Today the number of people whose body weight is higher than ideal is on the rise, particularly in developed countries. Excess body weight is one of the most important risk factors for all-cause of morbidity and mortality. The likelihood of developing such conditions as type 2 diabetes, heart disease, cancer and osteoarthritis of weight-bearing joints increases as the body weight increases(Reference Field, Coakley, Must, Spadano, Laird, Dietz, Rimm and Colditz1), and these conditions lead to substantial economic costs for the overall health care budget. The short- and long-term effects of conventional weight-management programmes have been unsatisfactory and thus obese people, and society as a whole, repeatedly call for alternative therapies, including dietary supplements. Although the use of dietary supplements is widespread, efficacy and safety have not been convincingly documented(Reference Pittler and Ernst2). It is clear that safe, effective and acceptable new therapeutic options are needed because there is currently limited evidence to support the effectiveness of any pharmacotherapeutic option other than rimonabant, orlistat and sibutramine(Reference Arterburn and Noël3). Nevertheless, while it has long been known that, in overweight patients, compliance is critical to low-energy diets, there are not yet enough studies on this factor even though it is truly the lynch-pin to the treatment of obesity(Reference Melin, Reynisdottir, Berglund, Zamfir and Karlstrom4). Many scientific works are now reconsidering the importance of evaluating what factors can affect compliance with a diet, and consequently the success of the treatment, both in terms of their ability to predict the results(Reference Teixeira, Going, Houtkooper, Cussler, Metcalfe, Blew, Sardinha and Lohman5, Reference Teixeira, Palmeira, Branco, Martins, Minderico, Barata, Silva and Sardinha6) and during the course of the diet. In evaluating the success of any diet, besides considering the weight loss and evaluating body composition, metabolic pattern modifications, variations in the sense of satiety and modifications in mood, it is extremely important also to evaluate the number of subjects that drop out of any low-energy regimen. A dietary supplement that could improve compliance with the low-energy diet would be a useful instrument in achieving the therapeutic goal. On the basis of these considerations, a study was conducted to determine compliance with a low-energy diet when associated with intake of a dietary supplement made up of two natural active substances: epigallocatechin-3-gallate (EGCG) and N-oleyl-phosphatidylethanolamine (NOPE). The catechin extracts from green tea, known for its antioxidant activity(Reference Higdon and Frei7), proved able to reduce excess cholesterol in the blood(Reference Mitscher, Jung, Shankel, Dou, Steele and Pillai8), to improve insulin action(Reference Kao, Hiipakka and Liao9), to help control weight homeostasis(Reference Kao, Hiipakka and Liao9), to increase fat oxidation and thermogenesis(Reference Dulloo, Duret, Rohrer, Girardier, Mensi, Fathi, Chantre and Vandermander10) and to reduce food intake(Reference Kao, Hiipakka and Liao9). Among the green tea catechins tested, the most effective proved to be EGCG(Reference Mitscher, Jung, Shankel, Dou, Steele and Pillai8) at doses comprised between 90 and 300 mg/d in man(Reference Williamson and Manach11). Unfortunately, when administered per os, EGCG is poorly adsorbed by the gastroenteric tract(Reference Chen, Lee, Li and Yang12). NOPE is a phospholipid present in many foods of animal and plant origin(Reference Schmid, Schmid and Natarajan13) and, in particular, it is plentiful in foods such as soya, eggs and chocolate(Reference Chapman and Moore14, Reference Di Tomaso, Beltramo and Piomelli15); moreover, it is normally produced and metabolized by man. The phospholipase D in the cell membrane hydrolyses NOPE into N-oleyl-ethanolamide (NOE) and phosphatidic acid. NOE has recently aroused great interest because it counteracts the orexigenic effect of anandamide (N-arachidonyl-ethanolamine), a well-known agonist of both the peripheral and central CB1 cannabinoid receptors whose activation leads to an increase in appetite and, consequently, in intake of food(Reference Williams and Kirkham16). The intra-peritoneal injection of NOE in rats, in fact, was recently demonstrated to promote an anorexic effect through the activation of intestinal PPARα, GPR119 and TRPV1 vanilloid receptors(Reference Fu, Gaetani and Oveisi17, Reference Overton, Babbs and Doel18). Unfortunately, when administered per os, NOE, too, is inadequately available to the enteric tract because it is rapidly broken down into oleic acid and ethanolamine(Reference Broccali, Berti, Pistolesi and Cestaro19). In a previous study it has been demonstrated that an oily dispersion of EGCG complexed with NOPE was more active than only NOPE or only EGCG in: (1) reducing food intake, (2) ameliorating in vivo plasma availability of EGCG, and (3) increasing the intestinal levels of NOPE, and consequently of NOE, in a group of diet-induced obese rats(Reference Broccali, Berti, Pistolesi and Cestaro19). This evidence prompted us to design a double-blind randomized parallel group, placebo-controlled trial to determine whether the NOPE–EGCG complex formula could improve compliance with diet during a weight-loss programme in overweight subjects.
