Nitric oxide (NO) has a short half-life but a profound relaxant effect on vascular smooth muscle(Reference Jin and Loscalzo1,Reference Mellion, Ignarro and Ohlstein2) . Through its dilatory properties, NO regulates vascular tone and blood pressure (BP)(Reference Ignarro3), which has prompted an investigation into vascular NO availability and consequent impacts on BP and blood flow. NO levels can be raised by consuming dietary nitrate (NO3-), which is reduced to nitrite (NO2-) and then NO, in a stepwise process involving NO3- reducing bacteria(Reference Lundberg, Carlström and Weitzberg4). The richest sources of dietary NO3- include beetroot (BR), rocket and lettuce, with BR receiving the most attention as a NO promoter(Reference Lidder and Webb5). Webb and colleagues(Reference Webb, Patel and Loukogeorgakis6) were the first to demonstrate BP-lowering effects of BR ingestion in young, healthy volunteers, which led to further exploration into the manipulation of NO levels through the intake of NO3--rich foods.
BR juice, as a rich source of dietary NO3-, has been repeatedly found to reduce BP and improve blood flow(Reference Siervo, Lara and Ogbonmwan7,Reference Lara, Ashor and Oggioni8) . The prevalent use of BR juice in dietary NO3- research has been propelled by the development of a NO3- free placebo juice. There has been, however, a wide variety of NO3- doses provided and a preponderance of research on young, healthy volunteers. Young, healthy adults are at a low risk of CVD but are efficient in the breakdown of dietary NO3- to NO and have a greater endogenous production of NO than older adults(Reference Ashor, Chowdhury and Oggioni9). This population, therefore, provides an elegant portrayal of the physiological effects of NO following dietary NO3- intake, but their low risk of CVD makes the clinical application of supplementation less relevant. Older adults, who are more at risk of CVD because of the ageing process, are a more suitable cohort in which to investigate the potential effects of dietary NO3- intake on vascular health.
Although most research on BR has focused on juice form, there are many other ways to consume the vegetable, such as in gel form(Reference Morgado, de Oliveira and Vasconcellos10), incorporated into bread(Reference Hobbs, Kaffa and George11) or as a whole food. BR in its whole form is underused and although NO3- levels vary depending on factors such as time of harvest, it provides a bioavailable source of NO3- and may be more cost-effective, palatable and easier to incorporate into the daily diet than juice or gel forms. However, no study has yet examined whether BR food is an acceptable and practical form of dietary NO3- that can be consumed to reduce BP and improve blood flow in both young and older adults.
Consequently, this study explores the short-term effects of incremental doses of BR food on resting BP and microvascular function in young and older participants and investigates whether these are correlated with surrogate markers of NO production (plasma NO3- and NO2- concentrations). We hypothesised that the positive effect on resting BP and vascular function associated with NO3- would be attenuated in the older population and that the responses would occur in a dose-dependent manner.
Methods
Participants
Twenty-four (twelve young and twelve older), healthy, non-smoking, participants with no co-morbidities volunteered for this study (physical characteristics are presented in Table 1). Participants were excluded if they consumed >30 units alcohol/week (equivalent to 240 g of pure alcohol), had a BMI > 30 kg/m2, BP > 140/90 mmHg, history of repetitive gastric reflux or intolerance or allergy to BR, or were taking the following medications: corticosteroids, sildenafil, aspirin, non-steroidal anti-inflammatory drugs, anti-hypertensives (Ca2+ channel blockers, angiotensin converting enzymes (ACE)-e inhibitors), diuretics, beta-blockers, antacids, anticoagulants, antibiotics, nitrate-derived agents and anti-cholinergics. Volunteers provided informed written consent before participating, and the study was approved by the Cambridge Central Research Ethics Committee (16/EE/0376) and registered with ISRCTN (86706442). The primary aim of the trial was to evaluate the pharmacokinetic profile of incremental oral doses of dietary nitrate in young and older adults, which have been reported elsewhere(Reference Capper, Siervo and Clifford12); this manuscript reports the results from the analyses of secondary outcomes such as BP and microvascular function. The study was conducted in the Clinical Trial Unit of the Newcastle University Royal Infirmary Hospital.
Table 1. Baseline characteristics of study participants*
(Mean values and standard deviations)

SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Comparison between age groups was performed by Independent t tests.
