Obesity and type 2 diabetes (T2D) constitute a major health problem in our society. In 2014, the number of diabetics worldwide reached 387 million and was forecasted to reach 592 million by 2035, with T2D accounting for 90 % of the cases( 1 ). In addition to a sedentary lifestyle, diet is a major player in the development and control of metabolic diseases. Various foods, such as the Mediterranean diet( Reference Perez-Martinez, Garcia-Rios and Delgado-Lista 2 ), fibre-rich diets( Reference Heikkila, Krachler and Rauramaa 3 ), dairy products( Reference Hirahatake, Slavin and Maki 4 ), coffee( Reference Jiang, Zhang and Jiang 5 ) and marine oils( Reference Wu, Micha and Imamura 6 , Reference Yanai, Hamasaki and Katsuyama 7 ), have been associated with protective effects against metabolic disorders( Reference Ran-Ressler, Bae and Lawrence 8 ); however, the active ingredients in foodstuff and their mechanisms of action are largely unknown( Reference Kotarsky, Nilsson and Flodgren 9 ). NEFA( Reference Itoh, Kawamata and Harada 10 , Reference Briscoe, Tadayyon and Andrews 11 ) are known to exert biological effects by acting as precursors of various oxidised messenger molecules and by acting directly on both intracellular and cell surface receptors( Reference Ran-Ressler, Bae and Lawrence 8 ). Their established biological activities suggest fatty acids as interesting potential candidates for active ingredients responsible for dietary health effects. The fatty acid receptors FFA1, FFA2, FFA3 and FFA4 are G protein-coupled 7-transmembrane receptors activated by different groups of NEFA and have all been associated in various ways with T2D and other metabolic and inflammatory disorders. FFA1 and FFA4 are activated by medium- to long-chain NEFA and are believed to be possible therapeutic targets for the treatment of T2D and obesity( Reference Kotarsky, Nilsson and Flodgren 9 – Reference Hirasawa, Tsumaya and Awaji 12 ). FFA2 and FFA3 are activated by SCFA( Reference Le Poul, Loison and Struyf 13 – Reference Nilsson, Kotarsky and Owman 15 ) and are highly expressed in the intestines where SCFA are produced by bacterial fermentation of dietary fibre( Reference Natarajan and Pluznick 16 , Reference Cani, Everard and Duparc 17 ), and may therefore be involved in mediating some of the beneficial effects of dietary fibre on obesity and T2D( Reference Ulven 18 , Reference Offermanns 19 ).
FFA1 is highly expressed in pancreatic β-cells and enhances glucose-stimulated insulin secretion in response to various medium- and long-chain NEFA( Reference Itoh, Kawamata and Harada 10 , Reference Briscoe, Peat and McKeown 20 , Reference Del Guerra, Bugliani and D'Aleo 21 ). The receptor has been clinically validated as a target for treatment of T2D by a phase 2 clinical study with the synthetic agonist fasiglifam( Reference Burant, Viswanathan and Marcinak 22 ). FFA1 is also expressed in enteroendocrine cells where it has been associated with release of glucose- and appetite-regulating hormones such as glucagon-like peptide-1, glucose-dependent insulinotropic polypeptide and cholecystokinin( Reference Luo, Swaminath and Brown 23 – Reference Edfalk, Steneberg and Edlund 25 ). FFA4 is expressed in intestinal enteroendocrine cells, where activation is reported to increase secretion of glucagon-like peptide-1, although this is controversial, and to inhibit secretion of the orexigenic hormone ghrelin( Reference Hirasawa, Tsumaya and Awaji 12 , Reference Engelstoft, Park and Sakata 26 – Reference Paulsen, Larsen and Hansen 28 ). The receptor is also expressed in the pancreas, adipose tissue, macrophages and the brain, where it has been associated with the protection of islets, improvement of insulin sensitivity and the mediation of anti-inflammatory and appetite-lowering effects( Reference Oh, Talukdar and Bae 29 – Reference Wellhauser and Belsham 33 ). Notably, a lack of FFA4 in mice or dysfunctional FFA4 in humans has been linked to increase the risk of obesity( Reference Ichimura, Hirasawa and Poulain-Godefroy 34 ). These observations suggest that FFA4 may protect against diet-induced obesity and improve glycaemic control. In the present study, we examined the activity of dietary fatty acids on FFA1 and FFA4. Of these, pinolenic acid was selected for additional in vitro characterisation, and the potential of pine nut oil and pinolenic acid as anti-diabetic agents was evaluated in mouse studies.
Experimental methods
Materials and compounds
Acetic acid was acquired from VWR, 22 : 5n-6 from Santa Cruz Biotechnology and 5-oxo-6E,8Z,11Z,14Z–eicosatetraenoic acid (5-oxo-ETE) was synthesised according to a published procedure( Reference Tyagi, Shimpukade and Blattermann 35 ). Pinolenic acid (5,9,12-18 : 3n-6), pinolenic acid ethyl ester, 18 : 4n-3, 20 : 3n-3, 22 : 3n-3 and c18, t11, t13-18 : 3n-5 were from Cayman Chemicals, and the remaining NEFA and dimethylsulphoxide (DMSO) were acquired from Sigma-Aldrich. The pine nut oils were acquired from Huilerie Beaujolaise (FA-60), Siberian Pine Nut Oil (FA-61), Siberian Pine Nut Oil enriched with 10 % resin (FA-62) and Siberian Tiger Natural, Inc. (FA-64). 10 % H2SO4 in methanol, butylated hydroxytoluene and water-free methanol were purchased from Sigma-Aldrich. n-Hexane was obtained from Fisher Scientific.