Materials and methods
Subjects
The subjects for this study were drawn from the Outpatient Unit for the Treatment of Obesity, Fondazione IRCCS Policlinico San Matteo, Presidio di Belgioioso, University of Pavia, Italy. The study was initiated in January 2004, the first subject recruited in February 2004, and the clinical part of the study was completed in September 2006. The subjects ranged in age between 18 and 50 years, of both sexes (females were required to be not currently pregnant and normally menstruating), with BMI (in kg/m2) ranging between 25 and 35. To be included in the study, the subjects could not present significant alterations in lipid and carbohydrate metabolism (glucose < 6·11 mmol/l, total cholesterol < 6·20 mmol/l, TAG < 2·28 mmol/l) or be affected by any acute or disabling conditions or by endocrinological, neoplastic and autoimmune diseases. Moreover, inclusion criteria dictated that the participating subjects have no history, signs or symptoms of heart disease. On the other hand, mild hypertension (systolic pressure 140–150 mmHg, diastolic pressure 80–95 mmHg) was allowed. Patients were excluded from the study if they met the Diagnostic and Statistical Manual-IV (DSM-IV)(Reference American Psychiatric Association20) criteria for a current diagnosis of major depressive disorder as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders. Patients were also ruled out if they had a history or current diagnosis of bulimia, panic disorder, obsessive compulsive disorder, post-traumatic stress disorder, bipolar I or II disorder, or schizophrenia. No psychoactive drugs, including anti-obesity agents, were permitted throughout the study. All subjects had to give complete medical histories, and underwent physical examination, anthropometric assessment and routine laboratory tests. Clinical data, alcohol intake, smoking habits and physical activity were recorded. Number of previous diets and weight history variables were also taken from a diet/weight history questionnaire developed specifically for the present study. Subjects gave their written consent to the study and the protocol was approved by the Ethics Committee of the IRCCS Policlinico San Matteo, Pavia, University of Pavia. All participants agreed to refrain from participating in any other weight-loss programme.
Study design
The subjects were randomly assigned to one of the two groups in a double-blind parallel study. The subjects were supplemented with the NOPE–EGCG complex or placebo. Subjects were randomized to receive one capsule of PhosphoLEAN™ orally twice daily, before lunch and dinner, or an identical placebo for 8 weeks. The commercially available supplement PhosphoLEAN™ is a soft-gel capsule containing 85 mg NOPE extracted from soya lecithin and 121 mg of a dry green tea extract standardized at 50 mg EGCG; the capsules were manufactured by GELFIPHARMA Lodi (Milan, Italy) on behalf of CHEMI Cinisello Balsamo (Milan, Italy). Bottles of identical capsules for each treatment group were assigned a subject number according to a coded (AB) block randomization table prepared by an independent statistician. Investigators were blinded to the randomization table, the code assignments and the procedure. As subjects were enrolled, they were assigned a progressive subject number. All measurements were performed at baseline and at week 8. In addition, 4 months after the study was interrupted, weight was assessed. Subject randomization and dropout throughout the study are shown in Fig. 1.

Fig. 1 Subject randomization and dropout throughout the study. EGCG, epigallocatechin-3-gallate; F, female; M, male; NOPE, N-oleyl-phosphatidylethanolamine.
Weight-loss programme
Subjects were trained to restrict their daily energy intake by a moderate amount, 3344 kJ/d less than daily requirements based on WHO criteria(21) with a regimen that maintained a prudent balance of macronutrients: 25 % of energy from fat, 60 % of energy from carbohydrates and 15 % of energy from protein. A registered dietitian performed initial dietary counselling.
