In Jamaica, pregnancy during adolescence has been found to be associated with a higher prevalence of low birth weight (LBW), which, in turn, is associated with a greater neonatal morbidity( Reference Thame, Wilks and Matadial 1 ). Challenges faced by adolescent mothers to adequately meet the nutrient demands for their own anatomical growth together with the additional needs associated with the growth of their reproductive tissues and fetus are especially marked in those aged 13–17 years( Reference Alves, Cisneiros and Dutra 2 – Reference Liran, Vardi and Sergienko 7 ). The increased deposition of maternal tissues and growth of the fetus and placenta during pregnancy require net protein deposition and ready availability of amino acids. The demand for amino acids increases as pregnancy progresses not only to sustain increased rates of protein deposition( Reference Duggleby and Jackson 8 ), but also to support ongoing availability of glucose, through gluconeogenesis, the primary fuel for the growing fetus( Reference Kalhan, Rossi and Gruca 9 ). Two consistent findings in healthy pregnant adult women are that protein synthesis and net protein deposition increase in the second and third trimesters when compared with that observed in the first trimester or in non-pregnant women( Reference Kalhan, Rossi and Gruca 10 ) and that amino acid oxidation is reduced when compared with that in non-pregnant women( Reference Duggleby and Jackson 11 – Reference Forrester, Badaloo and Persaud 13 ). Together these findings indicate that the partitioning of amino acids towards net protein deposition is enabled through a combination of an increase in maternal protein synthesis and an overall decrease in amino acid oxidation( Reference Kalhan, Rossi and Gruca 10 – Reference Forrester, Badaloo and Persaud 13 ). This raises the possibility that in the case of adolescent girls poorer fetal growth relative to that observed in pregnant adult women is the consequence of an inability to make the necessary adaptations in protein turnover and amino acid oxidation. This is the case especially during late gestation when fetal growth is fastest and the requirement for amino acids is highest( Reference Franco, Josephson and Moehn 14 – Reference Samuel, Moehn and Pencharz 16 ). The objective of the present study was to determine whether there were differences between adolescent girls and adult women during pregnancy with regard to amino acid supply and rates of amino acid oxidation as well as protein synthesis and degradation.
The significant lowering of plasma concentrations of amino acids after a brief fast during pregnancy suggests that the availability of maternal amino acids relative to the needs is marginal, especially for the gluconeogenic amino acids( Reference Felig, Kim and Lynch 17 , Reference Fitch and King 18 ). Dispensable amino acids represent the largest source of maternal amino acid N transferred to the fetus( Reference Felig, Kim and Lynch 17 ), and glucose is the primary fetal energy substrate, making the availability of dispensable amino acids to the fetus crucially important. The two dispensable amino acids that play major roles in intermediary metabolism are alanine and glutamine. As primary carriers of N and carbon from the peripheral to the central tissues of the body, they play pivotal roles linking amino acid, glucose and protein metabolism. Hence, in the fasted state, the flux of these amino acids can be considered to reflect the availability of labile N and carbon for de novo amino acid synthesis and gluconeogenesis. One important possibility that has not been explored is that adolescent girls may be constrained in their ability to synthesise sufficient quantities of dispensable amino acids to meet all their needs and this directly contributes to the increased risk of giving birth to LBW babies. The present study sought to test the hypothesis that adolescent girls, especially those aged < 17 years, would have slower fluxes of glutamine amide-N and alanine-N, indicating a decreased availability of labile N for the de novo synthesis of other dispensable amino acids in the fasted state. A further objective was to test the hypothesis that adaptive responses in protein turnover and amino acid oxidation are constrained in adolescent girls relative to adult women.
Subjects and methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the University of the West Indies and by the Institutional Review Board for Human Subject Research of Baylor College of Medicine & Affiliated Hospitals. Written informed consent was obtained from each study participant at recruitment.