Study design
This study employed a four-arm, randomised, open-label, cross-over design. Randomisation was conducted by an individual not involved in data collection using specialised online software (www.randomization.com). Participants consumed whole-cooked BR food in three portions: 100 g, 200 g, 300 g and a 200-ml solution containing 1000 mg of potassium nitrate (KNO3) in a random order, on four separate occasions over a 4-week period. The washout period was ≥7 d, and each visit was at the same time of day preceded by an overnight fast (≥10 h). For each trial, participants attended the laboratory in the morning (∼8:30 am), had a venous cannula fitted and baseline blood samples were collected. Participants consumed the intervention within 15 min and then had a standardised low NO3-content (∼2·8 mg) breakfast (cereal with milk, white toast and a natural yoghurt pot, providing ∼1882 kJ, 62 % carbohydrate, 16 % protein and 22 % fat). Blood samples, BP and microvascular vascular measures were taken periodically after each intervention over a 5-h period. The short version of the international physical activity questionnaire was used to monitor physical activity over the 7 d prior to each visit and consistency throughout the study. A combined total physical activity score was calculated and expressed in MET-min/week(Reference Craig, Marshall and Sjöström13).
Treatments and dietary control
The BR was provided by the same grower (G’s Fresh) as a pre-cooked product to reduce variation in NO3- levels due to differing cultivation practices. We could not standardise the NO3- levels in the BR, as they vary throughout the growing season, and we could not use the same batch due to the relatively short shelf-life of the product. Instead, we had samples of BR from three separate 100 g batches analysed for their NO3- content. On average (sd), the interventions contained: 100 g BR – 272 mg NO3- (sd, 37 mg), 200g BR – 544 mg NO3-, and 300 g BR – 816 mg NO3-. The KNO3 contained 1000 mg of NO3- and was administered in a 200-ml sterile solution prepared by the Newcastle University Hospitals pharmacy. Throughout the study, participants were instructed to avoid using anti-bacterial mouthwash and gum throughout the study as these can attenuate NO3- to NO2- conversion(Reference Lidder and Webb5). As in previous studies(Reference Keane, George and Constantinou14,Reference Clifford, Constantinou and Keane15,Reference Frank, Stintzing and Carle16) , the participants followed a low NO3- and (poly)phenol diet 2 d before each trial and during the 5 h of each testing day. Compliance was assessed with food-record diaries. The diet required the participants to avoid NO3--rich vegetables such as spinach and BR, fruits, cured meats, strong cheese, chocolate, wholegrain breads and grains, tea, coffee and alcohol.
Resting BP
Resting systolic and diastolic BP and heart rate were measured using an automated pressure cuff (Spot Vital Sign Device, Welch Allyn) after participants had rested for at least 15 min. The cuff was placed on the opposite upper arm to the cannula used for blood sampling with the participant in a seated and comfortable position. Baseline measurements were taken three times with a 1-min interval between each measurement, and one measurement was taken at each time point after that, that is, 30, 60, 90, 120, 180, 210, 240, 270 and 300 min. Measurements were concealed from the participants to ensure that their knowledge of their BP readings did not influence later measurements. Time points were grouped into baseline (0 min), early (30–90 min), mid (120–210 min) and late (240–300 min) average BP readings to better capture changes in BP after supplementation and improve the graphical presentation of the results across the time points.
Microvascular blood flow
A laser Doppler (Moor LDF, Moor Instruments) was used to assess cutaneous microvascular reactivity, which is based on the ability of the endothelium to release NO as a response to proximal arterial occlusion. After the end of occlusion, when peripheral perfusion is restored, the immediate reduction of vascular resistance results in an increase in the blood flow, that is, post-occlusive reactive hyperaemia (PORH). This is closely linked to NO release and integrity of endothelial function(Reference Carasca, Magdas and Tilea17). The measurement lasted 12 min and took place with participants lying supine in a 22–24°C temperature-controlled room. A pressure cuff was placed on the participants’ upper arm, on the opposite arm to the cannula when possible, and a laser Doppler probe was placed on the inner forearm of the same arm. A resting measurement lasting 5 min was taken, after which the cuff was inflated to 200 mmHg to obstruct the blood flow in the brachial artery and remained pressurised for 3 min. The pressure in the cuff was then released and the hyperaemic response, measured in perfusion units, was recorded using MoorVMS V3.1 software. The measurement continued for a further 4 min to allow the blood flow to return to baseline. Participants were instructed to keep their arm still during the measurement and tape was used to reduce the movement of the probe cable. The PORH index was derived as a functional measure of microvascular blood flow. The PORH index is the ratio of the post-AUC 1 min after the release of the pressure cuff, relative to the AUC of a 1-min period of pressure cuff inflation(Reference Yamamoto-Suganuma and Aso18). The laser Doppler was recalibrated every 6 months as instructed by the manufacturer.
