High cereal fibre intake is linked to reduced risk of developing type 2 diabetes, increased insulin sensitivity and reduced weight gain(Reference Ludwig, Pereira and Kroenke1–Reference Schulze, Schulz and Heidemann3). The source of cereal fibre in these studies is not clear, but the majority is probably wheat fibre; about 70 % of the servings of foods in the highest quintile of cereal fibre intake in US nurses consisted of dark bread, wheat germ and wheat bran(Reference Liu, Stampfer and Hu4), and, in Europe, cereal fibre intake was more strongly correlated with wholegrain bread (r 0·71) than muesli (r 0·34)(Reference Schulze, Schulz and Heidemann3). Recent studies have shown that various types of cereal fibre influence acute postprandial glucose, insulin and gut hormone responses(Reference Weickert and Pfeiffer5), and improve insulin sensitivity in studies lasting 1–3 d(Reference Weickert, Mohlig and Koebnick6, Reference Weickert, Möhlig and Schöfl7). However, controlled clinical trials lasting 4–12 weeks showed no significant effect of wheat fibre on glycaemic control or oral glucose tolerance in normal(Reference Munoz, Sandstead and Jacob8, Reference Kestin, Moss and Clifton9) or diabetic subjects(Reference Jenkins, Kendall and Augustin10, Reference Costabile, Klinder and Fava11). This suggests that the short-term effects of cereal fibre may not persist or that other mechanisms are involved. The discrepancy in results between prospective studies and 4- to 12-week clinical trials suggests that it may take a long time for the protective effects of cereal fibre to develop. Animal studies suggest that fibre may protect against diabetes due to an ability of the SCFA (acetic, propionic and butyric acids), produced during the colonic fermentation of dietary fibre(Reference Wong, deSouza and Kendall12), to increase the production of glucagon-like peptide-1 (GLP-1)(Reference Reimer and McBurney13, Reference Tappenden, Thomson and Wild14). GLP-1 stimulates insulin secretion, reduces the rate of gastric emptying, increases insulin sensitivity and reduces energy intake(Reference Drucker15).
The colon, containing thousands of bacterial phylotypes(Reference Samuel, Hansen and Manchester16), represents a complex ecosystem; when such systems are disturbed it takes a long time for equilibrium to be re-established(Reference Feng and Chai17). In rats fed resistant starch for 6 months, caecal SCFA concentrations continued to increase over the duration of the study(Reference Le Blay, Catherine Michel and Hervé18). In human subjects with diabetes it took 6–9 months for fasting plasma acetate to start rising after increased fibre intake(Reference Wolever, Schrade and Vogt19) or on long-term acarbose therapy(Reference Wolever, Radmard and Chiasson20). In human subjects, high-fibre diets(Reference Cummings, Pomare and Branch21) increased plasma acetate and butyrate, but not propionate. This may be because acetate is the most abundant SCFA(Reference Cummings, Pomare and Branch21) and high amounts of butyrate are produced when starch is fermented(Reference Cummings and MacFarlane22). Wheat bran is generally considered to be partly fermented(Reference Cummings, Kasper and Goebell23), with about 45 % disappearing from the human gastrointestinal tract over a 12 d feeding period(Reference Southgate, Branch and Hill24); however, wheat fibre fermentability may increase with long-term consumption due to adaptation of colonic bacteria.
Thus, our primary objective was to determine the time-course of the effects of increased wheat fibre intake on plasma acetate, butyrate and GLP-1 concentrations in hyperinsulinaemic human subjects. Hyperinsulinaemic subjects were studied because high plasma insulin is typically associated with insulin resistance(Reference Clausen, Borch-Johnsen and Ibsen25) and increased risk for diabetes(Reference Chiasson and Rabasa-Lhoret26); thus, they would be likely to benefit from any protective effect of fibre. We hypothesised that it would take 6–9 months for significant increases in plasma SCFA and GLP-1 concentrations to be detected.
Materials and methods
Healthy subjects (n 40) with fasting plasma insulin ≥ 40 pmol/l were recruited from the Toronto area through newspaper advertisements. No subjects had used antibiotics in the last 3 months, had any history of gastrointestinal problems, diabetes, hyperlipidaemia or a high-fibre diet.
