard errors. For a colour figure, see the online version of the paper.
Average daily gain
There was no effect of dietary treatment on the average daily gain (ADG) of progeny from days 0 to 42 postpartum (P>0·05; Table 6). The progeny of the I ewes had higher growth rates from days 42 to 70 postpartum (P=0·08) and from days 70 to 98 (P<0·05). Subsequently, the I progeny had increased growth rates (P<0·05) from days 0 to 98 postpartum (birth to weaning; 291 v. 271 (sem 9·4) g/d, respectively).
Table 6 The effect of prepartum dam nutritional treatment on lamb average daily gain (ADG), days-to-slaughter and kill-out percentage (n 48/treatment)
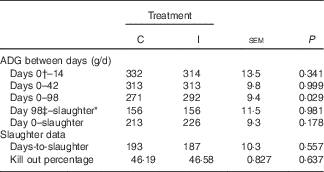
C, basal diet, no added supplement; I, ewes supplemented with 26·6 mg of I added as calcium iodate.
* Lambs were drafted for slaughter when they reached 45 kg body weight.
† Day 0=birth.
‡ Day 98 postpartum=weaning.
Slaughter data
There was no effect of excess I supplementation on the carcass weight of lambs (P=0·72; data not shown), the ADG from birth to slaughter or weaning to slaughter (P=0·18; Table 6) and the KO % of lambs at slaughter (P=0·64).
Faecal egg counts
The results of FECN for lambs are shown in Table 7. There was an overall effect of excess I supplementation, such that I lambs had lower mean FECN when compared with the progeny of C ewes (P=0·01). A treatment×time interaction was present whereby on day 42 I lambs had lower FECN than C lambs; however, this wasn’t present on days 56 or 70 postpartum. There was further evidence of a time effect whereby the number of faecal epg gradually increased with age at sampling. There was no effect of excess I supplementation on the number of FECOT observed from lambs (P>0·05; Table 7); however, there tended to be a time effect where the number of FECOT increased with age (P=0·08).
Table 7 The effect of prepartum dam nutritional treatment on the faecal egg count (FEC) for Nematodirus battus (FECN) and other trichostrongyle (FECOT) in lambs (Least-square means with their standard errors; geometric means and 95 % confidence intervals; n 48/treatment)

C, basal diet, no added supplement; I, ewes supplemented with 26·6 mg of I added as calcium iodate.
* Treatment P value for the log scale least-square means.
† Log scale least-square means; loge(X+25).
Discussion
Maternal I supplementation of the pregnant ewe has been shown to negatively influence serum IgG concentrations in her progeny immediately postpartum( Reference Mayer, Doleschall and Bender 11 ), however, neonatal lambs are dependent upon passively acquiring maternal antibodies from the colostrum to confer disease resistance in early life. Therefore, the primary objectives of this study were: (1) to determine the effect of excess I supplementation to the pregnant ewe on the thyroid hormone status of both the ewe and her progeny at parturition; (2) to determine potential mechanisms responsible for FPT by examining the expression profiles of selected genes in the ileum, duodenum, thyroid and perirenal adipose tissue of the lamb at 24 h postpartum; and (3) to assess the effect of FPT on the growing lambs’ response to gastrointestinal infection. The results from this study include a down-regulation of THRB and B2M and a reduction in VH in the ileum of I-supplemented progeny. Supporting previous work( Reference Mayer, Doleschall and Bender 11 ), which saw a decline in free T3, a decline in total T3 concentrations in the lamb were also observed in this study. These observations may explain in part the decline in IgG absorption at 24 h postpartum. However, despite the evident reduction in IgG absorption in lambs born to I-supplemented ewes, these lambs exhibited lower FECN in response to a natural parasitic infection and enhanced growth performance. These observations have led us to hypothesise that lambs born to I-supplemented ewes develop a superior ability to fight infection to N. battus in later life.
