Obesity continues to increase worldwide( 1 ) and poses a well-known risk for metabolic derangements such as insulin resistance and type 2 diabetes( Reference Kahn, Hull and Utzschneider 2 ). A potential dietary approach to curb weight gain and help regulate glycaemic control is to identify and encourage the consumption of satiating and low-energy-dense foods. Frequent consumption of whole pulses, the edible seeds of legumes or pod-bearing plants, including beans, chickpeas, yellow peas and lentils( 3 ), is associated with a higher-quality diet( Reference Mitchell, Lawrence and Hartman 4 , Reference Mudryj, Yu and Hartman 5 ), lower body weight( Reference Papanikolaou and Fulgoni 6 ) and improved markers of long-term glycaemic control( Reference Sievenpiper, Kendall and Esfahani 7 ). Acutely, whole pulses lower postprandial glycaemic response( Reference Jenkins, Wolever and Taylor 8 – Reference Mollard, Zykus and Luhovyy 13 ), reduce hunger( Reference Mollard, Wong and Luhovyy 12 , Reference Pai, Ghugre and Udipi 14 – Reference Holt 17 ) and suppress food intake( Reference Mollard, Wong and Luhovyy 12 , Reference Mollard, Zykus and Luhovyy 13 ) up to 2 to 6 h following their intake. Of these benefits, reduced blood glucose (BG) excursions are of particular importance( Reference Ceriello, Colagiuri and Gerich 18 ) as postprandial hyperglycaemia is an early abnormality of glycaemic control associated with type 2 diabetes( Reference Association 19 ). Despite their high nutrient profile and associated health benefits, pulse consumption remains notably low, especially in pulse-producing regions such as North America( Reference Mudryj, Yu and Hartman 5 ).
To increase pulse consumption, industrially processed pre-cooked ground pulses (pulse powders) are being developed for incorporation of pulses into a diverse range of foods( Reference Ryland, Vaisey-Genser and Arntfield 20 ). These powders require minimal cooking time and can be easily added to pastas, breads, cookies, energy bars and soups( Reference Han, Janza and Gerlatb 21 ). While processing generally affects the starch in pulses and can alter their biological effects( Reference Wursch, Del Vedovo and Koellreutter 22 – Reference O'Dea and Wong 25 ), the impact of industrial processing on the biological functionality of pulses has received little attention.
To date, studies that have prepared pulse powders by cooking, drying and grinding in a laboratory setting point to a higher glycaemic response from pulse powders compared with whole pulses( Reference Jenkins, Thorne and Camelon 23 , Reference Tovar, Granfeldt and Bjorck 24 ). However, these earlier results may not reflect the effects of industrial methods typically employed to process whole pulses to powder form( Reference Bellido, Arntfield and Cenkowski 26 , 27 ). Therefore, the objective of the present study was to compare the acute effects of commercially prepared pulse powders and whole pulses on glycaemic response in healthy young men.
Methods
Subjects
Healthy males aged 18–30 years old with a normal BMI (20–24·9 kg/m2)( 28 ) were recruited via advertisements around the University of Toronto Saint George Campus, in local newspapers and on student websites. To reduce between-subject variation and the effects of potential confounders, subjects were excluded if they had diabetes mellitus or any other metabolic disorders; were taking medications; were dieting; were frequent breakfast skippers, were smokers; or participated in any other nutrition-related studies within 4 weeks before the present study. Previous short-term studies have shown that a minimum of eight to ten participants are needed to assess the differences in glycaemic response( Reference Wolever and Bolognesi 29 , Reference Brouns, Bjorck and Frayn 30 ). In addition, sample size analysis using data from a fixed-meal whole-pulse study following a similar within-subject design determined that ten subjects are required to detect a difference of 1·0 mmol/l in BG concentration at 30 min (peak) between treatments at a power level of 0·8 (α = 0·05)( Reference Mollard, Wong and Luhovyy 31 ). Additional subjects were recruited to account for dropouts. Overall, seventeen subjects were recruited and completed Expt 1, and twelve subjects were recruited and completed Expt 2 and 3; no participants terminated the study. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the University of Toronto Health Sciences Research Ethics Board. Written informed consent was obtained from all subjects.