Subjects, stratified by sex, were randomised to a high-wheat fibre cereal (fibre group) (All Bran Original®; Kellogg Canada Inc., Mississauga, ON, USA) or a low-fibre cereal (control group) (Rice Krispies®; Kellogg Canada Inc.) using blocks of unequal sizes to enhance concealment. Random treatment allocations were created using the @RAND function of Lotus 123 (Lotus Development Corp., Cambridge, MA, USA) and sealed in opaque envelopes which were opened on the morning of the baseline metabolic profile in the order of each subject's arrival. Thereafter, subjects were asked to consume 60 g of the high-fibre cereal (750 kJ, 30 g carbohydrate, 24 g fibre, 2 g fat and 8 g protein) or 49 g of the control cereal (750 kJ, 41 g carbohydrate, 0·5 g fibre, 1 g fat and 3 g protein) daily for 1 year. Apart from the high-fibre cereal or the control cereal, subjects consumed self-selected diets with no restrictions, except that they were asked to maintain constant weight and physical activity throughout the study. At monthly intervals subjects were weighed, picked up supplies of cereal and returned unused cereal from the previous month.
At baseline, 3, 6, 9 and 12 months, overnight-fasted subjects came to St Michael's Hospital at about 08.00 hours for an 8 h metabolic profile. At baseline each subject received their assigned cereal for breakfast and chose the remainder of their breakfast and lunch from a fixed menu. The breakfast menu included white bread or toast with margarine, 2 % milk, orange or apple juice, and/or tea or decaffeinated coffee with sugar or sweetener. The lunch menu consisted of one of four frozen entrées, white bread, margarine, baby carrots, 2 % milk, orange or apple juice, cookies (digestive or chocolate chip), tea or decaffeinated coffee with sugar or sweetener, and/or pudding (vanilla or chocolate). Nutrient intakes during the metabolic-profile days for subjects on the fibre cereal v. the control cereal, respectively, were: energy, 4010 (sem 192) v. 4900 (sem 377) kJ; fat, 23 (sem 2) v. 28 (sem 3) g; protein, 37 (sem 1) v. 37 (sem 3) g; carbohydrate, 171 (sem 8) v. 196 (sem 14) g; dietary fibre, 26 (sem 0) v. 9 (sem 1) g. The only significant differences were for energy and fibre. For each subject, the exact amounts and types of foods chosen on the first test day were repeated on the 4 remaining test days. Three-day diet records for 2 weekdays and 1 weekend day were collected on test days. Nutrient intakes were analysed using Food Processor SQL® version 9.9.1 (ESHA Research, Salem, OR, USA). Throughout the present paper the term ‘carbohydrate’ means available carbohydrate defined as total carbohydrate minus dietary fibre. Physical activity was assessed on test days by asking subjects about type and frequency of regular exercise. This information was used to ensure that subjects maintained physical activity levels throughout the study.
On each metabolic profile day, subjects were weighed, gave a fasting blood sample, ate breakfast and had further blood taken 0·5, 1, 2, 3, 4, 5, 6, 7 and 8 h after the start of breakfast from a cannulated forearm vein (cannula kept open with sterile saline). Lunch was consumed after the 4 h blood sample. Blood for glucose, insulin, NEFA and SCFA was taken into fluro-oxalate tubes. Blood for GLP-1 was taken into ice-cooled EDTA tubes at 0, 0·5, 1, 2, 4 and 5 h, and 50 μl of dipeptidyl peptidase-4 (DPP-IV) inhibitor (Linco Research, Billerica, MA, USA) added within 30 s of collection. Blood samples were centrifuged at 4°C, and the plasma removed and stored at − 70°C until analysis.
Glucose was measured by a hexokinase method, insulin by electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany), NEFA enzymically (Wako Chemical Industries, Dallas, TX, USA) and GLP-1 by ELISA which captures active GLP-1 (7–36 amide) by a monoclonal antibody with specific binding to the N-terminal region of the molecule (Linco Research, Billerica, MA, USA).