A total of thirty-two pregnant adolescent girls and twenty-two pregnant adult women visiting the antenatal clinic at the University Hospital of the West Indies were invited to participate in the study and were enrolled consecutively. Women with any chronic illness, genetic abnormality or multiple gestations were excluded. The study was carried out in two phases. In phase 1 study, eleven adults and twenty-one adolescents (age range 14–17 years) were recruited. The participants were examined at approximately 13 weeks of gestation, and repeat examinations were performed at approximately 22 weeks of gestation. Spontaneous abortion occurred in one participant in each group before phase 2 study, leaving ten adults and twenty adolescents to be examined at 22 weeks of gestation. Data obtained from these two participants were excluded from the analysis. In phase 2 study, twelve adults and eleven adolescents, aged 16–17 years, were recruited. All adolescents were examined at 13 and 29 weeks of gestation. However, only ten adults were examined at 29 weeks of gestation because one had a spontaneous abortion and the other had a premature delivery at 24 weeks that did not survive. Data obtained from these two adults were excluded from the analysis.
Maternal weight and height were measured during each study as described previously( Reference Thame, Fletcher and Baker 19 ), and weight gain from the first to the second trimester (13 to 22 weeks of gestation, phase 1 study) and from the first to the third trimester (13 to 29 weeks of gestation, phase 2 study) was calculated. Gestational age was determined based on the last menstrual period and confirmed by an ultrasound measurement performed at the time of the first experimental examination. Birth weight, crown–heel length and head circumference were measured as described previously( Reference Thame, Fletcher and Baker 19 ).
Tracer infusion protocol
All participants were examined after an 8 h overnight fast on two occasions. The participants were admitted to the obstetrics ward in the evening and given their last meal at 22.00 hours. After 8 h, an intravenous catheter (Sesecure, 18 G; Morningside Pharmaceuticals Limited) was inserted into the antecubital vein of one arm for the infusion of isotopes, while a second catheter was inserted in an anti-flow direction into the dorsal vein of the contralateral hand for drawing blood samples. The cannula was kept patent with intermittent infusions of heparinised saline.
Sterile solutions of 1-13C-leucine, 15N-alanine, 5-15N-glutamine, 15N2-urea and NaH13CO3 (Cambridge Isotope Laboratories) were prepared in isotonic saline. In phase 1 study, baseline blood and breath samples were collected before the start of the infusion protocol: a primed-continuous infusion of NaH13CO3 (prime = 4 μmol/kg and infusion = 4 μmol/kg per h) was started and maintained for 2 h. Simultaneous primed-constant infusions of 15N-alanine and 5-15N-glutamine (prime = 6 μmol/kg and infusion = 6 μmol/kg per h, respectively) were started and maintained for 6 h. After 2 h, the NaH13CO3 infusion was stopped and a primed-continuous infusion of 1-13C-leucine (prime = 4 μmol/kg and infusion = 4 μmol/kg per h) was started and maintained for 4 h. Further samples of breath were collected at 10 min intervals during the last 30 min of the NaH13CO3 and 1-13C-leucine infusion periods. Further 3 ml blood samples were collected at 15 min intervals during the last 45 min of the tracer infusion period. In phase 2 study, in addition to the infusions used in phase 1 study, to derive another index of amino acid catabolism, a primed-continuous infusion of 15N2-urea (prime = 40 μmol/kg and infusion = 4 μmol/kg per h) was started and maintained for 6 h. At the end of each of the tracer infusion periods, the catheters were removed and the participants were given lunch and discharged.
Laboratory analyses
Blood was drawn in pre-chilled tubes containing sodium fluoride and potassium oxalate and centrifuged at 4°C to separate plasma, which was stored at − 70°C for later analysis. Plasma amino acid concentrations were determined as described previously( Reference Kao, Hsu and Bandi 20 ). The breath samples were analysed for 13C abundance in CO2 using gas-isotope-ratio MS as described previously( Reference Kurpad, Dwarkanath and Thomas 21 ). The isotopic enrichments of alanine and glutamine in the plasma were measured by negative chemical ionisation GC-MS analysis of their heptafluorobutyramide derivatives by selectively monitoring ions at m/z ratios 307–308 for alanine and 346–347 for glutamine( Reference Reeds, Burrin and Jahoor 22 ). The isotopic enrichment of α-ketoisocaproic acid in the plasma was measured by analysis of its pentafluorobenzyl derivative as described previously( Reference Hachey, Patterson and Reeds 23 ) and that of urea in plasma was determined by analysis of its 2-pyrimidinol N-tert-butyldimethylsilyl derivative( Reference Lee, Dennis and Healy 24 ).