Blood sampling
Whole blood samples were collected with a cannula at baseline (before supplementation) and at 30, 60, 120, 180, 240 and 300 min in two 6 ml lithium heparin vacutainer tubes. One lithium heparin tube was spun immediately at 5000 rpm for 3 min; plasma was subsequently aliquoted into dark-coloured microtubes and frozen at −80°C to preserve the NO2-. The microtube aliquots were pre-treated with a stop solution of 10 μl of diethylenetriaminepentaacetic acid and 6·5 μl of N-ethylmaleimide to prevent transition between compounds, as described previously(Reference Carasca, Magdas and Tilea17). Diethylenetriaminepentaacetic acid and N-ethylmaleimide prevent the destruction of plasma S-nitrosothiols to NO2-(Reference Nagababu and Rifkind19) and samples were spun and frozen as quickly as possible in order to maintain stability in the samples. The remaining vacutainer was spun at 3000 rpm for 10 min at 4°C and plasma was aliquoted and frozen at −80°C. The plasma samples were used to measure NO3- and NO2- concentrations.
Plasma NO3- and NO2- concentrations analysis
Plasma samples were first deproteinised before analysis using cold ethanol precipitation. Due to funding and personnel constraints, samples at the most relevant time points were analysed: 0, 60, 180 and 300 min. The BR was juiced and diluted to 1:1000 in deionised water prior to analysis of NO3- content. The ozone-based chemiluminescence method was used to measure plasma NO3- and NO2- concentrations using the Sievers gas-phase chemiluminescence nitric oxide analyser (NOA 280i), which has been described elsewhere(Reference Wylie, Kelly and Bailey20,Reference McIlvenna, Monaghan and Liddle21,Reference Shannon, Duckworth and Barlow22) .
Sample size calculation
The data presented are secondary analysis from a larger study for which this study was powered(Reference Capper, Siervo and Clifford12). The sample size calculation for that study was based on an acute (3 h) trial testing the effects of inorganic nitrate supplementation compared to placebo on plasma NO3- concentrations in young and older healthy individuals(Reference Ashor, Shannon and Werner23). The average (sd) concentrations of plasma NO3- in the placebo and interventions groups were 202 (22) μmol/l and 583 (39) μmol/l, respectively. With this data, it was calculated (in an ANOVA) that twelve participants per group (90/8 = 11·25) would be needed to detect a significant difference in plasma NO3- between doses and age groups with a power of 80 % and P < 0·05 (G Power for Windows 3.1). We did not perform a sample size calculation for changes in BP and acknowledge this as a limitation of the study. While this means the BP data presented should be treated as secondary, exploratory analysis, we do believe that the data is novel and valuable for researchers and clinicians. A post hoc calculation of the power achieved by the study for the observed changes in systolic BP across the incremental nitrate doses was performed in each age group. The effect size (partial η) was calculated for the time × intervention interaction term and used to determine the power in a repeated-measures ANOVA model using a P value less than 0·05 (G Power for Windows 3.1).
Statistical analysis
All statistical analyses were completed using IBM SPSS (version 24.0, IBM Corp.) and Statistica 10 for Windows (StatSoft. Inc.). Summary data are presented as means ± sd or means ± sem. Normality was assessed by visual inspection of Q–Q plots; all variables were overall normally distributed. Baseline differences between age groups were analysed using Independent t test. The analysis plan to evaluate the effects of the nitrate interventions included the following steps: (1) Test the effects of dietary nitrate interventions on systolic and diastolic BP, plasma NO3- and NO2- concentrations and PORH (young and old participants were combined due to missing data) over the 5-h period in each age group. Repeated-measures ANOVA was performed with time (baseline, 30–90 min, 120–210 min and late 240–300 min) and intervention (100 g BR, 200 g NR, 300 g BR, KNO3) as within-subject factors and time × intervention represented the interaction effect. Models were checked for sphericity assumptions using the Mauchly’s test. Post hoc test with Fisher’s least squared differences test was performed to evaluate differences between intervention groups at each time point; (2) Test whether young and old participants had different BP responses to each of the incremental NO3- doses. Systolic and diastolic BP changes were calculated as the difference between average BP during the 240–300 min period minus the respective baseline values. ANCOVA was performed with age as between-subject factor and adding baseline either systolic or diastolic BP as a covariate, respectively; and (3) Evaluate the correlation between changes in plasma NO3- and NO2- concentrations after 300 min (Δ = 300min – baseline) with changes in systolic and diastolic BP in young and old participants. The repeated-measures correlation (rmcorr) function was applied via the Shiny application ( × https://osf.io/4p3×6/) to compute and visualise the associations taking into account the paired, repeated measures data for each participant. Rmcorr is conceptually similar to a null multilevel model with a fixed slope and varying intercept by participant(Reference Bakdash and Marusich24,Reference Marusich and Bakdash25) .