Study design
The experiments conducted in the present study followed a within-subject, randomised, repeated-measure design. Healthy young men attended four sessions on a weekly basis during which they received three treatments (whole canned pulses, puréed canned pulses and pulse powders) or whole-wheat flour as a control in random order. Each experiment investigated commercially available whole and powdered forms of commonly consumed pulse types prepared with tomato sauce.
The treatments and control used in the present study were as follows: whole canned navy beans (188·0 g; FERMA® Import & Export Limited; 70·0 ml filtered water), puréed canned navy beans (188·0 g; FERMA® Import & Export Limited, 70·0 ml filtered water), whole navy bean powder (62·5 g, VegaFull® Instant Dehydrated Whole Navy Bean Powder; manufactured and donated by ADM® Edible Bean Specialties, Inc.; 195·5 ml filtered water; 0·90 g NaCl), or all-purpose whole-wheat flour (39·5 g, Robin Hood®, Smucker Foods of Canada Company; 208·0 ml filtered water; 0·94 g NaCl) in Expt 1; whole canned green lentils (183·8 g, NuPak®; Shah Trading Company Limited; 70·0 ml filtered water), puréed canned green lentils (183·8 g, NuPak®; Shah Trading Company Limited; 70·0 ml filtered water), pre-cooked Eston lentil powder (47·7 g; manufactured and donated by InfraReady® Products Limited; 204·9 ml filtered water; 1·21 g NaCl) and whole-wheat flour (39·4 g, Robin Hood®, Smucker Foods of Canada Company; 213·2 ml filtered water; 1·21 g NaCl) in Expt 2; and whole canned chickpeas (148·8 g, NuPak®; Shah Trading Company Limited; 104·7 ml filtered water), puréed chickpeas (148·8 g, NuPak®; Shah Trading Company Limited; 104·7 ml filtered water), pre-cooked garbanzo bean (chickpea) powder (49·7 g; manufactured and donated by InfraReady® Products Limited; 202·9 ml filtered water; 1·20 g NaCl), or whole-wheat flour (39·4 g, Robin Hood®, Smucker Foods of Canada Company; 213·2 ml filtered water; 1·21 g NaCl) in Expt 3.
Commercial navy bean powder was produced by ADM by washing, blanching, cooking, refrying, macerating, drying and grinding navy beans to powder form with a particle size for which 95 % passed through a no. 80 sieve. InfraReady produced the lentil and chickpea powders following a similar processing sequence of washing, tempering, micronisation and flaking, with the exception of short and intense heating in the microniser. Following flaking, flakes were milled in a pinmill to powder form. Overall, 96 % of the lentil powder passed through between no. 40 and 50 sieve sizes, and 85 % of the chickpea powder passed through between no. 30–60 sieves. Additional details were not provided by the manufacturers.
The tomato sauce served with the treatments and control was prepared identically in each study and consisted of the following ingredients: no-salt-added tomato paste (125 g, Hunt's®; ConAgra Foods Canada, Inc.); filtered water (110 ml); lemon juice from concentrate (1·2 ml, ReaLemon®; Cadbury Canada, Inc.); no-salt-added garlic and herb seasoning (1·2 ml, McCormick®; McCormick Canada); dried basil leaves (0·6 ml, Equality®; The Great Atlantic & Pacific Company of Canada Limited); ground black pepper (0·6 ml, Equality®; The Great Atlantic & Pacific Company of Canada Limited); garlic powder (1·2 ml, Equality®; The Great Atlantic & Pacific Company of Canada Limited); dried parsley flakes (2·5 ml, Equality®; The Great Atlantic & Pacific Company of Canada Limited); chilli powder (1·2 ml, Selection®; Metro Brands); white sugar (1·2 ml; Redpath® Sugar Limited).