Before SCFA analysis plasma samples were filtered through a micro-partition system with a 30 000 Da molecular-weight cut-off (Vivaspin 2; Vivascience, Hanover, Germany) by centrifuging at 5000 g at 4°C for 90 min and the protein-free filtrate stored at − 70°C. Before use, the filters were washed by centrifuging three times with doubly distilled, deionised water, to remove contaminants that interfered with butyrate and propionate peaks. Samples (225 μl) of protein-free plasma were vacuum distilled after adding 25 μl internal standard (1·25 mm-2-ethyl butyric acid and 1 m-[2H]formic acid; Cambridge Isotope Laboratories, Xenia, OH, USA). The distilled samples were stored on dry ice until analysis later the same day. An HP 7673 or HP 7683 auto-sampler (Hewlett Packard, Mississauga, ON, Canada) was used to inject 0·5 μl of sample into an HP 5890 Series II or HP 6890N gas chromatograph equipped with hot (130°C) on-column inlets, an Agilent 19095F-123 PEG column (30 m × 0·53 m × 1·0 μm film; Agilent, Mississauga, ON, Canada) and flame ionisation detector. The column temperature was initially 80°C and rose to 220°C. The carrier gas was He and the detector received He, H2 and air. Protein-free plasma samples were distilled in duplicate and each distillate injected in triplicate. SCFA concentrations were determined from the peak height ratio for each SCFA (average of 6) v. the internal standard, with reference to standard curves and corrected for dilution during sample preparation.
We studied thirty subjects because, based on a previous study(Reference Wong, deSouza and Kendall12), this number provided 80 % power to detect changes in postprandial acetate and butyrate responses. The results of subjects who dropped out of the study (n 12) before the 3-month metabolic profile were not included. Differences in subject characteristics, compliance and nutrient intakes at baseline and during the study (mean of 3, 6, 9 and 12 months) in the fibre group compared with the control group were assessed by the unpaired t test. Differences in dietary fibre intake were also assessed for the effect of group and time as a two-factor analysis of covariance (ANCOVA), where baseline was the covariate, using the SAS® (SAS Institute, Inc., Cary, NC, USA) procedure for mixed models.
Changes from baseline in SCFA and GLP-1 concentrations at individual time points during the metabolic profile were considered as secondary endpoints, and compared by the paired t test. The conclusions were based on the mean 0–8 h concentrations of glucose, insulin, NEFA and SCFA, and 0–5 h for GLP-1, determined as total area under the response curve divided by the time interval (8 h or 5 h). Missing values (four from the fibre group and five from the control group, due to drop-outs or subjects who missed tests) were imputed by averaging the preceding and following values or, if the missing value was the final value, then the last value was carried forward. The degrees of freedom were reduced by the number of missing values. Two 0–5 h mean GLP-1 values were removed from the analysis (one from each group) because they were>3 sd from the mean. Data were assessed for normality using the SAS® univariate procedure and a logarithmic transformation applied to non-normally distributed variables before conducting further statistical analyses. Differences in body weight and the metabolic endpoints were examined for the effect of group and time as a two-factor ANCOVA, where baseline was the covariate, using the SAS® procedure for mixed models. If the interaction between group and time was significant, unpaired t tests were performed at each time point to assess the effect of group.
Results are expressed as mean values with their standard errors or mean values with 95 % CI. Unless otherwise indicated, differences between means are considered significant at P < 0·05.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committees at the University of Toronto and St Michael's Hospital. Written informed consent was obtained from all subjects.
Results
A total of 171 individuals were screened to participate in the study, sixty-six were eligible, forty were recruited to participate and twenty-eight subjects were included in the final analysis (Fig. 1). At baseline, subjects randomised to the fibre group had lower fasting and postprandial insulin and lower homeostasis model assessment of insulin resistance (HOMAr) than those randomised to the control group, but did not differ significantly for any other anthropometric or metabolic variable (Table 1).