A sensitivity analysis was conducted to explore the effects of the interventions on PORH after replacing fifteen missing data and using the same statistical approach (repeated-measures ANOVA); the missing data were replaced by the next non-missing value. The potential confounding effects of the randomisation sequence on changes in systolic and diastolic BP were also evaluated by adding the randomisation sequence to the repeated-measures ANOVA with time, intervention and randomisation sequence included as within-subject factors. Statistical significance was set at P < 0·05.
Results
Recruitment and adherence
A total of twenty-six participants were enrolled to the study but one withdrew from participating prior to starting the study and one stopped the intervention; thus, twenty-four participants completed the study, and the baseline characteristics of the participants are presented in Table 1. A flow chart describing the recruitment of participants into the trial is provided in the Online Supplementary Material (Fig. S1). No adverse events were reported, and review of the food diaries revealed that participants adhered to the dietary instructions provided prior to the start of each test. The interventions were well tolerated; however, some of the participants reported mild urine and faecal discolouration after the BR, which has been reported previously(Reference Lidder and Webb5,Reference McIlvenna, Monaghan and Liddle21) .
Plasma NO3- and NO2- concentrations
These data are also part of a larger pharmacokinetic trial (see trial registration for further details). Changes in plasma NO3- and NO2- concentrations are shown in Fig. 1. The terms of the ANOVA models (time, intervention and time × intervention interaction) were all highly significant for plasma NO3- concentrations (all P < 0·001). Post hoc tests showed that plasma NO3- concentrations were higher than baseline at all time-points measured within the 200 g, 300 g and KNO3 dietary NO3- groups (P < 0·001), whereas changes for the 100 g intervention was only significant in the old group. Plasma NO3- concentrations increased in a dose-dependent manner, with the highest levels observed after KNO3 intake. There was a significant time, intervention and interaction effect on plasma NO2- concentrations post-ingestion (all P < 0·001). Plasma NO2- concentrations increased in a dose-dependent manner, with the highest levels observed after KNO3 intake. Irrespective of age and intervention, plasma NO2- concentrations peaked at 5 h post-intervention.

Fig. 1. Mean changes in plasma nitrate (a) and nitrite (b) concentrations following the administration of incremental doses of dietary nitrate as whole beetroot (100 g, 200 g, 300 g) and 1000 mg of potassium nitrate (PN) in young and old participants. Repeated-measures ANOVA was used to test the effects of time and interventions on plasma concentrations in young and old participants. Error bars are 1 sem. *Significantly different from baseline (P < 0·05) within each specific intervention group (see Methods for details of statistical analysis).
Systolic BP
Changes in systolic BP are shown in Fig. 2. There was a significant effect of time (P < 0·001) but no intervention (P = 0·552) or interaction effect (P = 0·813) in young participants. During the last hour of the intervention (240–300 min), the KNO3 showed the greatest drop in systolic BP (–7·4 ± 1·5 mmHg, –6·2 %) compared to baseline followed by 300 g (–5·3 ± 2·1 mmHg, –4·3 %), 100g (–4·7 ± 2·0 mmHg, 3·9 %) and 200 g (–3·5 ± 1·9 mmHg, –2·9 %) in younger participants.

Fig. 2. Short-term changes in mean systolic blood pressure following the administration of incremental doses of dietary nitrate as whole beetroot (BR, 100 g, 200 g, 300 g) and 1000 mg of potassium nitrate (PN) in young and old participants. Repeated-measures ANOVA was used to test the effects of time and interventions on plasma concentrations in young and old participants (see methods for details of statistical analysis). Error bars are 1 sem. aSignificantly different (P < 0·05) from BR 100 g within the specific time period. bSignificantly different (P < 0·05) from BR 200 g within the specific time period.
In older participants, there was no time (P = 0·501) or intervention effects (P = 0·312) on systolic BP but the interaction was significant (P = 0·047). During the last hour of the intervention (240–300 min), the KNO3 intervention showed the greatest drop in systolic BP (–4·8 ± 4·2 mmHg, −3·5 %) compared to baseline followed by 100 g (+1·7 ± 2·6 mmHg, +1·3 %), 200 g (+3·0 ± 2·7 mmHg, +2·5 %) and 300 g (+2·3 ± 2·9 mmHg, +2·1 %). The decrease in systolic BP in the KNO3 group achieved during the last period was significantly different compared with the 100 g portion (P = 0·024).