Calculations
Total leucine (or alanine or glutamine or CO2 or urea) flux (Q) and leucine oxidation (Leuoxd), an index of protein catabolism, were calculated as described previously( Reference Kurpad, Dwarkanath and Thomas 21 ). Endogenous leucine (or alanine or glutamine or CO2 or urea) flux, an index of body protein breakdown rate, was calculated by subtracting the leucine (or alanine or glutamine or sodium bicarbonate or urea) tracer infusion rate. Non-oxidative leucine disposal, an index of leucine used for protein synthesis, was calculated as follows:
Statistical analyses
Data are expressed as means with their standard errors. In both phase 1 and 2 studies, differences between the adult and adolescent groups were assessed using the non-paired t test. Differences in amino acid and urea kinetic variables between the groups were analysed using a mixed-model (repeated-measures two-factor) ANOVA. This model included the two age groups (adult and adolescent) and time of pregnancy (first, second or third trimester). Post hoc comparisons were made using Bonferroni's test. Because each group had different body weights, whole-body leucine kinetics and alanine, glutamine and urea fluxes were not compared among the groups. Only within-group comparisons were made in phase 1 and phase 2 (first to the second trimester and first to the third trimester) studies using the paired t test. Tests were considered statistically significant if P< 0·05. Statistical analyses were carried out using GraphPad Prism version 4 software (GraphPad Software, Inc.).
Results
The participants of phase 1 study were examined at 12·5 (se 0·3) weeks of gestation and at 21·7 (se 0·2) weeks of gestation. The participants of phase 2 study were examined at 13·1 (se 0·4) weeks of gestation and at 28·8 (se 0·4) weeks of gestation. The maternal characteristics and pregnancy outcomes of the participants of phase 1 study are given in Table 1. During the first-trimester examination, the adolescent participants were 16·3 (se 0·2) years old when compared with the adult participants, who were 25·5 (se 0·5) years old. There were no significant differences between the groups with regard to any of the physical parameters measured, although the adolescent participants tended to weigh less with a lower BMI when compared with the adult participants. Gestational ages during the first- and second-trimester examinations were 2 and 2·8 weeks longer, respectively, in the adult group (P< 0·05). There were no significant differences in any of the parameters related to pregnancy outcomes and newborn characteristics between the two groups. In phase 1 study, two of the ten babies born to adult mothers were of LBW, while one of the twenty babies born to adolescent mothers was of LBW. However, the weights of all the three LBW babies were appropriate for gestational age. There was one premature delivery in each group.
Table 1 Maternal characteristics and pregnancy outcomes of phase 1 study participants (Mean values with their standard errors)
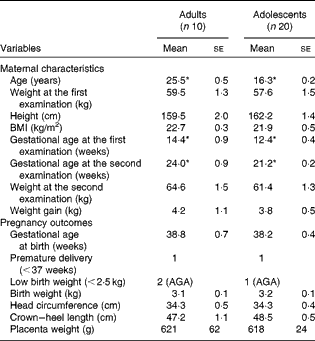
AGA, appropriate for gestational age.
* Mean value was significantly different (P< 0·05; unpaired t test).