The nutritional composition of the pulse treatments and control is listed in Table 1. All treatments and control were of similar available carbohydrate content (38·8 g), with 25 g from the test ingredient (pulses or whole-wheat flour) and 13·8 g from the tomato sauce. The pulse treatments and control were prepared the day before each session, transferred to airtight containers, combined with water to reach a final weight of 405·0 g, and sealed in airtight containers for overnight storage in an experimental fridge. To prepare the whole-pulse treatments, canned navy beans (Expt 1), lentils (Expt 2) and chickpeas (Expt 3) were poured into a strainer, washed thoroughly under running water for 30 s and drained well. Pulses were then combined with filtered water while the tomato sauce ingredients were cooked in a non-sticky pan on medium heat (3 min). The whole pulses were then added to the tomato sauce and mixed well, covered and brought to a boil (2 min). Heat was reduced and the pulse–tomato sauce mixture was simmered for 10 min and stirred every 2 min. The puréed navy bean, lentil and chickpea treatments were prepared following the same steps as the whole-pulse treatments except that canned pulses were blended in a food processor (KitchenAid Mod: KFP720OB2) for 60 s immediately before cooking and mixing with the tomato sauce. The pulse powder treatments were similarly produced, beginning with mixing the navy bean (Expt 1), lentil (Expt 2) and chickpea (Expt 3) powders with filtered water and NaCl while cooking the tomato sauce ingredients in a separate pan. The pulse powder mixture was added to the tomato sauce once it came to a boil, mixed well, covered and brought to a boil again (2 min). Heat was reduced and the pulse powder–tomato sauce mixture was simmered for 10 min and stirred every 2 min. The whole-wheat flour control was prepared following identical steps as the pulse powder treatments. Before serving, the treatments and control were heated in a microwave oven under high power for 90 s and served with 250 ml of filtered water.
Table 1 Nutritional composition of the treatments and control

* Fibre calculated as 0 kJ/g.
Protocol
The present study followed a similar protocol as reported in previous short-term studies from our group( Reference Anderson, Tecimer and Shah 32 , Reference Anderson, Catherine and Woodend 33 ). Following a 10–12 h overnight fast, participants were instructed to consume a standardised breakfast (1422·6 kJ) in the morning within 15 min and arrive at the laboratory 4 h later. The standardised breakfast was provided to them in advance and consisted of 26 g of Honey Nut Cheerios cereal (General Mills), 250 ml of Beatrice 2 % milk (Parmalat Canada) and 250 ml of Tropicana orange juice (Tropicana Products, Inc.). In addition, 500 ml of bottled water (Canadian Springs) were included, and participants were required to finish the bottle by 1 h before the session start time.
Upon arrival, participants were asked to complete a Sleep Habits and Stress Factors Questionnaire and an Activity Questionnaire. If they reported significant deviations from their usual patterns, they were asked to reschedule. Baseline blood samples were obtained by finger prick by a Monojector Lancet Device (Sherwood Medical), as described previously( Reference Mollard, Wong and Luhovyy 12 , Reference Mollard, Zykus and Luhovyy 13 ), and BG concentrations measured by using a glucose meter (Accu-Chek Compact Plus Glucose Monitor; Roche Diagnostics Canada). The second drop was placed on the testing strip after wiping off the first drop of blood due to contamination with alcohol and interstitial fluid. Each participant was provided with the same glucometer to use throughout the study to reduce intra-subject variation. A baseline BG concentration >5·5 mmol/l suggested that the participant had not complied with the protocol, and the session was rescheduled.
After taking baseline measurements for BG, participants were given 15 min to consume either one of the treatments or control provided to them in random order with 250 ml of filtered water. BG concentration was measured at 15, 30, 45, 60, 90 and 120 min. At 120 min, participants were asked to consume a fixed-size pizza meal (50·2 kJ/kg body weight, McCain Deep ‘N Delicious; McCain Foods Limited) with 500 ml of filtered water (which could be consumed ad libitum) within 20 min in order to measure post-second-meal glycaemic response without the variation introduced by ad libitum food intake. The pizzas averaged 7·6 g protein, 4·9 g fat, 29·3 g carbohydrate and 818·6 kJ/100 g. Each cooked pizza (8 min at 227°C and cut in quarters) was weighed before serving. Following the pizza meal, BG concentration was measured repeatedly at 140, 155, 170, 185 and 200 min.