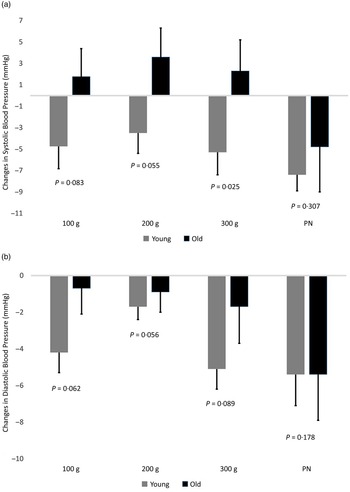
The systolic BP responses to the NO3- interventions were modified by age and they are shown in Fig. 3(a). Systolic BP did not change in older participants at lower NO3- intakes compared with young participants; decreases in systolic BP for both young and older participants were also not significantly different after the KNO3 intervention (young, −7·4 ± 1·5 mmHg; old, −4·8 ± 4·2 mmHg; P = 0·553). The influence of the randomisation sequence on changes in systolic BP was not significant (Table S2 of the Online Supplementary Material).

Fig. 3. Absolute mean changes in systolic (a) and diastolic (b) were measured at the end of each intervention period (calculated as the difference between average blood pressure at 240–300 min period minus baseline blood pressure). Results are shown for incremental doses of dietary nitrate as whole beetroot (100 g, 200 g, 300 g) and 1000 mg of potassium nitrate (PN) in young and old participants. Comparison between young and old participants within each intervention was performed by ANCOVA. Error bars are 1 sem.
Diastolic BP
Changes in diastolic BP are shown in Fig. 4. There was a significant effect of time (P < 0·001) but no intervention (P = 0·775) or interaction effects (P = 0·353) on diastolic BP in young participants. During the last hour of the intervention (240–300 min), the KNO3 showed the greatest drop in diastolic BP (–5·3 ± 1·6 mmHg, –6·9 %) compared with baseline followed by 300 g (–5·4 ± 1·1 mmHg, –6·8 %), 100 g (–4·2 ± 1·1 mmHg, –5·7 %) and 200 g (–1·7 ± 0·7 mmHg, –2·4 %).

Fig. 4. Short-term mean changes in diastolic blood pressure following in the administration of incremental doses of dietary nitrate as whole beetroot (BR, 100 g, 200 g, 300 g) and 1000 mg of potassium nitrate (PN) in young and old participants. Repeated-measures ANOVA was used to test the effects of time and interventions on plasma concentrations in young and old participants (see Methods for details of statistical analysis). Error bars are 1 sem. aSignificantly different (P < 0·05) from BR 100 g within the specific time period. bSignificantly different (P < 0·05) from BR 200 g within the specific time period.
In older participants, there was no time (P = 0·502) or intervention effects (P = 0·318) on diastolic BP but there was an interaction effect (P = 0·044). During the last hour of the intervention (240–300 min), the KNO3 showed the greatest drop in diastolic BP (–5·4 ± 2·4 mmHg, −6·5 %) compared with baseline followed by 300 g (–1·7 ± 2·0 mmHg, −1·6 %), 200 g (–0·8 ± 1·1 mmHg, −0·9 %) and 100 g (–0·7 ± 1·4 mmHg, −0·9 %). The drop in diastolic BP in the KNO3 group achieved during the last period was significantly different compared with the 100 g portion (P = 0·007). The diastolic BP responses to the NO3- interventions were modified by age and they are shown in Fig. 3(b). Changes in diastolic BP in older participants at lower NO3- intakes were not significant compared with young participants, whereas both young and older participants exhibited a similar decrease in diastolic BP after the KNO3 intervention (young, −5·3 ± 1·6 mmHg; older, –5·4 ± 2·4 mmHg; P = 0·991). The influence of the randomisation sequence on changes in diastolic BP was not significant (Table S2 of the Online Supplementary Material).
Cutaneous microvascular blood flow (PORH)
There was no significant effect of intervention (P = 0·158), time (P = 0·601) or their interaction (P = 0·243) on PORH in twelve young and older participants with complete data (Table 2). The results were confirmed in a sensitivity analysis conducted on all participants after imputation of missing data (n 23, one participant had all PORH values missing) (Table S1 of the Online Supplementary Material).