The maternal characteristics and pregnancy outcomes of the participants of phase 2 study are given in Table 2. During the first-trimester examination, the adolescent participants were 17·4 (se 0·1) years old when compared with the adult participants, who were 25·8 (se 0·5) years old. All participants, except 1 adolescent (BMI = 18·1 kg/m2), had BMI within the normal range. There were no significant differences in BMI or body weight between the groups, although the adolescent participants tended to weigh less with a lower BMI when compared with the adult participants. Gestational age at birth was significantly greater in the adolescent group (39·5 (se 0·3) weeks) than in the adult group (38 (se 0·4) weeks (P< 0·05)), but the average length of the newborn babies was significantly lower (crown–heel length 47 cm compared with 49·5 cm; P< 0·05). However, no significant differences were observed when the lengths of all the babies born to adolescent and adult mothers during both phase 1 and 2 studies were compared (47·9 (se 0·5) v. 48·5 (se 0·6), P= 0·49). In phase 2 study, one of the ten babies born to adult mothers was premature, while none of the babies born to adolescent mothers was premature. There was one LBW baby in each group. While the weight of the baby born to the adult participant was appropriate for gestational age, that of the baby born to the adolescent participant was small for gestational age.
Table 2 Maternal characteristics and pregnancy outcomes of phase 2 study participants (Mean values with their standard errors)
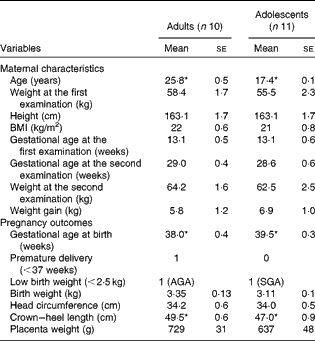
AGA, appropriate for gestational age; SGA, small for gestational age.
* Mean value was significantly different (P< 0·05; unpaired t test).
When leucine kinetic data were expressed per kg body weight, no significant differences were observed between the two groups with regard to any of the kinetic parameters measured in the first and second trimesters in phase 1 study (Table 3). However, there was a significant effect of time of pregnancy as the percentage of leucine flux oxidised decreased from the first to the second trimester in both groups (P< 0·05), while non-oxidative leucine disposal increased significantly (P< 0·05). When the kinetic parameters were expressed per whole body, leucine flux and non-oxidative leucine disposal were found to have increased significantly (P< 0·05) from the first to the second trimester in both groups. Neither age nor time of pregnancy had any effect on alanine or glutamine flux expressed per kg body weight (Table 3). There was no significant change in whole-body glutamine or alanine flux from the first to the second trimester in either group.
Table 3 Leucine kinetics in pregnant adolescent girls and adult women at 13 and 21 weeks of gestation in phase 1 study (Mean values with their standard errors)
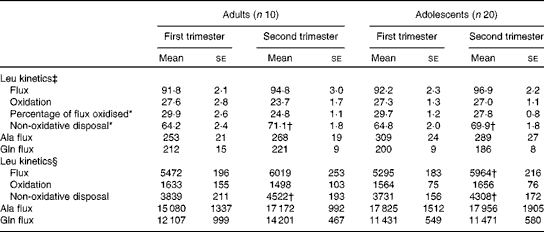
*There was a significant effect of time of pregnancy (P< 0·05; repeated-measures two-factor ANOVA).
† Mean value was significantly different from the corresponding value recorded in the first trimester (P< 0·05; paired t test).
‡ Leucine kinetics measured as per kg body weight (μmol/kg per h).
§ Leucine kinetics measured as per whole body (μmol/h).
Leucine flux expressed per kg body weight was significantly slower (P< 0·05) in the adult group than in the adolescent group at both 13 and 29 weeks of gestation in phase 2 study (Table 4). There was no significant effect of time of pregnancy on any parameter of leucine kinetics or on urea flux. When the kinetic parameters were expressed per whole body, leucine flux and non-oxidative leucine disposal were found to have increased significantly from the first to the third trimester in the adolescent group (P= 0·027 and P= 0·006, respectively). Although both parameters tended to be higher in the third trimester than in the first trimester in the adult group, the changes were not statistically significant.