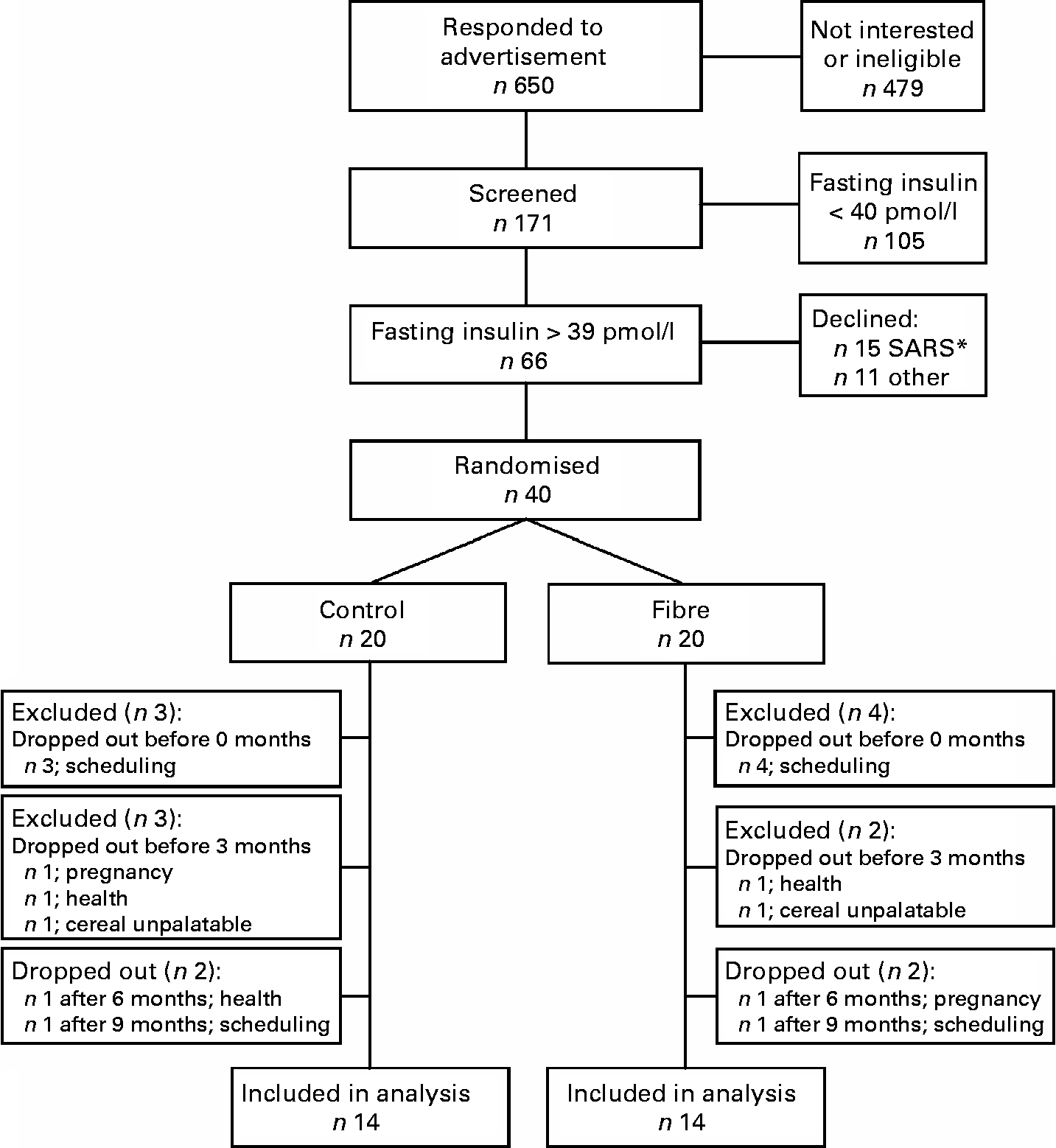
Fig. 1 Study flow chart. * The 2003 epidemic of severe acute respiratory syndrome (SARS) in Toronto resulted in a temporary halt of all clinical research. After the epidemic was over, fifteen previously recruited subjects declined to participate.
Table 1 Subject characteristics at baseline
(Mean values with their standard errors)

HOMAr, homeostasis model assessment of insulin resistance; GLP-1, glucagon-like peptide-1.
* Median value was significantly different from that of the high-wheat fibre cereal group (P < 0·05).
† Non-normally distributed variables.