Body composition
Nutritional status was assessed using anthropometric measurements. Body weight and height were measured and the BMI was calculated (kg/m2). Skinfold thicknesses (biceps, triceps, suprailiac, subscapular) were measured twice using a harpender skinfold caliper at 5 min intervals in each site following a standardized technique(Reference Frisancho22). Sagittal abdominal diameter was measured at the L4–5 level in the supine position and waist girth was also measured. Anthropometric variables were measured by a single investigator. Percentage change in body weight was calculated; the proportion of patients who lost at least 5 % of their baseline body weight was calculated because many studies have shown that the morbidity related to obesity-associated risk factors is significantly decreased by a 5–10 % reduction in weight, even if patients remain in the obesity range(Reference Blackburn23, Reference Goldstein24). After an overnight fast, body composition was assessed by a portable body impedance analyser (STA-BIA Akern, Florence, Italy). With appropriate equations, it was possible to estimate fat mass, fat-free mass, body cell mass, and the BMR using age, height and weight as variables in addition to resistance(Reference Kushner25).
Biochemical analyses
Fasting venous blood samples were drawn between 08.00 and 10.00 hours with the subjects in a sitting position. Blood collection and handling were carried out under strictly standardized conditions and in line with manufacturer recommendations. Blood for Clinical Chemistry parameters was collected into evacuated tubes without anticoagulant, left for 1 h at room temperature, and then centrifuged for 15 min at 1500 g. Following centrifugation the serum was transferred into plastic tubes, rapidly frozen and stored at − 80°C until analysis (less than 1 month later). Whole blood (EDTA as anticoagulant) was used for haematological parameters. Clinical Chemistry parameters were detected on the Roche Cobas Integra 400 plus analyser (Roche Diagnostics, Basel, Switzerland), with dedicated commercial kits provided by the manufacturer. Cobas Integra 400 is a random, continuous access, sample selective analyser, which provides absorbance photometry for enzymes and substrates, turbidimetry for specific proteins and ion-selective electrode potentiometry for measuring serum electrolytes. In particular, total serum cholesterol, TAG, HDL-cholesterol, total proteins, total bilirubin, iron, glucose, uric acid, creatinine and liver enzymes, such as transaminase alanine aminotransferase, aspartate aminotransferase and γ-glutamyl transferase were measured by enzymatic-colorimetric methods. LDL-cholesterol was calculated according to the Friedewald formula(Reference Friedewald, Levy and Fredrickson26) for those specimens with TAG levels less than 4000 mg/l ( < 4·5 mmol/l). C-reactive protein levels were determined by a particle-enhanced turbidimetric method on a Cobas Integra 400. Erythrocyte, leucocyte and platelet counts, as well as Hb concentration, mean corpuscle volume and mean corpuscle Hb concentration were measured using a Coulter automated cell counter MAX-M (Beckman Coulter Inc., Fullerton, CA, USA). This instrument takes advantage of the VCS Technology, an acronym for Volume, Conductivity and Scatter. Thyroid-stimulating hormone, free thyroxine and free triiodothironine levels were detected in serum on a Roche Elecsys 2010 analyser (Roche Diagnostics) using dedicated commercial electrochemiluminescent immunoassays. Serum insulin levels were measured on a Roche Elecsys 2010 analyser (Roche Diagnostics) using dedicated commercial electrochemiluminescent immunoassays. To determine insulin resistance, subjects were instructed to fast for 12 h before blood was taken. Furthermore, the subjects refrained from any form of exercise for 48 h before the study. Female subjects were tested during the early follicular phase of their menstrual cycles (days 3–10). Insulin resistance was evaluated using the homeostasis model assessment (HOMA)(Reference Haffner, Kennedy, Gonzalez, Stern and Miettinen27) and the quantitative insulin sensitivity check index (QUICKI)(Reference Katz, Nambi, Mather, Baron, Follmann, Sullivan and Quon28) using the following formulas:
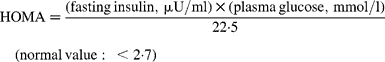
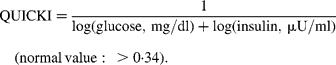
Satiating effect
The satiating capacity was assessed numerically, using a scoring system graded from minus 10, to represent extreme hunger, to plus 10, to represent extreme satiety. Subjects were shown a scale with twenty graduations and asked to indicate how they felt in respect to hunger or satiety by pointing to an appropriate place along the scale. The scale was dotted with phrases describing the various degrees of hunger or satiety, but subjects were free to choose any point along the scale(Reference Haber, Heaton, Murphy and Burroughs29).