NEFA stock solutions
The NEFA were dissolved in DMSO to 10 mm, unless otherwise stated. The solubility of each stock solution was checked by visual inspection after 100-fold dilution in 10 mm-phosphate buffer at pH 7·4. The stock solutions of the saturated NEFA were prepared on the basis of individual solubility: 6 : 0–10 : 0 were dissolved to 100 mm in DMSO, 11 : 0 was dissolved to 50 mm in DMSO, 12 : 0–14 : 0 were dissolved to 10 mm in DMSO, 15 : 0–18 : 0 were dissolved to 1 mm in DMSO, 19 : 0–22 : 0 were dissolved to 0·5 mm in DMSO and 23 : 0 was dissolved to a saturated solution in DMSO approximately 0·5 mm. The PUFA and oxidised NEFA 24 : 1n-9, 20 : 3n-6, 22 : 4n-6, t10, c12-18 : 2n-6, 16-OH-16 : 0 and 12-OH-18 : 0 were prepared as 5 mm in DMSO and perfluorotetradecanoic acid as 2 mm in DMSO.
Cell culture
Human embryonic kidney (HEK) 293T cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum at 37°C and 5 % CO2. In addition, stable cell lines with tetracycline-inducible expression of the receptor of interest were generated using the Flp-In™ T-REx™ 293 cell system (Life Technologies) as described previously( Reference Hudson, Shimpukade and Mackenzie 36 – Reference Shimpukade, Hudson and Hovgaard 38 ), and utilised to study NEFA receptor-induced Ca2+ mobilisation and dynamic mass redistribution (DMR).
Plasmids
Plasmids encoding either the human or mouse FFA1 or FFA4 (short isoform) receptors with enhanced yellow fluorescent protein fused to their C terminal and incorporating a N terminal FLAG epitope tag (FFA4 constructs only) in the pcDNA5 FRT/TO expression vector were generated as previously described( Reference Hudson, Shimpukade and Mackenzie 36 ).
β-Arrestin-2 interaction assay
β-Arrestin-2 recruitment to either human or mouse isoforms of FFA1 and FFA4 was measured using a bioluminescence resonance energy transfer (BRET)-based approach, as previously described( Reference Hudson, Shimpukade and Mackenzie 36 ). Briefly, HEK 293T cells were co-transfected with enhanced yellow fluorescent protein-tagged forms of each receptor in a 4:1 ratio with a β-arrestin-2 Renilla luciferase plasmid using polyethylenimine. Cells were then transferred into white ninety-six-well plates at 24 h post-transfection. At 48 h post-transfection, cells were washed to remove fatty acids that may be present in the culture medium and the culture medium replaced with Hanks' balanced salt solution immediately before conducting the assay. For FFA4, cells were incubated with 2·5 μm of the Renilla luciferase substrate coelenterazine h at 37°C for 10 min and the cells were then stimulated with NEFA samples for a further 5 min at 37°C. For FFA1, cells were incubated with NEFA samples for 15 min at 37°C. Coelenterazine h (2·5 μm) was then added to the cells for a further 15 min at 37°C. BRET, resulting from NEFA receptor–β-arrestin-2 interaction, was then determined by measuring the ratio of luminescence at 535 and 475 nm using a Pherastar FS fitted with the BRET1 optic module (BMG Labtech).
Ca2+ mobilisation
Ca2+ assays were carried out on Flp-In T-Rex 293 cell lines, generated to inducibly express either FFA4 or FFA1 upon treatment with doxycycline. One day before conducting the experiment, cells were seeded at 50 000 cells/well in black clear-bottom ninety-six-well microplates. Cells were allowed to adhere for 3–4 h before the addition of 100 ng/ml doxycycline to induce receptor expression. The following day, cells were incubated in culture medium containing the Ca2+-sensitive dye Fura2-AM (3 μm) for 45 min. Cells were then washed three times to remove fatty acids present in the culture medium and then allowed to equilibrate for 15 min in Hanks' balanced salt solution (HBSS) before conducting the assay. Fura2 fluorescent emission was measured at 510 nm following excitation at both 340 and 380 nm during the course of the experiment using a Flexstation plate reader (Molecular Devices). Ca2+ responses were then measured as the difference between 340:380 ratios before and after the addition of NEFA samples.
PPAR assay
A mouse embryo fibroblast cell line was used for PPARα, PPARδ or PPARγ transfections. Cells were propagated in Dulbecco's modified Eagle's medium supplemented with 10 % fetal calf serum and antibiotics. For transfections, cells were transfected in solution by Metafectene lipofection, essentially according to the manufacturer's (Biontex) instructions and seeded in Dulbecco's modified Eagle's medium supplemented with 10 % fetal calf serum and antibiotics in ninety-six-well dishes at 24 000 cells/cm2. The transfection plasmid mix included the Gal4-responsive luciferase reporter, the expression vector for the fusion between the Gal4 DNA-binding domain and the ligand binding domain of human PPARα, PPARδ or PPARγ, and a cytomegalovirus promoter driven Renilla normalisation vector. 6 h after seeding the transfected cells, new media containing the DMSO vehicle (0·1–0·5 %), positive control (GW7647 (30 nm) for PPARα, GW501516 (100 nm) for PPARδ or rosiglitazone (1 μm) for PPARγ) or the test compound was added. Approximately 18 h later, cells were harvested and lysates analysed for Photinus and Renilla luciferase activity by luminometry. All data points were performed in at least six replications. Luminometer raw data was analysed in Microsoft Excel spreadsheets and presented as column graphs depicting average values and standard deviations.
Label-free dynamic mass redistribution assay
Cell-based DMR assays were recorded as described previously in detail( Reference Schroder, Janssen and Schmidt 39 , Reference Schroder, Schmidt and Blattermann 40 ), using a beta version of the Corning® Epic® Biosensor (Corning) or the Enspire® benchtop optical label-free system in conjunction with the Mini Janus liquid handling station (Perkin Elmer). HEK 293 (HEK) cells were stably transfected with human FFA1 receptor or human FFA4 using the Flp-In™ T-REx™ system according to the manufacturer's instructions (Life Technologies).