The fibre group consumed 98 (sem 4) % of the prescribed amount of high-fibre cereal, resulting in an increase in total fibre intake of 20 g/d (Table 2). The control group consumed more cereal than requested, and so had significantly higher compliance than the fibre group (116 (sem 5) %; P = 0·01). At baseline there were no significant differences between the fibre and the control groups for mean daily intake of nutrients. During the trial the fibre group consumed significantly more total and soluble dietary fibre than the control group, but there were no other differences in nutrient intakes (Table 2). Body weight tended to increase in both groups throughout the trial, but neither the change with time, nor the difference between the fibre and the control group was statistically significant (baseline v. 1 year, respectively, for the fibre group: 78·5 (sem 4·2) v. 79·7 (sem 4·1) kg and the control group: 80·5 (sem 5·6) v. 81·8 (sem 6·1) kg).
Table 2 Nutrient intakes at baseline and during the study†
(Mean values with their standard errors)
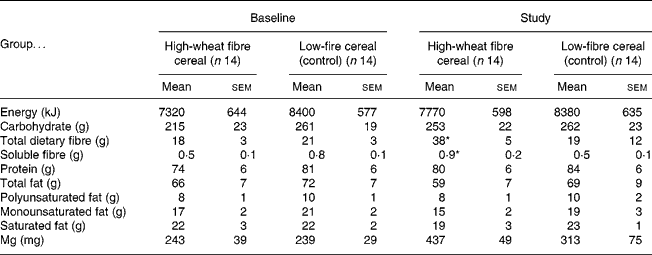
* Mean value was significantly different from that at baseline for the high-fibre group and from the study value for the control group (P < 0·05). No other differences were statistically significant.
† Data were obtained using 3 d food records once at baseline and at 3-month intervals for 1 year (values for study are means for 3 d food records collected at 3, 6, 9 and 12 months).
After 12 months on the control cereal, plasma acetate was significantly higher than baseline at several times throughout the day, while on the high-fibre cereal this was only significant at 8 h (Fig. 2). Propionate tended to increase from baseline in both the control and fibre groups, with significant differences at several times in the fibre group. Butyrate tended to be lower than baseline after 12 months on the control cereal, but higher than baseline after 12 months on the high-fibre cereal although none of the differences at individual time points was statistically significant (Fig. 2). After 12 months, plasma GLP-1 was no different from baseline on the control cereal, but on the high-fibre cereal increased significantly at fasting and 1 h (Fig. 2).

Fig. 2 Fasting and postprandial concentrations of plasma acetate (a and b), butyrate and propionate (c and d) and glucagon-like peptide-1 (GLP-1) (e and f) during 8 h metabolic profiles at baseline (●) and after 12 months (○) on a low-fibre cereal (a, c, e; n 14) or a high-wheat fibre cereal (b, d, f; n 14). ↑ , Times of meal consumption. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at 12 months (P < 0·05; paired t test).
After 12 months on the control cereal, fasting and postprandial glucose tended to be higher than at baseline (significant at 2 h), whereas after 12 months on the high-fibre cereal, mean fasting and postprandial glucose concentrations were virtually identical to those at baseline (Fig. 3). Fasting and postprandial insulin and NEFA did not change from baseline on either the high-fibre or the control cereal (Fig. 3).

Fig. 3 Fasting and postprandial concentrations of plasma glucose (a and b), insulin (c and d) and NEFA (e and f) during 8 h metabolic profiles at baseline (●) and after 12 months (○) on a low-fibre cereal (a, c, e; n 14) or high-wheat fibre cereal (b, d, f; n 14). ↑ , Times of meal consumption. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that at 12 months (P < 0·05; paired t test).
There were significant time × treatment interactions for baseline-adjusted 0–8 h mean concentrations of acetate (P = 0·008) and butyrate (P = 0·01), which indicates that the differences between the control and fibre groups changed with time. There was no significant difference in baseline-adjusted mean 0–8 h acetate between the fibre and control groups until 9 months, when the value for the fibre group, 8 (95 % CI − 4, 21) μmol/l, was greater than that for the control group, − 5 (95 % CI − 10, 0) μmol/l (P < 0·05; Fig. 4). Similarly, after 9 months, mean 0–8 h butyrate had increased more for the fibre group, 0·16 (95 % CI − 0·15, 0·46) μmol/l, than for the control group, − 0·14 (95 % CI − 0·25, − 0·02) μmol/l (P < 0·05; Fig. 4).