Table 4 Leucine kinetics and urea flux in pregnant adolescent girls and adult women at 13 and 29 weeks of gestation in phase 2 study (Mean values with their standard errors)
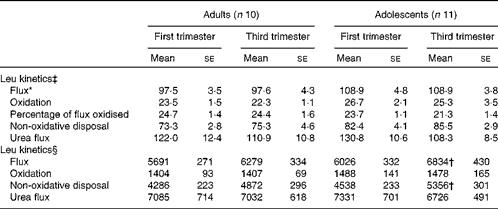
* There was a significant effect of time of pregnancy (P< 0·05; repeated-measures two-factor ANOVA).
† Mean value was significantly different from the corresponding value recorded in the first trimester (P< 0·05; paired t test).
‡ Leucine kinetics measured as per kg body weight (μmol/kg per h).
§ Leucine kinetics measured as per whole body (μmol/h).
In phase 1 study, a significant effect of time of pregnancy was observed on the plasma concentrations of five indispensable amino acids (leucine, methionine, tryptophan, valine and threonine), with concentrations of leucine, methionine, tryptophan and valine being lower and that of threonine being higher in the second trimester than in the first trimester (Table 5). There was a significant effect of time of pregnancy on the plasma concentrations of four dispensable amino acids (glycine, ornithine, serine and tyrosine), with a decrease being observed from the first to the second trimester. There was a significant effect of age on the plasma concentrations of aspartic acid and ornithine, with higher concentrations being observed in the adult group than in the adolescent group. Significant interactions between age and time of pregnancy (P< 0·05) were observed for plasma ornithine and serine concentrations, with a decrease being observed in the adult group, but no changes in the adolescent group from the first to the second trimester.
Table 5 Plasma amino acid concentrations (μmol/l) in pregnant adolescent girls and adult women at 13 and 21 weeks of gestation in phase 1 study (Mean values with their standard errors)

* There was a significant effect of time of pregnancy (P< 0·05; repeated-measures two-factor ANOVA).
† There was a significant effect of age (P< 0·05; repeated-measures two-factor ANOVA).
‡ There was a significant effect of time of pregnancy × age interaction (P< 0·05; repeated-measures two-factor ANOVA).
§ Mean value was significantly different from that of the adult group in the same trimester (P< 0·05; Bonferroni post hoc tests).
In phase 2 study, there was a significant effect of age on the plasma concentrations of seven indispensable amino acids (histidine, leucine, isoleucine, lysine, methionine, threonine and valine), with higher concentrations being observed in the adult group than in the adolescent group (Table 6). There was a significant effect of time of pregnancy on the plasma concentrations of seven indispensable amino acids (leucine, isoleucine, lysine, methionine, phenylalanine, tryptophan and valine), with a decrease being observed from the first to the third trimester. There was a significant effect of age on the plasma concentrations of seven dispensable amino acids (asparagine, citrulline, glutamine+glutamate, glycine, ornithine, proline and serine), with higher concentrations being observed in the adult group than in the adolescent group. There was a significant effect of time of pregnancy on the plasma concentrations of six dispensable amino acids (asparagine, glutamine+glutamate, glycine, ornithine, serine and tyrosine), with a decrease being observed from the first to the third trimester.
Table 6 Plasma amino acid concentrations (μmol/l) in pregnant adolescent girls and adult women at 13 and 29 weeks of gestation in phase 2 study (Mean values with their standard errors)

* There was a significant effect of age (P< 0·05; repeated-measures two-factor ANOVA).
† There was a significant effect of time of pregnancy (P< 0·05; repeated-measures two-factor ANOVA).
Discussion
The results of the present study show that during a normal pregnancy adolescent girls can make adaptations in amino acid and protein metabolism similar to those observed in pregnant adults, with an increase in protein synthesis and a decrease in protein oxidation, in the overnight fasted state. However, all the participants in the present study had normal body weights, comprehensive antenatal care and good-quality pregnancies and nearly all of them delivered at term. Hence, it cannot be assumed that the results obtained in underweight adolescent mothers would be similar to those obtained in those who did not receive adequate antenatal care.