Statistical analyses
All statistical analyses were performed using SAS version 9.2 (Statistical Analysis Systems; SAS Institute). Effects of time, treatment and treatment × time interaction on BG response were analysed using two-way repeated-measures ANOVA. If there was a treatment and/or interaction effect on BG response, one-way repeated-measures ANOVA and Tukey–Kramer's post hoc test were conducted to determine between-treatment differences at each time point. The effect of treatment on BG AUC was tested via one-way repeated measures ANOVA. All results are presented as means with their standard error of the mean. Differences were considered statistically significant with P< 0·05.
Results
Subject characteristics
A total of seventeen subjects with a mean BMI of 22·9 (sem 0·3) kg/m2 and age of 22·1 (sem 0·8) years completed Expt 1. Overall, twelve subjects with a mean BMI of 23·2 (sem 0·4) kg/m2 and age of 22·2 (sem 0·9) years completed Expt 2. A total of twelve subjects with a mean BMI of 22·3 (sem 0·4) kg/m2 and age of 23·6 (sem 1·0) years completed Expt 3.
Blood glucose
Expt 1 (navy beans)
Pre-meal (0–120 min) mean BG concentrations were significantly affected by time (P< 0·0001) and time × treatment interaction (P< 0·0001), but no main effect of treatment was detected (P= 0·28) (Table 2; Fig. 1(A)). Pre-meal BG concentration was lowest at 0 min and increased after treatment consumption. The interactions occurred because the BG response reached peak concentrations at 30 min, but there was a difference in pattern among the treatments over 120 min. At 15 min, BG concentration was lower after the consumption of whole navy beans (P= 0·004) and puréed navy beans (P= 0·02), but not the powder, compared with the whole-wheat flour control. All the treatments resulted in lower BG concentrations at 30 min compared with the control (P< 0·05). After the peak, navy bean treatments slowed the decrease in BG concentration compared with the whole-wheat flour control. Navy bean powder led to lower BG concentrations at 45 min than puréed navy beans (P= 0·03); yet at 60 min, the consumption of whole-wheat flour control resulted in lower BG concentrations compared with the consumption of whole navy beans (P= 0·03). Main effects of time (P< 0·0001) and treatment (P= 0·02) were observed following the fixed-energy pizza meal, but there was no time × treatment interaction (P= 0·25) (Table 2; Fig. 1(A)). All navy bean treatments and the control increased BG concentrations at 155 min (peak) and gradually decreased BG concentrations until 200 min; however, only whole navy beans suppressed BG concentrations compared with the whole-wheat flour control (P= 0·01).
Table 2 Absolute pre- and post-meal blood glucose concentrations (Mean values with their standard errors)
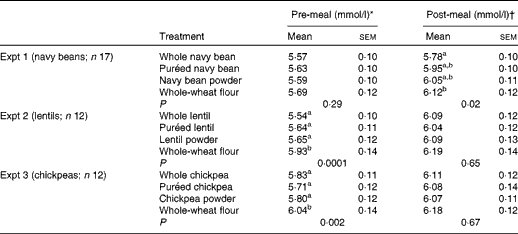
a,bMean values within a column with unlike superscript letters were significantly different from each other (P< 0·05; two-way ANOVA, Tukey–Kramer post hoc test).
* Pre-meal values are means of all observations before the pizza meal: 0, 15, 30, 45, 60, 90 and 120 min.
† Post-meal values are means of all observations after the pizza meal: 120, 140, 155, 170, 185 and 200 min.