Assessment of binge eating severity and depressive symptoms
The severity of binge eating was assessed using the Gormally Binge Eating Scale(Reference Gormally, Black, Daston and Rardin30). Patients scoring 17 or lower on the Binge Eating Scale were classified as non-binge eaters, those with a score of 18–26 as moderate binge eaters, and those scoring 27 or higher as severe binge eaters. The Binge Eating Scale includes sixteen items measuring the severity of the binge eating. It examines both behavioural manifestations (eating large amounts of foods) and feeling/cognition during a binge episode (loss of control, guilt, fear of being unable to stop eating). A Beck Depression Inventory (BDI-II)(Reference Steer, Ball, Ranieri and Beck31) was taken to assess depressive symptoms; a score of 10–30 was indicative of depressive symptoms. The tests were conducted under standardized conditions of comfort and silence, with a study technician always in attendance.
End-points of the study and sample size
Primary end-point
The effect of NOPE–EGCG complex on compliance with diet in healthy, moderately obese people, was considered the main outcome measure. Patients who dropped out of the study were defined as non-compliant.
Sample size
The sample size calculation was derived from a previous pilot study. Based on an expected proportion of dropouts of 26 and 6 %, in the placebo and the treated group, respectively, sixty-two patients per group would have made it possible to detect a two-sided significant difference between the two groups (OR 5·505) at the 5 % level and with a power of 80 %. NQuery Advisor 4 (Statistical Solutions, Ireland) was used for computation.
Secondary end-points
As a prespecified subgroup analysis, the proportion of dropouts was assessed separately in males and females. The other secondary end-points were the evaluation of: body composition (with anthropometric and impedenzometric variables), metabolic parameters (using the lipid profile, HOMA and QUICKI), sensation of appetite (evaluated by Haber analogue scale), depressive symptoms (evaluated by Beck Depression Inventory), and severity of binge eating (evaluated by Gormally Binge Eating Scale).
Safety
The primary measure of safety was the incidence of adverse events. At each clinical visit, the investigator queried patients about adverse events, defined as any untoward medical occurrence regardless of its suspected cause. Vital signs were also measured at each clinical visit.
Statistics
Data are presented as means and standard deviations if continuous or counts and as percentages if categorical. Patients were compared according to the initial assignment to the treatment arm, in adherence to the intention to treat principle. The proportion of dropouts was compared between groups by means of the Fisher exact test. The mean difference in proportions and its 95 % CI were computed to quantify the treatment effect. The treatment effect was adjusted for sex in a logistic model in a secondary analysis of the primary end-point. The OR and its 95 % CI were also computed to measure the age-adjusted association of treatment with the primary end-point. The Student's t test or the Mann–Whitney U test were used to compare secondary end-points on a continuous scale. Mean changes over time and 95 % CI were calculated in each group, as final value minus baseline value. Mean differences between changes were computed with their 95 % CI to quantify the treatment effect. Stata 9.2 (StataCorp, USA) was used for computation. A two-sided P value < 0·05 was considered statistically significant.
Results
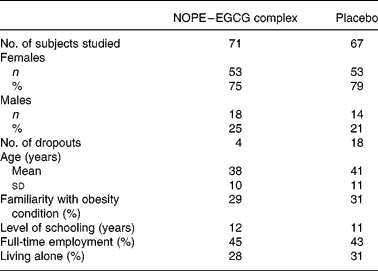
Population characteristics were similar in both groups (Table 1).
Table 1 Characteristics of subjects studied
EGCG, epigallocatechin-3-gallate; NOPE, N-oleyl-phosphatidylethanolamine.