When leucine kinetic data were expressed per kg body weight, it was found that leucine flux had increased, the percentage of leucine flux oxidised had decreased and non-oxidative leucine disposal had increased from the first to the second trimester in both groups in phase 1 study. Similarly, when the kinetic data were expressed per whole body, both leucine flux and non-oxidative leucine disposal were found to have increased significantly from the first to the second trimester in both groups. Overall, these data indicate an increase in both protein breakdown and synthesis rates in all groups as pregnancy progressed from the first to the second trimester. Furthermore, the decrease in the percentage of leucine flux oxidised after an overnight fast indicates that the extra amino acids required to maintain metabolic processes as pregnancy progresses from the first to the second trimester are provided as a result of an increased efficiency in the utilisation of the amino acids released from a faster breakdown of body proteins. These findings are in agreement with our previous findings in underweight and normal-weight pregnant Indian women( Reference Kurpad, Dwarkanath and Thomas 21 ) and corroborate similar findings reported by others in pregnant normal-weight adult women( Reference Kalhan, Rossi and Gruca 10 – Reference Forrester, Badaloo and Persaud 13 ). Thus, adolescent girls with a normal BMI, even those as young as 14 years, can increase both protein breakdown and synthesis rates as pregnancy progresses from early- to mid-pregnancy.
Studies carried out in pigs have shown that during late pregnancy when fetal growth is fastest the requirement for amino acids is highest( Reference Franco, Josephson and Moehn 14 – Reference Samuel, Moehn and Pencharz 16 ). Therefore, it was surprising that the magnitude of change in leucine kinetics from the first to the third trimester was not as great as that observed from the first to the second trimester. There were no significant changes in leucine kinetics expressed per kg body weight from the first to the third trimester in phase 2 study. However, when the kinetic data were expressed per whole body, both leucine flux and non-oxidative leucine disposal were found to have increased significantly from the first to the third trimester in the adolescent group and to be higher in the adult group. In addition, urea flux, an index of protein and amino acid catabolism, was found to have decreased from the first to the third trimester in both groups, a finding that is in agreement with earlier findings reported by others( Reference Duggleby and Jackson 12 , Reference Forrester, Badaloo and Persaud 13 ). Together these findings suggest that the adaptations observed in protein turnover and amino acid metabolism during the second trimester persist into the third trimester, but appear less intense. This finding is in agreement with previous observations that increases in arginine flux and NO synthesis reach a peak during mid-pregnancy with a decline during later pregnancy towards postpartum values( Reference Goodrum, Saade and Belfort 25 ).
Another aim of the present study was to test the hypothesis that in the fasted state adolescent girls would have slower fluxes of glutamine amide-N and alanine-N, indicating a decreased availability of labile N for the de novo synthesis of other dispensable amino acids and of amino acid carbon for gluconeogenesis. The absence of differences in the flux of either alanine or glutamine between the adolescent and adult groups demonstrates that the adolescent girls were synthesising adequate amounts of these two amino acids. Hence, in normal-weight adolescent girls, it is unlikely that the availability of these amino acids is limiting for fetal growth. However, if it were to be assumed that the plasma free pool of an amino acid marks the balance between the supply of the amino acid to the body and the demands, then the lowered plasma concentrations of most amino acids in the adolescent participants compared with that in the adult participants in phase 2 study suggest a marginal state. The finding that the concentrations of dispensable amino acids decreased after an overnight fast suggests that the supply from protein breakdown and de novo synthesis is insufficient to meet metabolic demands even in well-nourished pregnant teenagers. Furthermore, the lowering of plasma concentrations of most amino acids in both groups as pregnancy progressed into the second and third trimesters corroborates earlier reports that the balance between maternal amino acid supply and utilisation is very tight during pregnancy( Reference Felig, Kim and Lynch 17 , Reference Fitch and King 18 ).