Fig. 4 Residuals of baseline-adjusted mean 0–8 h concentrations of plasma acetate (a), propionate (b), butyrate (c), glucose (d), insulin (e) and NEFA (f) and mean 0–5 h concentrations of glucagon-like peptide-1 (GLP-1) (g) in fourteen subjects who consumed a low-fibre control cereal (●) and fourteen subjects who consumed a high wheat-fibre cereal (○) for 1 year. Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that of the high-fibre cereal group (P < 0·05). Significant time × treatment interactions existed for acetate (P = 0·008), butyrate (P = 0·01) and GLP-1 (P = 0·033).
There was a significant time × treatment interaction for 0–5 h mean GLP-1 concentration (P = 0·033). Mean 0–5 h plasma GLP-1 tended to fall with time on the control cereal, but rise with time on the high-fibre cereal (Fig. 4), so that by 12 months the baseline-adjusted mean 0–5 h GLP-1 for the fibre group was greater than baseline by 1·3 (95 % CI 0·4, 2·2) pmol/l (P < 0·05), and greater than the value for the control group by 1·4 (95 % CI 0·1, 2·7) pmol/l (P < 0·05); this difference represents a 25 % increase in GLP-1 on the high-fibre cereal. Baseline-adjusted mean 0–8 h plasma glucose tended to be lower on the high-fibre cereal than the control cereal, and the difference tended to increase with time, but this was not significant (time × treatment interaction; P = 0·78) (Fig. 4). Mean 0–8 h plasma insulin and NEFA appeared to be higher on the high-fibre cereal than on the control cereal, but the differences were not significant (P = 0·99 and P = 0·06, respectively).
There were no significant correlations between changes in mean 0–8 h concentrations of GLP-1, glucose, insulin or NEFA and changes in SCFA or body weight. Neither fibre intake, nor carbohydrate intake (g), nor percentage compliance, was correlated with any of the SCFA or GLP-1 mean concentrations throughout the study.
Discussion
The results showed that when hyperinsulinaemic human subjects increased wheat fibre intake by 20 g/d for 1 year, mean 0–8 h plasma acetate did not change except for a transient increase at 9 months; it took 9 months for plasma butyrate to increase and 12 months for plasma GLP-1 to increase significantly compared with the control treatment.
One limitation of measuring SCFA in peripheral blood is that plasma SCFA originate from exogenous (i.e. colonic fermentation) and endogenous sources. Acetate and butyrate are produced by fat oxidation, while propionate comes from branched-chain amino acid and methionine metabolism(Reference Brindle, Schooley and Tsai27). Thus, increased plasma SCFA concentrations occur during states of enhanced fat and amino acid oxidation, such as prolonged starvation(Reference Scheppach, Pomare and Elia28), diabetes(Reference Smith, Humphrys and Hockaday29) and impaired glucose tolerance(Reference Wolever, Josse and Leiter30). It has generally been considered that, under normal circumstances, colonic fermentation is the primary source of blood acetate(Reference Smith, Humphrys and Hockaday29). However, in the present and previous studies(Reference Adam, Levrat-Verny and Lopez31) plasma acetate concentrations fell after breakfast, rebounded before lunch, and then fell again after lunch, a pattern of response identical to that of NEFA which are suppressed by postprandial insulin. This suggests that a substantial proportion of fasting plasma acetate is endogenously produced from fat oxidation. In this context, it is of interest that after 9 months on the high-fibre cereal, acetate increased postprandially, particularly from 4–8 h, a time of day when increased colonic SCFA production is thought to occur(Reference Adam, Levrat-Verny and Lopez31). However, the tendency for plasma acetate to rise on the control cereal at 12 months was due to an increase in fasting and early postprandial acetate (Fig. 2) which might indicate increased endogenous production associated with the tendency toward increased plasma glucose at this time (Fig. 3). By contrast, plasma butyrate did not fall after eating, suggesting little or no contribution of endogenous production to the fasting butyrate level, and the increased mean 0–8 h concentrations after 9 and 12 months on the high-fibre cereal were due to increases after breakfast and particularly after lunch.