Inter-group differences in the primary outcome variables
Forty-nine (73 %) patients in the placebo group and sixty-seven (94 %) in the treated group reached the primary end-point of compliance to diet, with a mean difference of 21 % (95 % CI 9, 33, P < 0·001) and a sex-adjusted OR of 6·2 (95 % CI 2·0, 19·4). For all subjects who dropped out prematurely from the study, whether in the NOPE–EGCG group or in the placebo group, the reason for dropping out was their declared inability to continue following the prescribed dietary regimen because of hunger. Only one subject in the NOPE–EGCG group stated that non-compliance with the study was caused by important family problems. No other reasons for dropping out were reported by patients nor identified by the treating physician upon specific investigation; all subjects dropped out of the study on or before week 4.
Inter-group differences in secondary outcomes
Subgroup analysis by sex
Among the 106 females, 73 and 94 % of the placebo and treated groups, respectively, reached the primary end-point of compliance to diet, with a mean difference of 21 % (95 % CI 7, 34, P < 0·006). Similar proportions were reached among the thirty-two males (71 and 94 %) and a similar (although not significant) mean difference was observed 23 % (95 % CI − 3, 49, P < 0·142).
Body composition
As regards changes in body composition, differences between the treatment groups were found for the mean change in the waist/hip ratio (difference − 0·01, 95 % CI − 0·02, 0·00, P < 0·026). The other parameters were found to be similar in the NOPE–EGCG group and in the placebo group (Table 2). As regards bioimpedenzometric parameters studied in the NOPE–EGCG complex and placebo groups, these were found to be similar.
Table 2 Secondary end-points: anthropometric variables studied in the N-oleyl-phosphatidylethanolamine–epigallocatechin-3-gallate (NOPE–EGCG) complex and placebo groups*

* For details of procedures and subjects, see the Materials and methods section and Table 1.
Biochemical analysis
The counts and indexes of blood cell as well as total proteins, total bilirubin, iron, glucose, uric acid, creatinine, C-reactive protein and liver enzymes did not vary significantly in both groups (data not shown). In Table 3 are reported the mean changes of lipaemia, glycaemia, insulin, HOMA, QUICKI and thyroid hormones. The mean changes of these parameters were similar in the NOPE–EGCG group and in the placebo group, with the exception of insulin, HOMA, QUICKI and free thyroxine.
Table 3 Secondary end-points: biochemical parameters studied in the N-oleyl-phosphatidylethanolamine–epigallocatechin-3-gallate (NOPE–EGCG) complex and placebo groups*

HOMA, homeostasis model assessment; QUICKI, quantitative insulin sensitivity check index.
* For details of procedures and subjects, see the Materials and methods section and Table 1.
Feeling of fullness, binge eating severity and depressive symptoms
The feeling of fullness score was higher in the NOPE–EGCG group than in the placebo group (1·42 (sd 1·95) v. 0·63 (sd 1·18), respectively, P < 0·05 by t test). As regards binge eating severity and depressive symptoms, we found differences between the groups with mean changes in the Binge Eating Scale score ( − 5·17, 95 % CI − 7·51, − 2·83, P < 0·000) and Beck Inventory Scale ( − 2·85, 95 % CI − 4·78, 0·91, P < 0·005) (Table 4).
Table 4 Secondary end-points: scores of Beck Inventory Scale, Binge Eating Scale and Haber test studied in the N-oleyl-phosphatidylethanolamine–epigallocatechin-3-gallate (NOPE–EGCG) complex and placebo groups*

* For details of procedures and subjects, see the Materials and methods section and Table 1.
Safety
The NOPE–EGCG complex was well tolerated, and there were no serious adverse events over the 8 weeks of the study.
Discussion
In this randomized, double-blind controlled study, the first to our knowledge, in obese patients, the NOPE–EGCG complex, together with a 3344 kJ/d-deficit diet, was significantly more effective than the placebo as regards compliance with diet.
In any long-term diet, patient compliance is crucial to the successful treatment of obesity. Most studies on weight loss show a high number of dropouts indicating that subjects had significant difficulty in adequately changing their eating and activity habits(Reference Melin, Reynisdottir, Berglund, Zamfir and Karlstrom4). Interventions to improve long-term weight-loss programmes are needed to treat obesity effectively. Given the limited effectiveness of conventional weight management (dietary intervention, physical activity and behavioural therapy), alternative weight-reduction strategies must be developed in order to increase the diet compliance and in this field a rapidly growing therapeutic area is the use of natural supplements, two of which are EGCG and NOE.