Cells were seeded at a density of 18 000 cells/well (FFA1-HEK, FFA4-HEK and HEK 293) on fibronectin-coated biosensor plates and were cultivated overnight (37°C, 5 % CO2) to obtain confluent monolayers. Afterwards, cells were washed twice with Hanks' balanced salt solution (HBSS) containing 20 mm-HEPES and 0·1 % bovine serum albumin and incubated for at least 1 h in the Epic® reader at 37°C. The sensor plate was then scanned and a baseline optical signature was recorded. Hereafter, compound solutions were transferred into the biosensor plate and DMR was monitored for at least 4000 s. All optical DMR recordings are buffer-corrected. Quantification of DMR signals for concentration effect curves was calculated by maximum response within 1800 s. Data calculation was performed using GraphPad Prism 5·04 (GraphPad Software).
Fatty acid profiling by GC analysis
Fatty acid methyl esters were prepared by acid-catalysed transesterification from TAG of pine nut oil or maize oil( Reference Christie 41 ). Briefly, 1 μl of oil was derivatised at 60°C overnight with 1 ml of 2·5 % methanolic H2SO4 and 20 μl 2 mg/ml butylated hydroxytoluene dissolved in dry methanol. After cooling to room temperature, 1 ml of water and 500 μl of n-hexane were added to the glass vials. Samples were centrifuged and 400 μl of the n-hexane-containing upper phase were transferred into a 1 ml auto-sampler vial for GC analysis. GC analysis was carried out using a Clarus 500 Gas Chromatograph (Perkin Elmer) equipped with a flame-ionisation detector and a capillary column (TR-FRAME, 60 m × 0·25 mm inner diameter, 0·25 mm film thickness). Helium was used as a carrier gas at a constant flow rate of 0·8 ml/min. Samples (5 μl) were injected with 10:1 split ratio. The column temperature was maintained at 140°C for 5 min and then raised at a rate of 3°C/min up to 240°C and maintained for 20 min. The injection port and detector temperature were set to 250 and 260°C, respectively. Total chromatographic run time was 58 min. Chromatograms were processed using Total Chrome Navigator software, peak areas were used to achieve relative quantification of identified fatty acid methyl esters.
Oral glucose tolerance test in mice
Animal procedures were conducted in accordance with the University of Buckingham project licence under the UK Animals (Scientific Procedures) Act (1986) and as approved by the University's Ethics Review Board. Male C57BL/6 mice (Charles River) aged 6–7 weeks on arrival were fed a standard laboratory chow diet that contained 10 % fat, 70 % carbohydrate and 20 % protein by energy (Beekay Feed; B&K Universal Limited). They were housed at 21–23°C with lights on from 07.00 to 19.00 hours. The mice were fasted for 5 h before receiving an oral glucose load (3 g/kg); 30 min before receiving glucose, the mice were given pine nut oil (1 g/kg), pinolenic acid (100 mg/kg) or ethyl pinolenate (100 mg/kg) by gavage. Control mice received maize oil (1 g/kg) and the FFA1 agonist TUG-905 (10 mg/kg) was used as a positive control. The dosing vehicle consisted of 10 % DMSO, 90 % (1:1 PEG400:100 mm-phosphate buffer pH 7·4). The dosing volume was 10 ml/kg. Blood samples were taken from the tail tip for glucose measurement at 30 min before the glucose load and after 30 min. Further samples for glucose only were obtained at 0, 30, 60 and 120 min after the glucose load. Blood samples (10 μl) were mixed with haemolysis reagent and blood glucose measured in duplicate using the Sigma Enzymatic (Glucose Oxidase Trinder; ThermoFisher Microgenic) colorimetric method at 505 and 575 nm using a SpectraMax250 (Molecular Devices Corporation).
Statistical analysis
Data analysis and curve fitting were carried out using the GraphPad Prism software package version 5.0. Potency (pEC50) and efficacy (E max) values for the NEFA were calculated from the BRET and Ca2+ data by fitting to three-parameter sigmoidal concentration–response curves. Reported pEC50 and E max values represent the mean with their standard errors of two to four independent experiments. For statistical comparison of the pinolenic acid curve-fit parameters obtained between human and mouse orthologues or between Ca2+ or arrestin-BRET assays, curve fits were generated for independent experiments and t tests used to establish statistical difference between the mean pEC50 values obtained. For statistical comparison of PPAR data, t tests of treatments against vehicle control were used. Results from fatty acid composition analysis are reported as means and standard deviation. Glucose tolerance data were analysed by two-way ANOVA followed by Bonferroni multiple comparisons against the vehicle-treated group. Results are presented as means with their standard errors. Statistical significance is indicated as * P< 0·05, ** P< 0·01 and *** P< 0·001.
Results
Screening and characterisation of NEFA
Since the solubility is a limiting factor in biological testing of NEFA, the solubility of the compounds was investigated by dilution of DMSO solutions by 100-fold with PBS (pH 7·4). The concentration of the DMSO solution was reduced if PBS dilution resulted in precipitation or clouding. This gave DMSO solutions in the 0·5–100 mm range (see above). Saturated NEFA with longer chain length ( ≥ C24) were insufficiently soluble for testing. Most unsaturated NEFA were prepared as 10 mm-DMSO stock solutions and tested at a maximal concentration of 30 μm. Compounds were generally screened at the highest possible concentration, and below their estimated critical micelle concentrations( Reference Serth, Lautwein and Frech 42 – Reference Mukerjee and Mysels 44 ), on FFA1 in a Ca mobilisation assay and on FFA4 in a β-arrestin-2 interaction BRET assay. Compounds exhibiting a response higher than 20 % relative to the reference compounds (lauric acid for FFA1 and TUG-424 for FFA4) were characterised in full concentration–response curves (online Supplementary Figs. S1 and S2).