Another limitation of measuring SCFA in peripheral blood is that butyrate is extensively metabolised in the colonic mucosa(Reference Adam, Levrat-Verny and Lopez31) and all three SCFA are taken up by the liver(Reference Rémésy, Demingé and Chartier32); thus peripheral blood levels may not reflect colonic production. The concentration of butyrate, in particular, is very low in peripheral blood(Reference Wolever, Josse and Leiter30); nevertheless, we consistently have detected rises in serum butyrate on high-fibre diets(Reference Cummings, Pomare and Branch21) and acarbose therapy(Reference Wolever and Chiasson33). In addition, studies in pigs suggest that peripheral blood butyrate concentrations mimic production rates in the colon(Reference Knudsen, Serena and Canibe34). We did not measure breath H2 because it may not be a marker of fibre fermentation. Consuming 15 g of various types of fermentable and non-fermentable fibre elicited no rise in breath H2 in any case(Reference Wolever and Robb35). When we simultaneously measured serum acetate and breath H2, we found that 20 g guar gum increased serum acetate in the absence of a breath H2 response(Reference Wolever, ter Wal and Spadafora36).
The substrates for the increased colonic fermentation on the high-fibre cereal probably include both wheat fibre and starch. Wheat fibre intake reduces intestinal transit time(Reference Cummings, Southgate and Branch37), which, in turn, increases the amount of starch delivered to the colon(Reference El Oufir, Barry and Flourie38). Also, in vitro fermentation of starch and wheat fibre produces both acetate and butyrate(Reference Christensen, Bach Knudsen and Wolstrup39). The changes in acetate and butyrate over time may represent gradual adaptation of the colonic ecosystem to a sustained increase in the amount of carbohydrate entering the system. Ecosystems are self-adaptive systems which exist under specific environmental constraints, containing many different living organisms which compete for the available supply of light, nutrients and water required to sustain their populations(Reference Le Blay, Catherine Michel and Hervé18). The colonic ecosystem is complex not only because it contains thousands of species of bacteria but also because there are several distinct environments in the caecum and proximal and distal colons(Reference Cummings, Kasper and Goebell23). A sustained change in the inputs to a complex ecosystem leads to a complex chain of events which ultimately results in a new equilibrium being reached with respect to the number and type of inhabitants of the ecosystem. The more complex the ecosystem, the longer it takes to reach equilibrium, with several hundred steps being required for land-based ecosystems(Reference Le Blay, Catherine Michel and Hervé18).
Little is known about how the colon adapts to changes in diet, and how long it takes to reach equilibrium. However, experiments in which rats were fed resistant starch for 6 months(Reference Jenkins, Kendall and Augustin10) illustrate the complexity of the changes in SCFA production which occur. Relative to the control diet, on resistant starch, at 2 weeks, 2 months and 6 months, respectively, in the caecum acetate increased by 50, 20 and 100 %, propionate did not change and butyrate increased by 130, 490 and 870 %. In the proximal colon acetate increased by 60, 60 and 120 %, propionate did not change and butyrate increased by 130, 600 and 990 %, while in the distal colon acetate increased by 200, 50 and 240 %, propionate by 50, 20 and 60 % and butyrate by 40, 600 and 1400 %, respectively. The magnitude of changes in specific SCFA varied with time and in different parts of the colon; however, the most consistent change, in agreement with the present results, was a gradual increase in the butyrate concentration with time in all parts of the colon. Also in agreement is a study showing an increase in the levels of butyrate in the distal colon of rats fed wheat fibre(Reference Boffa, Lupton and Mariani40).
The decline in acetate at 12 months in the fibre group was not associated with any significant reduction in fibre intake. However, it may reflect long-term adaptation of the colonic ecosystem. Certain butyrate-producing bacteria metabolise acetate produced by other bacteria(Reference Duncan, Holtrop and Lobley41). Thus, it is possible to speculate that a sustained increase in acetate production leads to the proliferation of bacterial species which convert acetate to butyrate, making less acetate available for absorption into the systemic circulation. We were unable to assess whether the colonic flora changed in the present study because the faecal samples collected for this purpose were lost. However, previous research has shown that colonic bacteria change with changes in diet; for example, feeding rats starch led to increases in Lactobacillus acidophilus, L. plantarum and Bifidobacterium sp., whereas other species were unaffected(Reference Kleessen, Stoof and Proll42). In addition, the specific ability of inulin and fructo-oligosaccharides to increase Bifidobacterium sp. in the human colon is well known(Reference van Loo, Cummings and Delzenne43).