Previous studies demonstrated that bioavailability of both these compounds is low when administered alone as an oral supplement(Reference Chen, Lee, Li and Yang12, Reference Broccali, Berti, Pistolesi and Cestaro19). Therefore, it is of interest to estimate whether the per os use of a NOPE–EGCG complex could in any way increase the efficiency of the obesity treatment. In a previous study, it was found that the complexed form was able to ameliorate both the plasma availability of EGCG and the intestinal levels of NOPE in rats(Reference Broccali, Berti, Pistolesi and Cestaro19) through mechanisms that increase EGCG intestinal permeability and protect NOPE from digestive enzyme-induced hydrolysis. Though data on the absorption rate of NOPE–EGCG in man are not yet available, it is reasonable to suppose that similar mechanisms could be operative also in man.
NOPE-derived NOE is a natural compound structurally similar to anandamide, an endogenous orexigenic mediator that acts through the activation of both peripheral and central cannabinoid receptor CB1. Like anandamide, NOE is produced in cells in a stimulus-dependent manner and is rapidly eliminated by enzymatic hydrolysis, suggesting that it has a function in cellular signalling. NOE is produced primarily in the small intestine and acts through receptors different from cannabinoids. In particular, NOE was shown to alter expression of several PPARα target genes in the jejunum of wild-type but not PPARα null mice, including a repression of the inducible nitric oxide synthase gene, and it was proposed that the resulting reduction in nitric oxide could lead to stimulation of vagal afferents and, consequently, to reduction in food intake(Reference Fu, Gaetani and Oveisi17). Wang et al. (Reference Wang, Miyares and Ahern32) demonstrated that short-term food intake is reduced in response to NOE in wild-type but not TRPV1 null mice and postulate that TRPV1 is an additional target for NOE, mediating an immediate suppression of feeding via direct excitation of vagal sensory neurons. Overton et al. (Reference Overton, Babbs and Doel18), at last, found that NOE also acts as an agonist at gastrointestinally expressed orphan receptor GPR119, that is involved in satiety regulation, suggesting a third possible mechanism by which its hypophagic effects might be mediated. Though the relative contribution of these three potential targets to the hypophagic action of NOE is still to be determined, nevertheless any parallelism with rimonabant can be excluded, since this synthetic hypophagic compound acts blocking both peripheral and central CB1 cannabinoid receptors.
The main finding of the present study was that administration of the NOPE–EGCG complex significantly improved the compliance with diet in a group of healthy, overweight or obese subjects, as demonstrated by the patient dropout rate in comparison to that of the placebo group. Compliance with diet is a key point for the successful treatment of weight loss in obesity. We suppose that the compliance with diet in the NOPE–ECGC group was an outcome of various successful health effects.
Three lines of evidence suggest that the NOPE–ECGC complex modulates compliance with a diet. First, it was demonstrated that there was significant difference in satiety between the two groups, as shown by the scores on the Haber test. After 8 weeks, feelings of fullness and satiety were significantly increased and feelings of hunger were significantly decreased in the NOPE–ECGC group as compared to the placebo group. This determined a decrease in the desire for food and, consequently, in food intake thus improving compliance with diet. This is in agreement with the literature demonstrating that both NOPE and EGCG have inhibitory effects on food intake, forming part of a satiety sensor system(Reference Kao, Hiipakka and Liao9, Reference Fu, Gaetani and Oveisi17, Reference Overton, Babbs and Doel18).
Second, the NOPE–ECGC group showed a significant amelioration of mood and a decrease in depressive symptoms, as shown by the Beck Inventory score. Although the nature of the causal relationship, if any, between obesity and depressive symptoms remains to be elucidated(Reference Khaodhiar, McCowen and Blackburn33, Reference Lamberg34), the literature suggests that the two co-occur more often among patients seeking treatment for obesity than in either the general population or among non-treatment-seeking obese patients(Reference Fitzgibbon, Stolley and Kirschenbaum35, Reference Wadden, Womble, Stunkard, Anderson, Wadden and Stunkard36). It has been well demonstrated that successful weight loss is associated with a reduction in the severity of reported psychological aspects(Reference MacLachlan, Connacher and Jung37). In the present study we demonstrated that the administration of NOPE and EGCG, as an oral weight loss supplement, favourably affects mood alteration symptoms.