Screening of saturated NEFA on FFA1 and FFA4 resulted in the selection of compounds with a chain length of C10–C16 for detailed analysis. The compounds displayed similar potency on each receptor, although 10 : 0 and 11 : 0 appeared 10-fold more potent on FFA1 and vice versa for FFA4, and 14 : 0 and 15 : 0 where somewhat more potent on FFA4 (Table 1). There was a general trend towards higher efficacy for the medium-chain fatty acids and decreased efficacy towards the long-chain congeners for both receptors.
Table 1 Potency (pEC50) and efficacy (E max) values for medium- to long-chain saturated NEFA on hFFA1 and hFFA4
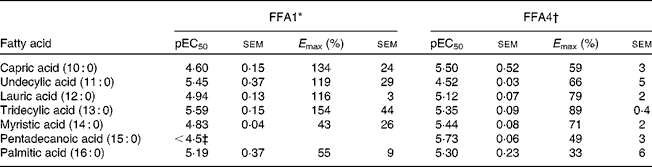
* Determined in a Ca2+ assay, efficacy is given as % response relative to 100 μm-lauric acid (n 2 apart from undecylic acid (n 4), myristic acid (n 3) and pentadecanoic acid (n 3)).
† Determined in a β-arrestin-2 assay, efficacy is given as % response relative to 100 μm-TUG-424 (n 2 apart from capric acid (n 3)).
‡ The response did not saturate; therefore, accurate measure of pEC50 and E max could not be obtained.
Myristoleic acid (14 : 1n-5) and palmitoleic acid (16 : 1n-7) were the most active MUFA with regard to both potency and efficacy on FFA1 and FFA4 (Table 2). Oleic acid (18 : 1n-9), petroselinic acid (18 : 1n-12) and cis-vaccenic acid (18 : 1n-7) displayed reduced efficacy on FFA4. MUFA longer than C18 were not sufficiently active on FFA4 to qualify for full curve testing. All MUFA acted as full agonists at FFA1 except the industrial trans-fatty acid (TFA) elaidic acid (trans-18 : 1n-9), which behaved as a partial agonist (online Supplementary Fig. S1), and nervonic acid (24 : 1n-9), which was inactive. Vaccenic acid (trans-18 : 1n-7), a TFA naturally present in ruminants, showed increased efficacy on FFA1 relative to lauric acid (12 : 0) and the other MUFA (online Supplementary Fig. S1). The low potency of several MUFA precluded accurate calculation of pEC50 and E max.
Table 2 Potency (pEC50) and efficacy (E max) values for MUFA, including trans-MUFA, on hFFA1 and hFFA4
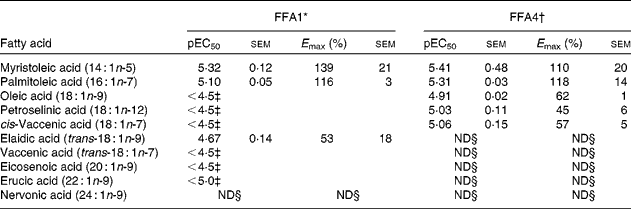
ND, not determined.
* Determined on a Ca2+ assay (n 2), efficacy is given as % response relative to lauric acid.
† Determined on a β-arrestin-2 assay (n 3), efficacy is given as % response relative to TUG-424.
‡ The response did not saturate; therefore, accurate measure of pEC50 and E max could not be obtained.
§ Activity less than 20 % of reference compounds at maximal possible concentration.
The n-6 PUFA linoleic acid (18 : 2n-6) and γ-linolenic acid (GLA, 18 : 3n-6) were both comparably potent dual agonists on FFA1 and FFA4, with GLA tending towards higher potency (Table 3). Linolelaidic acid (all-trans-18 : 2n-6), an industrial TFA, was a full agonist of FFA1, but only a partial agonist of FFA4. Dihomo-γ-linolenic acid (20 : 3n-6), arachidonic acid (20 : 4n-6) and adrenic acid (22 : 4n-6) were equally potent agonists on FFA1 and slightly more potent on FFA4, but displayed decreased efficacy on FFA4 with increasing unsaturation and chain length. The longest n-6 PUFA tested, adrenic acid was a moderately potent full agonist of both FFA1 and FFA4. The ethylene interrupted n-6 PUFA pinolenic acid (5,9,12-18 : 3n-6) was one of the most potent NEFA on both FFA1 and FFA4 and displayed high efficacy on both receptors.
Table 3 Potency (pEC50) and efficacy (E max) values for PUFA on hFFA1 and hFFA4

LA, linoleic acid; GLA, γ-linolenic acid; DGLA, dihomo-γ-linolenic acid; AA, arachidonic acid; ALA, α-linolenic acid; SDA, stearidonic acid; ND, not determined; CLA, conjugated linoleic acid.
* Determined on a Ca2+ assay, efficacy is given as % response relative to lauric acid (n 2, apart from DGLA, adrenic acid, pinolenic acid, eicosatrienoic acid, α-eleostearic acid and ximenynic acid for which n 3).
† Determined in a β-arrestin-2 assay, efficacy is given as % response relative to TUG-424 (same replicate numbers as for the Ca2+ assay).
‡ The response did not saturate, therefore accurate measure of pEC50 and E max could not be obtained.
§ Activity less than 20 % of reference compounds at maximal possible concentration.
The n-3 PUFA α-linolenic acid (18 : 3n-3) and stearidonic acid (18 : 4n-3) were also potent dual agonists. The more highly unsaturated EPA (20 : 5n-3) appeared to be more than twice as potent on both receptors compared with 20 : 3n-3. Of the longer n-3 PUFA, 22 : 3n-3 was the only selective FFA4 agonist among the NEFA, whereas DHA (22 : 6n-3) was a potent dual agonist.