Table 2. Changes in microvascular forearm blood flow measured by laser Doppler following post-occlusive reactive hyperaemia (PORH) in twelve young and old participants with complete data collected at baseline, 150 min and 300 min*
(Mean values and standard deviations)

BR, whole beetroot; KNO3, potassium nitrate (positive control); PORH, post-occlusion reactive hyperaemia.
* Repeated-measures ANOVA was used to test the effects of time, intervention and their interaction (independent factors) on PORH (dependent variable).
Correlation between plasma biomarkers and BP
There was no correlation in young participants between changes in plasma NO3- concentrations and changes in systolic (r rm(35) = –0·17, 95 % CI (–0·479, 0·17), P = 0·306, Fig. 5(a)) or diastolic BP (–0·19, 95 % CI (–0·495, 0·149), P = 0·251, Table S3 of the Online Supplementary Material) over the 300-min measurement period. In older participants, however, a significant negative correlation between changes in plasma NO3- concentrations and systolic BP was observed (r rm(35) = -0·37, 95 % CI (–0·626, –0·043), P = 0·024, Fig. 5(c)); the association between changes in plasma NO3- concentrations and changes in diastolic BP was not significant (r rm(35) = –0·24, 95 % CI (–0·528, 0·105), P = 0·159, Table S3 of the Online Supplementary Material).

Fig. 5. Repeated-measures correlation (r rm) analysis testing the association between changes (Δ, calculated as 300 min–baseline) in systolic and diastolic blood pressure and changes in plasma nitrite and nitrate concentrations measured at 300 min. Analyses were conducted separately for young and old participants. Regression lines were fitted to each set of data for each participant (see Methods for further details).
In young participants, a significant association between changes in plasma NO2- concentrations and changes in systolic BP (r rm(35) = −0·42, 95 % CI (–0·661, −0·102), P = 0·010, Fig. 5(b)) was observed. Similarly, in older participants changes in systolic BP were significantly associated with changes in plasma NO2- concentrations (r rm(35) = −0·45, 95 % CI (–0·678, −0·133), P = 0·006, Fig. 5(d)).
Discussion
Summary of findings
The main finding of the study is that incremental doses of dietary NO3- reduced systolic and diastolic BP in healthy young adults, whereas in the older group a significant decrease was only observed with the highest dose. None of the interventions modified microvascular blood flow, as measured by PORH, in older or younger adults. This was the first study to show that whole BR, an inexpensive and readily available food, can reduce BP in healthy young adults, and such changes are associated with increased plasma NO2- concentrations.
Plasma NO3- and NO2-
All interventions increased plasma NO3- and NO2- concentrations in the older and young groups. The increases were dose dependent, and much larger after KNO3 ingestion, which provided 1000 mg of NO3-. A dose-dependent increase in plasma NO3- and NO2- concentrations was shown previously with KNO3 and BR juice(Reference Wylie, Kelly and Bailey20,Reference Kapil, Milsom and Okorie26) . While plasma NO3- levels were similarly elevated by dietary NO3- in the older and young adults, plasma NO2- levels were significantly more elevated in the young. This suggests that NO formation is more efficient in younger adults. As such, older adults may have a reduced capacity to generate NO2- and presumably NO, as suggested previously(Reference Lara, Ashor and Oggioni8,Reference Lyons, Roy and Patel27) . This apparent age-related impairment in NO2- formation may occur in the oral cavity, as NO3- is converted to NO2- in the mouth. A recent study found that the expression of sialin, thought to be a co-transporter of NO3- in various tissues, including the salivary glands(Reference Qin, Liu and Sun28), decreases with age, and this could partly explain why basal NO2- and NO2- formation after NO3- intake is lower in older adults(Reference Ashor, Chowdhury and Oggioni9). Salivary flow rate is also attenuated by the ageing process(Reference Affoo, Foley and Garrick29), and this could also influence NO2- formation. It is possible that several days of NO3- ingestion is required to further increase NO2- in older adults; a recent study showed that 10 d of NO3- ingestion (12 mmol/d) increased plasma NO2- concentrations in older (>70 years) than younger (<22 years) adults(Reference Vanhatalo, Blackwell and L’Heureux30). Strategies to boost the conversion of NO3- to NO2- in older adults should be examined in the future.