Fig. 1 Effect of the treatments on absolute blood glucose concentration over time. (A) Expt 1, (B) Expt 2 and (C) Expt 3. Values are means (n 17 in Expt 1, n 12 in Expt 2 and n 12 in Expt 3), with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different at each measured time point (P< 0·05; one-way ANOVA, Tukey-Kramer post hoc test). (A) ![]() , Whole navy bean;
, Whole navy bean; ![]() , puréed navy bean;
, puréed navy bean; ![]() , navy bean powder;
, navy bean powder; ![]() , whole-wheat flour. (B)
, whole-wheat flour. (B) ![]() , Whole lentil;
, Whole lentil; ![]() , puréed lentil;
, puréed lentil; ![]() , lentil powder;
, lentil powder; ![]() , whole-wheat flour. (C)
, whole-wheat flour. (C) ![]() , Whole chickpea;
, Whole chickpea; ![]() , puréed chickpea;
, puréed chickpea; ![]() , chickpea powder;
, chickpea powder; ![]() , whole-wheat flour.
, whole-wheat flour.
Pre-meal BG net AUC was lower for the navy bean powder treatment (82·9 (sem 11·4) mmol × min/l) than for the whole-wheat flour control (116·0 (sem 12·8) mmol × min/l) (P< 0·05), but was intermediate for the whole bean (97·2 (sem 11·3) mmol × min/l) and puréed (102·1 (sem 10·8) mmol × min/l) treatments. Postprandial BG net AUC was lower for the whole navy bean treatment (94·5 (sem 10·1) mmol × min/l) than for the control (123·9 (sem 15·4) mmol × min/l) (P< 0·05); puréed and powdered navy bean treatments led to intermediate responses (102·0 (sem 10·4) mmol × min/l and 114·5 (sem 11·5) mmol × min/l, respectively).
Expt 2 (lentils)
Pre-meal mean BG concentration was significantly affected by time (P< 0·0001), treatment (P= 0·0001) and time × treatment interaction (P= 0·0008) (Table 2; Fig. 1(B)). Pre-meal BG response was lowest at 0 min and increased after treatment consumption. During the pre-meal period (0–120 min), mean BG concentrations were lower for all lentil treatments than for the whole-wheat flour control (P< 0·05). The interaction is explained by the variation in the effect of the treatment at specific time points. At 15 and 30 min, the whole and purée lentil treatments led to a lower BG response compared with the whole-wheat flour treatment (P< 0·05), while the lentil powder led to an intermediate response. Postprandial BG concentration (120–200 min) was affected by time (P< 0·0001), but there was no main effect of treatment (P= 0·65) or time × treatment interaction (P= 0·43) (Table 2; Fig. 1(B)). All lentil treatments and the control increased BG concentrations similarly at 155 min (peak) and gradually decreased BG concentrations until 200 min.
Whole lentils (97·1 (sem 10·1) mmol × min/l) and lentil powder (95·8 (sem 9·6) mmol × min/l) led to lower pre-meal BG net AUC compared with the whole-wheat flour control (125·8 (sem 15·0) mmol × min/l) (P< 0·05); puréed lentils led to an intermediate response (102·4 (sem 8·4) mmol × min/l). Postprandial BG AUC was not affected by treatment (108·3 (sem 16·0), 94·2 (sem 10·4), 113·4 (sem 17·1) and 125·5 (sem 16·4) mmol × min/l for whole lentils, puréed lentils, lentil powder and control, respectively).
Expt 3 (chickpeas)
Pre-meal mean BG concentration was significantly affected by time (P< 0·0001), treatment (P= 0·002) and time × treatment interaction (P< 0·0001) (Table 2; Fig. 1(C)). Pre-meal BG response was lowest at 0 min and increased after treatment consumption. During the pre-meal period (0–120 min), BG concentration was lower following the consumption of whole, puréed and powdered chickpeas compared with the whole-wheat flour control (P< 0·05). BG response at 15 and 30 min was lower for the whole and puréed chickpea treatments than for the control; chickpea powder led to a marginally lower response (P= 0·057). Following the pizza meal (120–200 min), a main effect was observed for time (P< 0·0001) but not for treatment (P= 0·67) or time × treatment interaction (P= 0·36) (Table 2; Fig. 1(C)). BG concentration peaked comparably for all the treatments and control at 155 min and gradually decreased until 200 min.