Most dietary I becomes incorporated as a constituent of the thyroid hormones T4 and T3 in mammalian species( Reference Mayer, Zolnai and Frenyo 32 , Reference Zhu, Peng and Raychowdhury 33 ). In agreement with this, increased concentrations of both the thyroid hormones were observed in ewes supplemented with excess I in the present study. While placental transfer of both T3 and T4 is absent during the final two-thirds of gestation, the production of thyroid hormones by the fetus remains dependent of the uptake of iodide from maternal circulation( Reference Forhead and Fowden 34 ). However, in the present study, lambs born to I-supplemented ewes had lower concentrations of total T3 at 1 and 24 h postpartum and lower expression of THRB in the ileum at 24 h postpartum. This finding is in agreement with previous studies( Reference Boland, Hayes and Sweeney 6 , Reference McGovern, Magee and Browne 12 , Reference Cabello and Wrutniak 35 ) and may be partially explained by a disruption to the conversion of T4 to the biologically active T3. In comparison with previous work, lower concentrations of total T4 were observed in this study( Reference Boland, Hayes and Sweeney 6 ). Neonatal T4 concentrations are dependent on a thyroid-stimulating hormone surge which is further reliant on the intake of milk by the neonate after birth( Reference Forhead and Fowden 34 ). Therefore, the lack of milk consumption by the newborn lamb before blood sample collection at 1 h postpartum may in part explain the lower concentrations of T4 in the lambs at 1 h postpartum.
The level of I offered to ewes in the present study was in excess of dietary I requirements for both the C and I treatment groups( 36 – Reference Korhonen, Marnila and Gill 38 ) but did not exceed the stated toxicity level of 50 mg/ewe per d( 39 ). Despite this increase in I intake, in excess of requirement and the elevated production of the thyroid hormones, there were no effects on the performance parameters of the ewes including colostrum yield, composition and IgG concentration. However, in accordance with previous studies, FPT was evident in lambs at 24 h postpartum( Reference Boland, Hayes and Sweeney 6 , Reference Boland, Brophy and Callan 13 , Reference Boland, Brophy and Callan 40 – Reference Rose, Wolf and Haresign 42 ). Because of the hypo-immunocompetent nature of the newborn ungulate( Reference Boland, Hayes and Sweeney 6 ), FPT has been linked to increases in susceptibility to infection and subsequent declines in health( Reference Donovan, Dohoo and Montgomery 43 , Reference Quigley 44 ). However, in the present study there was no decline in the number of lambs treated for either joint-ill or E.coli between birth and day 14 postpartum. Previous authors have suggested that the decline in serum IgG concentration is a result of a ‘preprogramming’ effect due to excess I supplementation and mediated through the thyroid hormones in the developing fetus( Reference Boland, Keane and Nowakowski 10 , Reference McGovern, Magee and Browne 12 ). In this study, the lower concentration of serum IgG, observed among lambs born to I-supplemented ewes, was evident until day 32 postpartum, after which the lamb normally produces its own internal antibodies( Reference Dominguez, Perez and Puyol 45 ). This highlights the persistency of the pre-programming effect on serum IgG in the lamb; which had been previously reported at 24 and 72 h postpartum( Reference Boland, Hayes and Sweeney 6 ). Supplementation of the ewe with minerals and more specifically I prepartum may lead to increased concentrations of the mineral in both the colostrum and milk of the ewe postpartum; however, previous work has shown that the progeny of I-fed dams had reduced serum IgG concentrations when fed either artificial colostrum( Reference Rose, Wolf and Haresign 42 ) or colostrum from control-fed ewes( Reference Boland, Keane and Nowakowski 10 ).