NO3 – on BP in younger adults
In agreement with previous research, we found that incremental doses of dietary NO3- intake determined progressively greater reduction in BP in healthy young adults(Reference Siervo, Lara and Ogbonmwan7). Plasma NO3- concentrations were not associated with decreases in BP in the young group, but plasma NO2- concentrations were consistent with a previous study using KNO3 (Reference Kapil, Milsom and Okorie26). This suggests the degree to which a NO3- intervention elevates plasma NO2- concentrations may partly determine the magnitude of the BP-lowering effect. This is the first study to show that BR, consumed in its whole-cooked form, can significantly reduce systolic and diastolic BP in this population. There was evidence of a dose-response effect on BP after the interventions; indeed, the 300 g BR dose and KNO3 evoked the greatest decreases in systolic and diastolic BP in the young; however, the differences were not statistically significant. A previous study found dose-dependent effects of NO3- on BP (KNO3, 248 v. 744 mg of NO3-) in young adults(Reference Kapil, Milsom and Okorie26). Another study found a dose-response decrease in BP, but only up to a dose of ∼520 mg of NO3- (via BR juice)(Reference Wylie, Kelly and Bailey20), which is similar to the amount in the 200 g BR intervention in the present study. Taken together, these data suggest that NO3- decreases BP in a dose-dependent manner in young healthy adults, although more research is needed to find the threshold above which no further decreases are observed.
NO3- on BP in older adults
Although plasma NO3- and NO2- concentrations were negatively correlated with BP in older adults, BR did not significantly reduce systolic BP or diastolic BP in this group. However, KNO3 did reduce systolic and diastolic BP by ∼4·8 mmHg and ∼5·4 mmHg between 240 and 300 min post-ingestion. Although the study evaluated the short-term effects of dietary NO3- on BP, the observed drop in BP can be considered clinically meaningful as a decrease of 5 mmHg in either systolic or diastolic BP has been associated a 10 % decreased risk of cardiovascular events even in people with normal BP and those who never had a heart attack or stroke(31). This finding suggests that higher doses of NO3- are likely needed to markedly decrease BP in older adults. The BP responses in older adults were more variable than in younger adults, and this could partly explain why we did not detect statistically significant differences in this population. However, these findings agree with several previous studies, which have also found no effects of dietary NO3- on BP in an older population(Reference Coggan, Hoffman and Gray32,Reference Siervo, Lara and Jajja33) . Gilchrist and colleagues(Reference Gilchrist, Winyard and Fulford34) found no effect of BR juice on BP in older (∼67 years) participants with type 2 diabetes and Bondonno et al.(Reference Bondonno, Liu and Croft35) found no effect of BR juice on BP in older adults (∼65 years) taking anti-hypertensive medication. Nonetheless, some studies have found NO3- capable of reducing BP in older populations; in a healthy cohort, BR juice (595 mg of nitrate) reduced both systolic and diastolic BP in adults aged ∼64 years(Reference Kelly, Fulford and Vanhatalo36) and Kenjale and colleagues(Reference Kenjale, Ham and Stabler37) found BR juice to reduce diastolic BP in older patients (∼67 years) with peripheral arterial disease. The difference between our results and that of Kenjale et al. (Reference Kenjale, Ham and Stabler37) could be related to the fact that they provided a higher dose of NO3- than we did (1116 mg nitrate) and/or because our participants were healthy and had lower resting BP. It is less clear why our results differed from those of Kelly et al. (Reference Kelly, Fulford and Vanhatalo36), as the doses used and participants were more comparable, although they did provide BR in juice and not food form. Regardless of the precise reasons, the inconsistent findings in studies to date suggest that NO3- ingestion is less effective at reducing BP in older than younger adults. The reasons for this remain unclear, but in addition to those already mentioned regarding diminished NO2- formation, one explanation is that older adults generate more reactive oxygen species, such as superoxide, that can attenuate NO formation and thereby blunt its physiological effects(Reference Taddei, Virdis and Ghiadoni38). We did not measure reactive oxygen species in this study to confirm this, but the fact NO2- was attenuated in the older adults compared with the younger adults suggest that NO was less available in this cohort.