The conjugated linoleic acids c9, t11-18 : 2n-7 and t10, c12-18 : 2n-6 showed moderate dual agonism and slightly higher potency on FFA4 than FFA1, whereas the all-trans isomer t9, t11-18 : 2n-7 was equally potent but exhibited low efficacy on both receptors. The c9, t11, t13-18 : 3n-5 conjugated NEFA was approximately 10-fold less potent on FFA1 compared with the conjugated linoleic acids and more potent but less efficacious on FFA4. Ximenynic acid, a conjugated enyne, was a potent agonist on FFA1 but only a partial agonist on FFA4.
A selection of oxidised, branched and other NEFA was evaluated on FFA1 and FFA4 (Table 4). The keto-NEFA 5-oxo-ETE, a metabolite of arachidonic acid involved in inflammatory processes by activation of the OXE receptor( Reference Powell and Rokach 45 ), was found to be inactive on FFA1 and a potent partial agonist on FFA4. Of the saturated hydroxy-NEFA, only juniperic acid (16-OH-16 : 0) showed activity on FFA1, whereas both 16-OH-16 : 0 (10-fold more potent) and 12-OH-18 : 0 were partial agonists on FFA4.
Table 4 Potency (pEC50) and efficacy (E max) values for oxidised, branched and other NEFA on hFFA1 and hFFA4
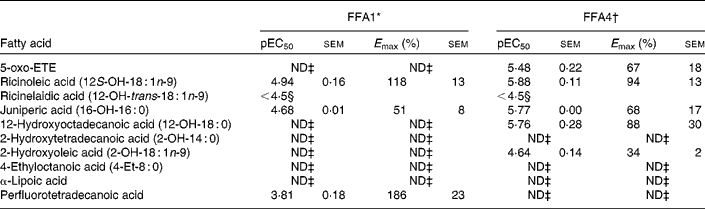
5-oxo-ETE, 5-oxo-6E,8Z,11Z,14Z-eicosatetraenoic acid; ND, not determined.
* Determined on a Ca2+ assay, efficacy is given as % response relative to lauric acid (n 2, apart from ricinoleic acid (n 3), rircinelaidic acid (n 3) and 2-hydroxyoleic acid (n 3)).
† Determined on a bioluminescence resonance energy transfer assay, efficacy is given as % response relative to TUG-424 (n 3).
‡ Activity less than 20 % of reference compounds at maximal possible concentration.
§ The response did not saturate; therefore, accurate measure of pEC50 and E max could not be obtained.
The 12-OH MUFA ricinoleic acid (12S-OH-18 : 1n-9) stood out among the hydroxy NEFA with high potency and efficacy on both FFA1 and FFA4 with EC50 in the low micromolar range and high efficacy, whereas the corresponding TFA ricinelaidic acid (12-OH-trans-18 : 1n-9) was found to be more than an order of magnitude less potent. The perflourotetradecanoic acid is a representative synthetic perfluoroalkyl acid, e.g., found in non-stick coatings in food packing and cookware and suspected to be harmful( Reference Vestergren, Berger and Glynn 46 ). Perflourotetradecanoic acid was a poorly soluble low potency but high efficacy agonist on FFA1.
In vitro characterisation of pinolenic acid
Pinolenic acid was chosen because of its combined high potency and high efficacy on both receptors, and was thus further evaluated in both the Ca2+ and the β-arrestin-2 interaction BRET assay on the human and mouse orthologues of FFA1 and FFA4 (Table 5). Pinolenic acid showed similar potency between human and mouse orthologues of both FFA1 and FFA4, as no statistical differences (P>0·05) were observed between the pEC50 obtained for the two species compared within the same assay format. When comparing between assay formats, it was apparent that pinolenic acid did tend to exhibit lower potency in the β-arrestin-2 BRET assay than in the Ca2+ assay, with significantly lower β-arrestin-2 BRET pEC50 values obtained for human FFA1 (P <0·01), mouse FFA1 (P <0·05), mouse FFA4 (P< 0·05), but not human FFA4 (P>0·05). Overall, the results indicated that pinolenic acid shows similar pharmacology between human and mouse orthologues, and therefore should be suitable for in vivo evaluation in mice.
Table 5 Potency (pEC50) and efficacy (E max) values for pinolenic acid on human (h) and mouse (m) orthologues of FFA1 and FFA4
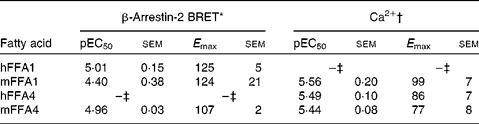
BRET, bioluminescence resonance energy transfer.
* Efficacy is given as % response relative to TUG-424 (n 3 for hFFA1 and mFFA1, n 4 for mFFA4).
† Efficacy is given as % response relative to lauric acid (n 8 for hFFA1, n 4 for mFFA1 and n 7 hFFA4).
‡ See values in Table 3.
Pinolenic acid has previously been reported to activate the nuclear receptors PPARα and PPARδ( Reference Le, Shin and Tu 47 ). We tested the compound at these two receptors and PPARγ, and confirmed full activation of PPARα at 50 μm with a small but significant response already at 10 μm (online Supplementary Fig. S3). Likewise, pinolenic acid was confirmed to activate PPARδ at 50 μm, but only to approximately 20 % of the level of the selective agonist GW501516. A very small but significant response was also observed at 10 μm. Pinolenic acid did not significantly activate PPARγ at up to 50 μm and did not significantly activate any PPAR at concentrations corresponding to the EC50 values at FFA1 and FFA4.