Except for the finding that teenagers gave birth to shorter babies in phase 2 study, there were no other differences in any of the pregnancy outcomes measured between the adult and adolescent participants. Furthermore, when the lengths of all the babies born to adolescent and adult mothers in both phase 1 and 2 studies were compared, this difference was found to be no longer significant. The absence of differences in any of the pregnancy outcome variables measured was somewhat surprising, as we( Reference Thame, Wilks and Matadial 1 ) and others( Reference Alves, Cisneiros and Dutra 2 – Reference Liran, Vardi and Sergienko 7 , Reference Amini, Catalano and Dierker 26 , Reference Gortzak-Uzan, Hallak and Press 27 ) have reported an increased risk of adverse pregnancy outcomes, including LBW, among pregnant adolescents. On the other hand, in the Montreal Diet Dispensary study of 2406 pregnant adolescent girls who received adequate antenatal care that included individualised nutritional supplements based on nutritional status and other health risks, there was a 55 g improvement in birth weight as well as marked reductions in the rates of LBW and very LBW in the intervention group compared with the non-intervention group( Reference Dubois, Coulombe and Pencharz 28 ). Hence, the relatively good pregnancy outcomes of the thirty-one adolescent participants in the present study could be because of the comprehensive prenatal care and normal body weights that they had at the time of becoming pregnant, as a closer examination of the published data suggests that the increased prevalence of LBW is especially prominent in adolescents who have poor antenatal care and a low BMI( Reference Thame, Wilks and Matadial 1 , Reference Amini, Catalano and Dierker 26 , Reference Gortzak-Uzan, Hallak and Press 27 ). This is also the case in underweight adult women ( < 51 kg) who have a 42 % risk of giving birth to a LBW baby( Reference Mavalankar, Gray and Trivedi 29 ), suggesting that underweight mothers are challenged in providing adequate nutrients to support increased deposition of maternal tissues and growth of the fetus. With respect to amino acid availability, Duggleby & Jackson( Reference Duggleby and Jackson 11 ) reported that protein turnover increases to a greater extent in pregnant women whose BMI exceeds 25 kg/m2 compared with those with a BMI lower than 25 kg/m2, suggesting that amino acid supply is directly related to maternal BMI and protein turnover. This finding suggests that underweight women and teenagers will be unable to synthesise enough amino acids to satisfy the demands of pregnancy. Hence, an underweight mother has to restrain the growth of the fetus to allow a successful pregnancy within her nutritional and metabolic constraints( Reference Duggleby and Jackson 11 ). In the present study at the time of the first measurement during the first trimester, all adolescent girls, except one, had BMI within the normal range, indicating that they were well nourished at the time of pregnancy. Furthermore, both groups of adolescents gained weight at the same rate as their adult counterparts. Even the one adolescent participants with a low BMI (17·8 kg/m2) gave birth at term to a normal-weight (3·21 kg) baby. Hence, endogenous capacity to provide amino acids for the synthesis of maternal and fetal protein plus synthesis of other compounds needed to facilitate fetal growth was adequate. From these results, we conclude that similar to their adult counterparts, adolescent girls with a normal BMI can synthesise the extra amino acids required for increased maternal protein synthesis during pregnancy by increasing protein breakdown and decreasing oxidation in the fasted state. This may explain why their pregnancy outcomes are not different from those of adult women.
Acknowledgements
The authors are grateful to the nursing staff of the obstetrics ward at the University Hospital of the West Indies for their care of the participants.
The present study was supported with federal funds from the US Department of Agriculture, Agricultural Research Service under Cooperative Agreement Number 58-6250-6001, and funds from the International Atomic Energy Agency and by the NIHR to Southampton Biomedical Research Centre.
All authors contributed to different aspects of the study, including the design of the study, data collection, sample analysis, data interpretation, and writing of the manuscript as follows: F. J., M. M. T. and A. A. J. designed and supervised various aspects of the study; M. M. T., R. G., T. M. B., A. V. B. and H. M. F. recruited the participants, conducted the experiments, processed the samples, and took care of the participants; G. J. T. and J. W. H. analysed the samples and calculated the data; M. M. T., A. A. J., F. J. and J. W. H. analysed and interpreted the data and wrote the manuscript.
None of the authors has any conflicts of interest to declare.