The mechanism by which the NOPE–EGCG complex ameliorates mood in obese individuals has not been determined. A plausible explanation could be the co-administration with NOPE of EGCG: it has been demonstrated, in fact, that EGCG has sedative and hypnotic effects in the brain, partially through γ-aminobutyric acid receptors, and consequently moderates acute stress responses(Reference Adachi, Tomonaga, Tachibana, Denbow and Furuse38). Behavioural tests have indicated that EGCG exerted anxiolytic effects just as benzodiazepines do(Reference Vignes, Maurice, Lanté, Nedjar, Thethi, Guiramand and Récasens39). Moreover, the Binge Eating Scale score demonstrated a significant improvement in binge eating events in the NOPE–EGCG group as compared to the placebo group. For this purpose it is important to note that, in contrast to rimonabant, NOPE and NOE do not antagonize the central CB1 cannabinoid receptors: since the blockade of these receptors promotes long-term depression(Reference Hoffman, Oz, Caulder and Lupica40), it is quite probable that some of the adverse side-effects of rimonabant, such as anxiety and depression, could be attributable to this different mechanism of action.
The third line of evidence concerning NOPE–EGCG treatment regards weight loss and metabolic parameters. The treatment induced a significant weight reduction in both groups ( − 3·28 kg and − 2·67 kg in NOPE–EGCG and placebo, respectively) but there was no significant difference in weight change between the two groups. On the contrary, insulin resistance was significantly decreased only in the NOPE–ECGC group. This is surprising since weight loss is an important cause of insulin reduction. Also in this case, the possible explanation could be attributable to EGCG co-administration, since it is well known that this catechin can improve insulin action(Reference Kao, Hiipakka and Liao9). Another metabolic parameter that decreased significantly in the treated group, though remaining in the physiological range, was free thyroxine levels. This is in good agreement with previous findings that changes in thyroid volume and function are correlated with weight loss, though these changes are not clinically and biologically significant(Reference Sari, Balci, Altunbas and Karayalcin41).
Following the evaluation of the observed data, it is possible to make a comparison between the two anorexic compounds remonabant and NOPE–EGCG for what concerns weight reduction, insulin resistance and compliance with a low-energy diet. In the RIO-North America trial(Reference Pi-Sunyer, Aronne and Heshmati42), 5 and 20 mg rimonabant once daily for one year in addition to a mild hypo-energetic diet (2508 kJ/d deficit) significantly increased diet-induced weight loss. Rimonabant at 20 mg, but not 5 mg, produced a significantly greater improvement than placebo of insulin resistance, but the number of dropouts (mostly due to common adverse events such as anxiety, depression, mood disorder, nausea and diarrhoea) was very high. The administration of NOPE–EGCG in addition to a mild hypo-energetic diet (3344 kJ/d deficit) for only 2 months did not significantly increase the diet-induced weight reduction of placebo, but it promoted a significant amelioration of insulin resistance together with a very low number of dropouts. This last evidence is probably due to the improvement of the sensation feelings of fullness, the reduction of depressive symptoms and the severity of binge eating.
Collectively, the present results indicate that the assumption of a NOPE–EGCG complex improved compliance with diet in a group of healthy, overweight or obese subjects, as demonstrated by the rate of dropout patients v. the placebo group, making an important link between various pathways.
Further study in this area is warranted and in particular long-term studies with more patients are needed to draw definitive conclusions.
Acknowledgements
The authors thank the subjects for their interest in the study, and Professor Benvenuto Cestaro and Dr Tiziano Oldoni for their dedicated assistance and supervision in the preparation and development of the study. M. R. and A. O. recruited the subjects, conducted the intervention study at the clinic, analysed the data, and wrote the study. R. C. S. B. S. and M. R. were the investigators who designed the study and provided supervision. R. T. and R. C. were responsible for sample collection of biochemical markers and provided supervision. C. K. was responsible for statistical issues and provided advice on interpretation of results. None of the authors had a financial or personal interest in any company or organization sponsoring this study (CHEMI S.p.A. (Italfarmaco Group, Italy)).