The DMR assay is a label-free technology that captures integrated responses of living cells in real time in a pathway-unbiased yet pathway-sensitive manner. Changes of cytoskeletal rearrangement as a consequence of cell signalling alter the refractive index in the sensing zone above the optical biosensor, which can be monitored by light refraction measurement, and thereby circumvent the need for fluorescent tagging and other labelling that may interfere with the natural cellular processes. Due to the holistic nature of this detection system, it is ideally suited to unravel mechanistic differences of test compounds that mediate their pharmacological effect via targets with pleiotropic signalling( Reference Schroder, Janssen and Schmidt 39 ) but also to expose off-target effects of test compounds under controlled conditions. We therefore characterised pinolenic acid on cells transfected with FFA1, FFA4 or empty vector DNA as control and compared real-time signalling patterns with those induced by the FFA1 agonist TUG-424( Reference Christiansen, Urban and Merten 48 ) and the FFA4-selective agonist TUG-891( Reference Shimpukade, Hudson and Hovgaard 38 ) that we previously developed for both receptors and that have shown beneficial effects on glucose tolerance in rodent models (online Supplementary Fig. S4). We observed robust and concentration-dependent activation by pinolenic acid of both FFA1 and FFA4 but no evidence for divergent modes of receptor activation compared with the synthetic small molecules (Fig. 1). Importantly, the lack of cell responses in mock-transfected control cells indicates selective agonism via FFA1 and FFA4 but also the absence of non-specific perturbation of cell function.

Fig. 1 Concentration–response curves of pinolenic acid from the dynamic mass redistribution assay in FFA1-transfected (a), FFA4-transfected (b) and mock-transfected HEK 293 cells. Values are means, with their standard errors of three independent experiments represented by vertical bars. (a) –○–, hFFA1-HEK;–●–, HEK 293. (b) –○–, hFFA4-HEK; –●–, HEK 293.
Analysis of pine nut oils
Pine nut oil has the highest proportion of pinolenic acid of any natural oil known. The concentration of pinolenic acid in pine nuts from different regions and pine species is known to vary, with the most common nuts used for food oils being Korean pine nuts and Siberian pine nuts containing 13·9–15·0 % and 18·1–18·5 %, respectively( Reference Wolff, Pedrono and Pasquier 49 ). Therefore, four different Siberian pine nut oils were selected and the fatty acid composition analysed using the GC method to determine the amount of pinolenic acid (Table 6). FA-61 was found to contain the highest amount of pinolenic acid and was selected for in vivo studies in mice. FA-60 and FA-62 contained only slightly lower amounts of pinolenic acid, whereas the amount was less than half in FA-64. Maize oil was chosen as a reference. Analysis confirmed a fatty acid composition as reported in the Danish Food Composition Database( 50 ). The oil did not contain pinolenic acid and only trace amounts of other 18 : 3 fatty acids, and compensatory increased levels of 16 : 0, 18 : 1n-9 and 18 : 2n-6.
Table 6 Fatty acid (FA) composition of pine nut oils and maize oil determined by GC analysis* (Mean values and standard deviations)
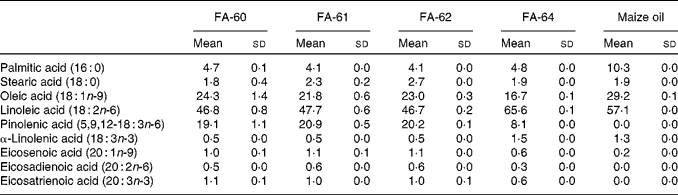
* Means and sd are calculated from three independent replicates.
Oral glucose tolerance test with pine nut oil and pinolenic acid in mice
The effects of pine nut oil and corresponding doses of pinolenic acid and pinolenic acid ethyl ester on acute glucose tolerance were investigated in mice using oral administration (Fig. 2). Maize oil contains a distribution of fatty acids that, apart from pinolenic acid, closely resembles pine nut oil, and was therefore used as a control. The FFA1 agonist TUG-905, an orally bioavailable potent and selective agonist on both human and murine FFA1( Reference Hudson, Shimpukade and Mackenzie 36 , Reference Christiansen, Due-Hansen and Urban 51 ), was used as positive control. Pine nut oil significantly reduced the plasma glucose concentration 30 min after glucose challenge relative to maize oil (P< 0·05). Pinolenic acid ethyl ester and TUG-905 significantly lowered the plasma glucose concentration compared with the maize oil-treated group (t= +30 min, P< 0·001, t= +60 min, P< 0·05) (Fig. 2(a)). The free pinolenic acid was compared in a head-to-head study with the pinolenic acid ethyl ester and demonstrated similar glucose-lowering effects (Fig. 2(b)).

Fig. 2 Oral glucose tolerance test in mice, compounds dosed orally 30 min before glucose challenge. Values are means, with their standard errors represented by vertical bars (n 8). Mean value was significantly different: * P <0·05, ** P <0·01, *** P <0·001. In (b), one high value (>12 mm) excluded at t= +30 in free acid group. (a) –○–, Control (1 g/kg maize oil); –●–, 1 g/kg pine nut oil; –Δ–, 100 mg/kg pinolenic acid ethyl ester; ![]() , 100 mg/kg TUG-905. (b) –○–, Control (1 g/kg maize oil); –●–, 100 mg/kg pinolenic acid (free acid);
, 100 mg/kg TUG-905. (b) –○–, Control (1 g/kg maize oil); –●–, 100 mg/kg pinolenic acid (free acid); ![]() , 100 mg/kg pinolenic acid (ethyl ester).
, 100 mg/kg pinolenic acid (ethyl ester).
Discussion
The receptors FFA1 and FFA4 have previously been shown to respond to long-chain NEFA and are linked to several physiological processes that could have beneficial effect on metabolic diseases, including enhancement of glucose-dependent insulin secretion for FFA1, anti-inflammatory and insulin-sensitising effects for FFA4 and regulation of secretion of incretins and other hormones affecting appetite and plasma glucose( Reference Offermanns 19 , Reference Watterson, Hudson and Ulven 52 ). Both receptors are regarded as potential therapeutic targets for the treatment of metabolic diseases and FFA1 is clinically validated through studies with the selective agonist fasiglifam/TAK-875( Reference Burant, Viswanathan and Marcinak 22 ). As nutrient-sensing receptors, they are likely mediators of effects of food components counteracting obesity and metabolic diseases( Reference Milligan, Ulven and Murdoch 53 , Reference Dranse, Kelly and Hudson 54 ). Apart from the screening reported with the deorphanisation of the receptors( Reference Kotarsky, Nilsson and Flodgren 9 – Reference Hirasawa, Tsumaya and Awaji 12 ), the activity of dietary NEFA on these receptors has not been investigated. Here, we elucidate the agonist properties of a broad selection of long-chain NEFA and further elaborate the structure–activity relationships of NEFA on FFA1 and FFA4. Since it is probable that the two receptors can act co-operatively or synergistically against T2D, we have focused on the effect of the NEFA that co-activate FFA1 and FFA4.