GLP-1, produced from post-translational modification of proglucagon, is secreted by intestinal L-cells located throughout the intestine, but whose concentration increases progressively from the distal jejunum to the distal ileum and from the proximal colon to the distal colon(Reference Eissele, Göke and Willemer44). The rise in plasma GLP-1 on wheat fibre is consistent with animal data showing that high-fibre diets or butyrate administration increase ileal and colonic proglucagon mRNA in rats(Reference Drucker15) and dogs(Reference Massimino, McBurney and Field45) and an increase in postprandial GLP-1 concentrations in rats(Reference Drucker15). SCFA stimulate the growth of the intestinal mucosa(Reference Kripke, Fox and Berman46) and oligofructose doubled the number of L-cells in the proximal colon of rats(Reference Cani, Hoste and Guiot47), possibly by promoting differentiation via the SCFA receptor GPR43 expressed on L-cells(Reference Karaki, Tazoe and Hayashi48). Therefore, the gradual rise in plasma GLP-1 after wheat fibre feeding seen in the present study may have been due to a proliferative effect of SCFA on the intestinal lumen or a direct effect on intestinal L-cells.
GLP-1 secretion may be reduced in insulin resistance(Reference Rask, Olsson and Soderberg49) and obesity(Reference Verdich, Toubro and Buemann50), but it is not known if decreased GLP-1 secretion is a cause or an effect of obesity and insulin resistance. GLP-1 suppresses appetite, increases insulin secretion and reduces insulin resistance(Reference Feng and Chai17). On the other hand, the high plasma insulin concentrations associated with insulin resistance may inhibit the release of gut glucagon-like immunoreactivity (an indicator of L-cell secretory activity)(Reference Brubaker and Vranic51) and leptin resistance, a common feature of obesity, may have the same effect(Reference Anini and Brubaker52). The subjects in the present study were insulin resistant and some were obese, and therefore probably leptin resistant; nevertheless, increasing wheat fibre intake increased GLP-1 secretion. This may help to explain why cereal fibre protects against the development of obesity and diabetes(Reference Ludwig, Pereira and Kroenke1, Reference Liu, Willet and Manson2). In addition to increasing GLP-1, there may be other mechanisms by which cereal fibre or colonic fermentation may prevent diabetes; for example, some investigators have found that short-term consumption of cereal fibre (24–72 h)(Reference Weickert, Mohlig and Koebnick6, Reference Weickert, Möhlig and Schöfl7) or resistant starch (4 weeks)(Reference Robertson, Bickerton and Dennis53) may improve insulin sensitivity without affecting GLP-1. The fact that subjects in the fibre group did not lose weight is not inconsistent with our hypothesis since our objective was not to induce weight loss but rather to examine a possible mechanism by which fibre may maintain weight or lessen weight gain over time. Colonic SCFA provide energy to the body which may explain why weight was not lost despite increased GLP-1. Since high-fibre diets increase the weight of the intestine(Reference van Loo, Cummings and Delzenne43), there may have been a small loss of body fat in subjects in the fibre group which was offset by increased intestinal mass. Finally, bran products vary in their content of starch, lipids, proteins and micronutrients, and thus different types may not have the same effects as the source of wheat fibre used here.
We conclude that a sustained increase in wheat fibre intake increases plasma butyrate and GLP-1 concentrations in hyperinsulinaemic subjects, but that it takes 9–12 months for these changes to occur. These results may provide a mechanism for the epidemiological association between high cereal fibre intake and reduced risk for diabetes.
Acknowledgements
The present study was registered at clinicaltrials.gov (no. NCT00247455; http://clinicaltrials.gov/).
The study was supported by a grant from the Canadian Diabetes Association in honor of the late Kenneth B. Palmer and by a grant from the Canadian Institutes for Health Research, Institute of Nutrition, Metabolism and Diabetes – Obesity/Healthy Body Weight Grant no. OOP-64648.
K. R. F. and T. M. S. W. conceived of the study; T. M. S. W. obtained funding; K. R. F. and C. W. collected the data; K. R. F. and T. M. S. W. performed the statistical analysis; K. R. F. drafted the paper; T. M. S. W. and C. W. revised and reviewed the paper for critical content.
None of the authors declares any conflict of interest.