Post-occlusive reactive hyperaemia
Cutaneous microvascular blood flow, as measured by PORH, was unaffected by NO3- ingestion, irrespective of age. The lack of change in microvascular blood flow in young adults is somewhat at odds with the decreases in BP. Nonetheless, changes in BP and vascular function do not necessarily correlate(Reference Keane, George and Constantinou14), and it has been suggested that longer intervention periods might be required to detect microvascular changes(Reference Keane, George and Constantinou14). It is also possible that there were changes in global vascular function that was not evident in the cutaneous microvasculature and/or we could not detect them with laser Doppler imaging. Similar to the present study, Kapil and colleagues(Reference Kapil, Khambata and Robertson39) also saw no change in PORH following 4 weeks of daily consumption of BR juice containing 397 mg of NO3- in hypertensive older adults, but they did see reductions in BP and improvements in augmentation index and PWV over a 4-week period. Ashor and colleagues(Reference Ashor, Shannon and Werner23) also found no effect of KNO3 (7 mg/kg) on PORH in younger or older adults 3 h post-ingestion. In contrast, de Oliveira et al.(Reference de Oliveira, Morgado and Pierucci40) reported increased reactive hyperaemia following a single bolus of ∼756 mg NO3- from a BR gel in older adults with CVD risk factors. The contrasting findings in our study could be due to the different form of NO3- administered (gel v. food), older age (∼70 years) and higher CVD risk of the participants, or differences in the protocol used for measuring reactive hyperaemia. A recent systematic review suggested that NO3- significantly reduced arterial stiffness, as evidenced by reductions in augmentation index and PWV(Reference Jackson, Patterson and MacDonald-Wicks41); it may be that these markers are more sensitive to the acute effects of NO3- consumption than PORH. The lack of change in PORH in the present study could also be due to the small number of data points available for this measure due to equipment failures and lack of compliance measurement protocols during testing.
Strengths and limitations
This study is the first to assess the effects of a whole BR food source on BP and microvascular function in healthy adults. The study was well controlled, the young and older participants were well-matched and the pre-study screening ensured none of the volunteers had any co-morbidities that could interfere with the study outcomes. However, there are some potential limitations of this research that need to be acknowledged. The study was powered to detect statistically significant changes in plasma NO3- and not BP. Thus, it is possible that our sample size was not sufficient to detect minor changes in BP between the different interventions and age groups. A post hoc calculation of the power achieved by study for the differences in change in systolic BP across the four incremental doses of NO3- showed that a power of 59 % in the young group and 87 % in the older group. We also did not recruit enough participants to perform a sub-analysis of whether gender affected our results. There is some suggestion that the systolic BP-lowering effects of dietary NO3- are more pronounced in males(Reference Kapil, Milsom and Okorie26), although this is not supported by all studies(Reference Wickham and Spriet42,Reference Bonilla Ocampo, Paipilla and Marín43) . While speculative, the greater number of females in the older cohort could have rendered the intervention less effective, and this warrants further research. The study did not include a NO3--free control group but the design had a positive control group (1000 mg of KNO3) instead. This approach was specifically chosen to suit the dose–response study design and the lack of an appropriate control group to mimic the administration of whole BR. Single measurements of resting BP were taken in the clinic, which is the most commonly used method, but may introduce measurement bias due to white-coat syndrome(Reference Bloomfield and Park44), operator bias and protocol variations. These were minimised by a single operator conducting the BP measurement according to the written protocol, using standardised clinical equipment and concealing BP readings from participants. A circadian influence on BP was also minimised in this study by having participants attending the research facility at the same time. This study did not employ pulse wave velocity (PWV) or flow mediated dilation (FMD) to measure vascular function, which are considered the gold standards for measurement. Although the reactive hyperaemia measure used in this study is considered a useful marker of vascular function, it may not be as sensitive as other measures of vascular function. In addition, the interpretation of this data was limited by the low number of data points collected due to equipment errors and reduced compliance. Lastly, it should be acknowledged that BR contains (poly)phenols and other compounds with putative vascular effects(Reference Clifford, Howatson and West45), and thus, without a NO3- only BR control, we cannot solely ascribe the BP-lowering effects of the BR interventions to its NO3- content, as synergistic effects with these other compounds are possible.
Conclusions
In conclusion, ingestion of whole BR food in amounts achievable through the diet increased plasma NO3- and NO2- concentrations and reduced systolic and diastolic BP in healthy young adults. However, higher doses were needed to elicit BP reductions in older participants, which suggests there may be mechanistic differences in the production of NO from dietary NO3- in young and older populations. The results of the present study may be used to inform dosages for longer term intervention studies with whole BR food and provide a rationale for evaluating their vascular effects in hypertensive populations.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522001337
Acknowledgements
We wish to thank the study participants for volunteering for this research.
This project was funded by Gs Fresh Ltd as part of a doctoral degree. The funders had no role in the study design, data collection, analysis and interpretation, the preparation of the manuscript or in the decision to submit the article for publication.
T. E. C., E. S. and M. S. designed the research. T. E. C., G. T. and W. I. conducted the research. T. E. C. and M. S. performed the statistical analyses. T. E. C., M. S. and T. C. interpreted the data and wrote the paper. All authors critically revised the manuscript, read and approved the final manuscript.
The authors declare no conflicts of interest.