A Ca2+ assay was employed for screening of FFA1, since increased intracellular Ca2+ is the pathway leading to insulin release( Reference Fujiwara, Maekawa and Yada 55 ). β-Arrestin recruitment is relevant to the function of FFA4 as this pathway has been implicated in the anti-inflammatory and insulin-sensitising effects of the receptor( Reference Oh, Talukdar and Bae 29 ); thus, FFA4 screening was performed using a β-arrestin-2 interaction BRET assay. Many of the NEFA investigated here have also been previously characterised on FFA1 and FFA4 by others( Reference Kotarsky, Nilsson and Flodgren 9 – Reference Hirasawa, Tsumaya and Awaji 12 ). Our data generally correspond well with these results. The saturated NEFA were found to be 7- to 10-fold more potent on FFA4 than the reported Ca2+ data by Hirasawa et al. ( Reference Hirasawa, Tsumaya and Awaji 12 ). However, they employed a Ca2+-mobilisation assay, whereas we have used a β-arrestin-2 recruitment assay, and the discrepancy could possibly be explained by a signalling bias towards β-arrestin-2 for these NEFA. None of the previous reports include efficacy data, which is a factor that can result in significant functional differences. For example, FFA1 agonists with high efficacy in Ca2+ response in cells expressing the receptor at physiological levels have been associated with the release of glucagon-like peptide-1, whereas partial FFA1 agonists appear to lack this property( Reference Luo, Swaminath and Brown 23 ). Discrepancies between the reported data for some of the NEFA can probably be explained by their relatively modest potency combined with poor solubility and risk of micelle formation. Furthermore, the amount of bovine serum albumin used in the different assays can dramatically affect the free concentration of NEFA.
The MUFA myristoleic acid was identified as a potent agonist on FFA1 with activity in the low micromolar range and high efficacy. In addition, four MUFA that have previously been reported to activate FFA1 and FFA4 were confirmed, including oleic acid (18 : 1n-9), especially abundant in Mediterranean diet, and palmitoleic acid (16 : 1n-7), a ‘lipokine’ mediating metabolic homeostasis between organs( Reference Cao, Gerhold and Mayers 56 ). The potencies obtained on FFA4 for the n-6 NEFA GLA, dihomo-γ-linolenic acid and adrenic acid corresponded to the values reported by Hirasawa et al. ( Reference Hirasawa, Tsumaya and Awaji 12 ), with GLA appearing to be more potent than the two others. The compounds varied considerably in efficacy, although for many compounds, the curves did not level sufficiently to determine the accurate potency and efficacy. For FFA1, lower potencies (approximately 2-fold) were found for the longer n-6 NEFA with the decreased potency being more pronounced for 20 : 2n-6 and dihomo-γ-linolenic acid (>4-fold) compared with previously reported data( Reference Briscoe, Tadayyon and Andrews 11 ). The positional isomer of GLA, pinolenic acid, was among the most potent and efficacious NEFA on both receptors. All n-3 NEFA tested on FFA1 have been reported to be agonists in the low micromolar range( Reference Kotarsky, Nilsson and Flodgren 9 – Reference Briscoe, Tadayyon and Andrews 11 ). This was confirmed with the exception of 22 : 3n-3, which showed low activity in our assay, but has previously been reported with EC50= 7 μm ( Reference Briscoe, Tadayyon and Andrews 11 ). The difference might be explained by assay variance or by 22 : 3n-3 being a low efficacy agonist relative to lauric acid (12 : 0). 22 : 3n-3 was also the only NEFA deviating substantially from the previously published data on FFA4, being a partial agonist in our assay but previously reported to be inactive( Reference Hirasawa, Tsumaya and Awaji 12 ). This could possibly be explained by a bias of 22 : 3n-3 towards the β-arrestin-2 pathway. Stearidonic acid, a precursor of EPA, was also identified as a particularly potent agonist on both FFA1 and FFA4.
In contrast to most of the other unsaturated fatty acid, TFA are generally associated with detrimental health effects, also in relation to metabolic diseases( Reference Micha and Mozaffarian 57 ). The TFA elaidic acid, vaccenic acid and linolelaidic acid all displayed relatively low potency on FFA1 and low efficacy or no activity on FFA4. The conjugated NEFA, in general, exhibited intermediate potencies on both receptors, apart from the all trans-isomer that was found to have low efficacy. Conjugated linoleic acids are associated with several beneficial health effects, but may have a detrimental effect on metabolic diseases( Reference Kennedy, Martinez and Schmidt 58 ). The conjugated linoleic acids have previously been reported as FFA1 agonists with potencies similar or somewhat lower to what we have shown( Reference Schmidt, Liebscher and Merten 59 ).
Oxidation products of fatty acids often act as potent and specific signalling molecules, including members of the prostanoid, leukotriene, lipoxin and resolvin classes. Ricinoleic acid (12S-OH-18 : 1n-9) appeared to be a more potent and efficacious agonist on FFA4 compared with the corresponding non-hydroxylated oleic acid. The same trend was also observed for the saturated NEFA juniperic acid (16-OH-16 : 0) compared with palmitic acid (16 : 0) and for 12-OH-18 : 0 compared with 18 : 0, however, to a smaller degree. Thus, hydroxylation of NEFA does in several cases seem to increase both potency and efficacy on FFA4. This is in agreement with a recent publication linking a hydroxy-MUFA to intestinal homeostasis through FFA1( Reference Miyamoto, Mizukure and Park 60 ).