Previous authors have hypothesised that the resultant FPT, following excess I supplementation of the pregnant ewe, may be due to the IgG being rechannelled back into the intestinal lumen( Reference Mayer, Zolnai and Frenyo 32 , Reference Cervenak and Kacskovics 37 , Reference Baumrucker and Bruckmaier 46 ). The absorption of IgG from the colostrum is mediated via the neonatal Fc Receptor (FcRn)( Reference Yvon, Levieux and Valluy 14 ), which has been linked to both the transcytosis and recycling of IgG( Reference Ghetie and Ward 47 , Reference Rath, Kuo and Baker 48 ). The primary site of IgG absorption in the newborn ruminant is in the lower ileum( Reference Yvon, Levieux and Valluy 14 ) where there are two primary pathways of IgG absorption. First, IgG is bound to FcRn at the apical surface, forming an endosome, within which it is transcytosed to the basal surface, or alternatively, the IgG is engulfed into intestinal cells via fluid phase endocytosis before binding to FcRn and being transcytosed across intestinal epithelial cells( Reference Baumrucker and Bruckmaier 46 , Reference Tzaban, Massol and Yen 49 , Reference Anderson, Chaudhury and Kim 50 ). Therefore, recycling of IgG back into the intestinal lumen requires transport from the basal to the apical surface( Reference Rath, Kuo and Baker 48 ). Previous authors have reported the localisation of FcRn, which are known to mediate the recycling of IgG back into the intestinal lumen, at the apical side of duodenal crypt epithelial cells( Reference Mayer, Zolnai and Frenyo 32 , Reference Cervenak and Kacskovics 37 ). In the present study, there was no difference between the expression of the FcRn heavy chain component, FCGRT, in the ileum and the duodenum; however, an up-regulation of RAB25 gene expression was observed in the duodenum. RAB25 is a member of the Rab GTPases family which are small intracellular proteins associated with the regulation of recycling or transcytosis of the endosome( Reference Baumrucker and Bruckmaier 46 ). An in vitro study found that suppression of RAB25 inhibited the bidirectional transcytosis of IgG by 25–50 %( Reference Anderson, Chaudhury and Kim 50 ). If the opposite effect were true, the up-regulation of RAB25 in the duodenal tissue examined in the present study may indicate an increase in IgG recycling from the basal to the apical surface of the intestinal tract.
A secondary function of the FcRn receptor is to protect IgG and albumin from catabolism, thus, indirectly increasing the serum half-life of both( Reference Junghans and Anderson 51 ). Higher serum concentrations of IgG can therefore be limited by the availability of FcRn, as unbound IgG is delivered to lysosomes where it is degraded( Reference Cervenak and Kacskovics 37 ). Maternal supplementation with excess I down-regulated B2M and tended to down-regulate ALB in the ileum of lambs in the present study. In mice, a deletion in the light chain component of the FcRn receptor, B2M, has previously been linked to loss of FcRn expression and depletion of maternal IgG transport( Reference Roopenian and Akilesh 52 ) resulting in lower levels of both serum IgG and albumin( Reference Blum 53 ). Hence, the down-regulation of B2M, in conjunction with the decline in serum IgG concentrations, could indicate reduced expression of functional FcRn and therefore increased levels of IgG catabolism in the intestine of the lamb. A previous study which looked at the expression of B2M in the ileum of the lamb, before colostrum consumption, reported a lower level of expression in C lambs at 1 h( Reference McGovern, Magee and Browne 12 ). The increase in B2M gene expression, from 1 to 24 h postpartum in control lambs, coincides with the ingestion of colostrum and is in agreement with previous work which found that B2M was up-regulated in the mammary gland during the period of active IgG transfer( Reference Mayer, Zolnai and Frenyo 32 ). Despite the enhanced expression of B2M at 1 h( Reference McGovern, Magee and Browne 12 ) a similar pattern in expression was not observed in I-supplemented progeny over time. Thus, it would be beneficial to examine the catabolism of IgG along the digestive tract of newborn lambs in future experiments.
Colostrum intake promotes dramatic morphological and functional changes in the gastrointestinal tract of neonates including epithelial cell proliferation, migration and differentiation( Reference Underwood and Suttle 54 ). In addition, the influence of the thyroid hormones on cellular differentiation, growth and development in the fetus and neonate( Reference Pluske, Hampson and Williams 55 ) has resulted in previous authors re-examining the impact of the thyroid hormones on the development and maturation of the gastrointestinal tract( Reference McGovern, Magee and Browne 12 , Reference Pluske, Hampson and Williams 55 ). Following maternal supplementation with I, negative changes in the intestinal architecture of the lamb were observed (decreased VH and VH:CD ratio in the ileum), in the present study. Villi are fundamental components of the digestive tract and their geometry acts as an indicator of the absorptive capacity of the small intestine( Reference Montagne, Pluske and Hampson 56 ). Hence, the VH:CD ratio has been identified as a useful parameter in the evaluation of intestinal health( Reference Sangild, Fowden and Trahair 57 ), with previous work reporting that the VH:CD ratio steadily increases in the lamb postnatally( Reference Besier and Love 58 ). This is inconsistent with results obtained from the progeny of the I-supplemented ewes where a reduction in the VH:CD ratio was observed at 24 h postpartum and is in accordance with the decline in the absorption of serum IgG.