BG net AUC was not affected by treatment either pre- or post-meal. The net AUC values before and after the pizza meal for the whole, puréed and powdered chickpea treatments were as follows: 112·2 (sem 11·4) and 91·6 (sem 14·8) mmol × min/l; 105·5 (sem 8·0) and 111·6 (sem 19·9) mmol × min/l; 110·9 (sem 10·5) and 108·0 (sem 12·3) mmol × min/l. The pre- and post-meal values for the whole-wheat flour control were 122·3 (sem 12·7) mmol × min/l and 127·4 (sem 13·0) mmol × min/l, respectively.
Discussion
Overall, no differences in BG response were observed between the pulse treatments due to processing between whole canned pulses, puréed canned pulses and pulse powders, indicating that puréeing and industrial processing of pulses to powder forms does not eliminate their benefits on short-term glucose regulation( Reference Wong, Mollard and Zafar 11 – Reference Mollard, Zykus and Luhovyy 13 ).
Similar to the puréed canned lentils and chickpea treatments, pre-cooked lentil and chickpea powders prepared by a similar process suppressed BG response over 120 min. In contrast, powdered navy beans, along with whole and puréed navy beans, had a less pronounced effect as evidenced by suppressing BG peak concentrations only at 30 min compared with whole-wheat flour control and leading to a more attenuated decline after peaking. The overall lower (4–6 %) mean pre-meal glycaemic response to the control in Expt 1 compared with Expt 2 and 3 may have also contributed to the findings that the navy bean treatments did not result in a lower overall BG response compared with the control, while the lentil and chickpea treatments did. Possible reasons for this inconsistency in the response to the control between the experiments include differences in the batches of whole-wheat flour used to prepare the control, small differences in the composition of the controls, and inter-subject variability.
The results of the present study contrast with a previous report indicating that lentil powder prepared in a laboratory setting by boiling lentils for 20 min, puréeing, drying for 12 h at 121°C and then grinding to a powder produced a significantly greater glycaemic response compared with boiled whole lentils( Reference Jenkins, Thorne and Camelon 23 ). This discrepancy could be due to the differences in processing. Cooking time of pulses positively correlates with starch hydrolysis rate( Reference Traianedes and O'Dea 34 ). It is possible that drying for 12 h at 121°C leads to increased starch gelatinisation, thus making starch granules more readily available for digestion( Reference Newsome 35 ). In addition, protein is located between starch granules in the cells; protein denaturation and digestion by enzymes and acids leads to increased accessibility of α-amylase to the starch, resulting in higher digestibility in pulses( Reference Wursch, Del Vedovo and Koellreutter 22 ). Heating also causes protein denaturation( Reference Newsome 35 ), which may have led to increased accessibility of enzymes to starch granules resulting in higher digestibility.
Processing times in industrial preparations are much shorter than those in home or laboratory settings, as they are optimised to soften and achieve the best textural characteristics of pulses. For example, the micronisation technique used to produce lentil and chickpea powders is a process widely used in the production of cooked, flaked cereals and instant pulse products( Reference Cenkowski and Sosulski 36 ). In this process, grains are exposed to electromagnetic radiation in the wavelength region of 1·8–3·4 μm( Reference Collier, McLean and O'Dea 37 ) for 2–3 min( Reference Arntfield, Scanlon and Malcolmson 38 ). These IR waves cause molecules to vibrate at 60 000–150 000 MHz, thereby producing a rapid internal heat. The temperature can reach approximately 140°C and all starches are gelatinised within 2–3 min( Reference Collier, McLean and O'Dea 37 , Reference Arntfield, Scanlon and Malcolmson 38 ). The grains are then flaked and milled into powders( Reference Collier, McLean and O'Dea 37 ). In contrast, the navy bean powder used in Expt 1 was processed differently compared with the lentil and chickpea powders. The navy bean powder was produced by the conventional method of blanching, cooking, refrying, macerating, drying, and size reduction; nevertheless, the product produced had similar effects to the whole bean.