The most potent dual agonists for FFA1 and FFA4 included the ethylene interrupted n-6 PUFA pinolenic acid (5,9,12-18 : 3n-6) and the n-3 PUFA stearidonic acid (18 : 4n-3), both with single digit μm EC50-values on both receptors. Pinolenic acid was chosen for further investigation partly due to a tendency towards higher efficacy for this compound. Stearidonic acid (18 : 4n-3) is an intermediate in the conversion of α-linolenic acid (18 : 3n-3) to EPA and the longer chain n-3 PUFA, and its low general abundance can be explained by its efficiency as an enzyme substrate( Reference Walker, Jebb and Calder 61 ). In contrast, pinolenic acid is not converted to arachidonic acid, and is therefore not a likely precursor of eicosanoids, nor has it been found to give rise to chain shortened metabolites( Reference Sugano, Ikeda and Wakamatsu 62 – Reference Tanaka, Uozumi and Morito 64 ). The C2-elongated pinolenic acid 7,11,14-20 : 3n-6 is, however, reported to be formed in macrophages( Reference Chuang, Tsai and Lee 65 ) and to decrease the formation of PGE2 production by competition with arachidonic acid for the cyclo-oxygensae-2 enzyme( Reference Huang, Tsai and Huang 66 ).
It is notable that pine nut oil, containing up to 20 % pinolenic acid, has been associated with effects that potentially can be explained by activity on FFA1 and FFA4. Supplementation of pine nut oil to mice on a high fat diet has been shown to reduce weight gain and intramuscular lipid accumulation compared with soyabean oil( Reference Le, Shin and Tu 47 ). This was explained at least partly by dual agonism on PPARα and PPARδ, nuclear receptors activated by NEFA that are involved in metabolism( Reference Nakamura, Yudell and Loor 67 ). In vivo experiments in rats using Korean pine nut oil also revealed beneficial effects on degenerative disorders such as hypercholesterolaemia, thrombosis and hypertension( Reference Sugano, Ikeda and Wakamatsu 62 ). Additionally, treatment of human hepatocytes with pinolenic acid-enriched NEFA extracts of hydrolysed Korean pine nut oil showed an LDL-lowering effect mediated by an increased cholesterol uptake( Reference Lee, Lee and Lee 68 ). The effect of Korean pine nut oil has also been investigated on overweight post-menopausal women and showed appetite suppressant effects and a significant increase in the levels of the satiety hormones cholecystokinin-8 and glucagon-like peptide-1 compared with olive oil-treated women( Reference Pasman, Heimerikx and Rubingh 69 ). We confirmed activity of pinolenic acid on PPARα and PPARδ at higher concentrations, but did not observe any activity at 3 μm concentration, corresponding to EC50 at FFA1 and FFA4. Furthermore, robust and similar activities were observed with pinolenic acid in the label-free DMR assay in FFA1- and FFA4-transfected cells, whereas the compound was inactive in non-transfected cells, indicating that pinolenic acid-induced cell activation is FFA1 and FFA4 dependent. Together with the expected higher exposure of cell surface receptors compared with nuclear receptors to pinolenic acid, this suggests FFA1 and FFA4 as primary targets for pinolenic acid. Moreover, the complex pharmacology of especially FFA1 has raised the question of whether NEFA and synthetic compounds engage the same signalling mechanisms( Reference Mancini and Poitout 70 ), and the similarity between DMR traces of pinolenic acid and synthetic receptor ligands suggests that they do in this case.
Effects such as glucose-dependent insulin secretion, protection of pancreatic islets, anti-inflammatory and insulin-sensitising effects and secretion of appetite- and glucose-regulating hormones have been linked to either FFA1 or FFA4. The combination of these effects could be expected to robustly counteract metabolic diseases. From this rationale, co-activation of FFA1 and FFA4 appears to be an attractive strategy for treatment of metabolic diseases. Even dual FFA1/FFA4 agonists with relatively moderate potency, such as pinolenic acid, could have potential to give robust effects due to synergistic activities between the receptors. Indeed, pinolenic acid is already associated with some of the effects that would be expected from dual FFA1/FFA4 agonism. Although further studies are required to confirm the effects of pinolenic acid and elucidate to which degree FFA1 and FFA4 are responsible for these, the compound appears to be an interesting candidate for an active ingredient in diets to prevent or counteract metabolic diseases.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451500118X
Acknowledgements
We thank Professor Karsten Kristiansen for useful discussions and Professor Nils J. Færgeman for access to GC equipment. We are grateful to Corning® and Perkin Elmer for providing us with support on the Epic® biosensor and the Enspire multimode microplate reader.
The present study was supported by the Danish Council for Strategic Research (grant 11-116196).
None of the authors has any conflict of interest to declare.
The authors' contributions are as follows: T. U. conceived the study; E. C. and T. U. selected compounds for the study; E. C. acquired or synthesised test compounds, performed solubility tests and dissolved NEFA; K. R. W. and L. J. performed Ca and β-arrestin-2 assays; M. G. and K. S. performed DMR assays; R. K. P. designed and performed PPAR assays; C. J. S. and E. T. W. performed animal studies; E. C., T. U., R. K. P., E. S., K. R. W., B. D. H., G. M., M. G., E. K., C. J. S. and M. A. C. analysed the data; E. C. and T. U. wrote the manuscript; G. M., M. A. C., E. K., C. S. E., K. R. W., B. D. H., C. J. S., M. G., E. S. and R. K. P. critically read and provided feedback; G. M., M. A. C., E. K., T. U., C. S. E. and B. D. H. designed and supervised the studies. All authors approved the final manuscript.