To date, the decline in serum IgG concentration following maternal supplementation with excess I has not been investigated alongside the neonatal lambs’ response to infection postpartum. The increasing prevalence of GIN infections and the evolution of anthelminthic resistant nematodes has increased the use of chemoprophylaxis in sheep production systems( Reference Hoste and Torres-Acosta 59 , Reference Eysker, Bakker and Kooyman 60 ); hence, there is growing interest in developing alternative parasite control strategies. N. battus is one of the earliest nematodes to cause infection in the growing lamb, establishing itself in the host animal from 6 weeks of age( Reference Beasley, Kahn and Windon 61 ) because of a temporary lapse in the immune-competence of the dam around the period of late pregnancy and early lactation( Reference Greer 62 ). Strategies which promote a robust immune response are therefore crucial to enhancing both the health and survival of the young lamb with regard to nematode infections( Reference Greer 62 ). Infection of growing lambs with GIN causes significant production losses through sub-optimal feed intake and nutrient utilisation( Reference Good, Hanrahan and Crowley 3 , Reference Greer 62 ). Coinciding with a decline in FECN, animals born to I-supplemented ewes had higher growth rates from days 56 to 98 postpartum. Interestingly, results obtained from the present study show that lambs from I-supplemented ewes had an overall reduction in FECN, which was also evident on d 42 postpartum. This coincides with a rise in serum IgG concentrations, which were higher on day 70, among these lambs. Therefore, it may be hypothesised that the progeny of the I-supplemented ewes were primed for a heightened response to N. battus infection due to FPT in the neonatal period and the requirement to begin producing antibodies at an earlier age v. C lambs that had increased serum IgG concentrations in the postpartum period. Alternatively, lambs born to I-supplemented ewes may have been exposed to higher concentrations of I in the colostrum and milk during the postpartum period; thus proving toxic to N. battus in the gastrointestinal tract of the growing lamb.
In conclusion, the data presented in this study indicates that maternal supplementation with excess I during the late gestation period reduces serum IgG concentrations in the lamb; a decline that was evident up to day 28 postpartum. This FPT of IgG may be due in part to impaired intestinal integrity and down-regulation of B2M; which has the potential to reduce the total area available for absorption and increase the degradation of IgG in the intestine. Alternatively, the gene expression data from the duodenum indicate the possibility of recycling the IgG back into the intestinal lumen; however, further investigation into this is merited. Collectively, the results are mediated by the decline in T3 concentrations and a down-regulation of THRB in the ileum. They provide new insights into the mechanisms governing FPT in the progeny of I-supplemented dams and highlight the effect this has on the lambs’ own ability to produce IgG and subsequent positive responses to N. battus infection.
Acknowledgements
The authors wish to thank Dr Cormac O’ Shea, Dr Maria Markiewicz-Keszycka and Dr Gaurav Rajauria for their assistance with laboratory analysis and Mr Philip Brady for his help with animal management. Thanks are also extended to Ms Denise Rafferty for her assistance with sample collection and the Irish Research Council from whom the research described herein was funded.
This study was funded by the Irish Research Council (grant no: RS/2012/103) under the Government of Ireland postgraduate scholarship scheme. The Irish Research Council had no role in the design, analysis or writing of this article.
The authors’ contributions are as follows: F. Mc. G. wrote the manuscript, collected the samples and carried out the laboratory and statistical analysis; T. M. B. was the principal investigator responsible for the design of the experiment, sample collection and supervision of the data collection and statistical analysis. T. M. B and T. S.corrected the manuscript; T. S. designed the study and supervised the laboratory analysis; M. T. R. assisted and supervised the laboratory analysis; F. P. C. and S. L. contributed to the sample and data collection. All authors approved the final version of the manuscript.
The authors declare that there are no conflict of interest.