In the present study, cooking first and then grinding to produce pulse powders did not change the low glycaemic property of the cooked pulses. Cooking whole pulses and then grinding to produce pulse powders disrupts the cotyledon structure yet preserves the integrity of the cell walls that encapsulate the starch granules( Reference Tovar, Bjorck and Asp 39 , Reference Tovar, Bjorck and Asp 40 ). When pulse powders are prepared by grinding pulses before cooking, the cell walls break down( Reference Wursch, Del Vedovo and Koellreutter 22 ) and release starch( Reference Wursch, Del Vedovo and Koellreutter 22 , Reference O'Dea and Wong 25 ). Thus, these powders contain mostly free starches( Reference Wursch, Del Vedovo and Koellreutter 22 ). Previously, it has been shown that pulse powders obtained by grinding raw pulses and then cooking result in a higher peak BG response than after consumption of cooked whole pulses( Reference O'Dea and Wong 25 ) and powders made from cooked whole pulses( Reference Tovar, Granfeldt and Bjorck 24 ).
The role of particle size in the pre-meal glycaemic response was not elucidated by the present study. Despite differences in particle size (navy bean 180 μm or less, chickpea 300–425 μm and lentil 250–600 μm) between the pulse powders, they led to similar BG responses to those for the whole and puréed pulse treatments. Particle size would have been greatest for the chewed whole pulses, and, thus, if it played a major role, BG would have been significantly lower after consumption of the whole pulses.
A lower BG response following the pizza meal was observed only after the consumption of whole navy beans, which suggests that smaller particle size may have accounted for the reduced effect of puréed and powdered navy beans after a second meal. Seed tissue architecture plays an important role in maintaining prolonged glucose absorption of intact pulses( Reference Tovar, Granfeldt and Bjorck 24 ). If particle size was a contributing factor, the larger particle size of the navy been powder should have resulted in a similar postprandial decrease to that of the whole beans following the pizza meal. Furthermore, postprandial BG responses for the lentil and chickpea powders, which were smaller in particle size, should have led to higher responses compared with the whole-pulse treatments. However, lentil and chickpea powders did not differ from either the whole and puréed treatments or the control in Expt 2 and 3, respectively. This observation warrants additional studies to examine the differences between pulse varieties and processing within the same experiments.
The strengths of the present study include that the treatments contained the same amount of available carbohydrate, had amounts of powders reflective of those that would be used as value-added ingredients, and featured powders currently available on the market. However, differences in processing, protein and dietary fibre contents, and particle size of the treatments may be confounders of the outcomes. Also, the experiments may have been underpowered to detect the differences in BG responses between the pulse treatments at specific time points. Finally, the study was a comparison of commercially produced canned pulses with powdered pulses. Previous studies have shown that canning pulses can lead to higher glycaemic responses( Reference Tovar, Granfeldt and Bjorck 24 , Reference Traianedes and O'Dea 34 , Reference Wolever, Jenkins and Thompson 41 ), while others have shown no difference( Reference Wong, Mollard and Zafar 11 ). Thus, to compare with previous studies( Reference Jenkins, Thorne and Camelon 23 , Reference Tovar, Granfeldt and Bjorck 24 ), it would have been informative to have included dried whole pulses cooked as they would be in the home to compare with the whole canned pulses. Finally, the present study was conducted in young normal-weight males, and, thus, further research into the effects of pea fractions on BG regulation in other populations, including females and overweight/obese adults, is necessary.
In conclusion, commercial processing of pulses to a powder form does not alter their low glycaemic characteristics. Pulse powders can therefore be used as value-added ingredients in home cooking and functional foods to improve postprandial glycaemic control. It is expected that the development of such foods will help promote consumption of pulses in convenience foods among individuals who normally avoid them due to taste or perceived inconvenience.
Acknowledgements
The authors acknowledge the participants who volunteered to participate in the study.
The present study was supported by Agriculture and Agri-Food Canada (AAFC) under the Pulse Science Cluster initiative.
The authors’ contribution are as follows: G. H. A., B. L. L., C. E. S. and Y. L. designed the study; Y. L. and T. T. L. conducted the experiments; Y. L., C. E. S., M. F. N., R. C. M. and G. H. A. contributed to the writing and reviewing of the manuscript; Y. L. conducted the primary analysis of the data; G. H. A. had primary responsibility for the final content of the manuscript.
The authors declare that there are no conflicts of interest.





