Flavonoids are a group of polyphenolic compounds with health-related properties that are widely distributed in fruits, vegetables, fruit juices, cocoa, teas and wines. Citrus fruits are rich in flavonoids that have been investigated for their biological activity. The use of citrus flavonoids as anti-inflammatory, anticarcinogenic and antitumour agents has been reported (Middleton & Kandaswami, Reference Middleton, Kandaswami and Harborne1994; Benavente-García et al. Reference Benavente-García, Castillo, Marin, Ortuño and Del Río1997; Montanari et al. Reference Montanari, Chen, Widmer, Manthey and Busling1998). Recent research shows that the citrus flavonoid naringenin stimulates DNA repair in prostate cancer cells (Gao et al. Reference Gao, Henning, Niu, Youssefian, Seeram, Xu and Heber2006), whereas the flavanone glycoside naringin has proved to be a potent inhibitor of angiogenic peptide vascular endothelial growth factor, which is released in human tumour cells (Schindler & Mentlein, Reference Schindler and Mentlein2006). These studies suggest a novel mechanism for mammary cancer prevention, which is considered the most common cancer in female dogs.
Several studies have shown that grapefruit juice elevates the blood levels of some orally taken drugs, primarily by inhibiting intestinal CYP3A4-mediated first-pass metabolism (Fuhr et al. Reference Fuhr, Muller-Peltzer, Kern, Lopez-Rojas, Junemann, Harder and Staib2002; Dahan & Altman, Reference Dahan and Altman2004; Lilja et al. Reference Lilja, Neuvonen and Neuvonen2004; Paine et al. Reference Paine, Criss and Watkins2004, Reference Paine, Criss and Watkins2005), CYP3A4 being a type of cytochrome P450. These studies suggest a potential therapeutic benefit from using the active constituents of grapefruit to increase drug bioavailability. Lowering the effective dose will also reduce drug costs, although potential clinical problems remain (Dahan & Altman, Reference Dahan and Altman2004).
One of the most common flavonoids found in grapefruit (Citrus paradisi) is the flavanone glycoside naringin (naringenin 7-O-neohesperidoside; Fig. 1). Naringin is also known to be the agent responsible for the bitterness of grapefruit juice. Narirutin and naringenin (Fig. 1) are also present in grapefruit but to a lesser extent (Macheix et al. Reference Macheix, Fleuriet and Billot1990).

Fig. 1 Structures and molecular weights (MW) of (A) naringin, (B) narirutin and C naringenin.
Most of the molecular forms of flavonoids that reach the peripheral circulation and tissues are different from those present in foods (Day & Williamson, Reference Day and Williamson2001; Day et al. Reference Day, Mellon, Barron, Sarrazin, Morgan and Williamson2001; Graefe et al. Reference Graefe, Wittig, Mueller, Riethling, Uehleke, Drewelow, Pforte, Jacobasch, Derendorf and Veit2001; Natsume et al. Reference Natsume, Osakabe, Oyama, Sasaki, Baba, Nakamura, Osawa and Terao2003; Zhang et al. Reference Zhang, Hendrich and Murphy2003). In general, the predominant forms in plasma are conjugates (glucuronates or sulphates, with or without methylation). These conjugates are chemically distinct from their parent compounds, differing in size, polarity and ionic form. Consequently, their physiological behaviour is likely to be different from that of the native compounds (Kroon et al. Reference Kroon, Clifford, Crozier, Day, Donovan, Manach and Williamson2004), and their biological effect will ultimately depend on the cellular effects of their circulating metabolites (Harada et al. Reference Harada, Kan, Naoki, Fukui, Kageyama, Nakai, Miki and Kiso1999; Spencer et al. Reference Spencer, Schroeter, Crossthwaithe, Kuhnle, Williams and Rice-Evans2001a, Reference Spencer, Schroeter and Rice-Evansb).
Very little is known about the biological activities of these conjugated metabolites. Glucuronides of isoflavones and epicatechin have been shown to have a much weaker oestrogenic activity and to provide no protection against oxidative stress in cells grown in vitro (Zhang et al. Reference Zhang, Song, Cunnick, Murphy and Hendrich1999; Spencer et al. Reference Spencer, Schroeter, Crossthwaithe, Kuhnle, Williams and Rice-Evans2001a, Reference Spencer, Schroeter and Rice-Evansb), whereas additional studies have shown that the 5-O-β-D-glucuronide of catechin and epicatechin excreted in rat urine does not interfere with their antioxidant properties, as assesed by their ability to scavenge superoxide (Harada et al. Reference Harada, Kan, Naoki, Fukui, Kageyama, Nakai, Miki and Kiso1999; Okushio et al. Reference Okushio, Suzuki, Matsumoto, Nanjo and Hara1999), thus suggesting that in plasma they may still act as antioxidants. Although glucuronides do not readily enter cells, it is also possible that they might be cleaved by the action of β-glucuronidases located in human tissues such as the liver (O'Leary et al. Reference O'Leary, Day, Needs, Sly, O'Brien and Williamson2001) or by neutrophils that release β-glucuronidases when activated (Shimoi et al. Reference Shimoi, Okada, Furugori, Goda, Takase, Suzuki, Hara, Yamamoto and Kinae1998; Simio et al. Reference Simio, Saka, Nozawa, Sato, Amano, Nakayama and Kinae2001).
Initially, only free flavonoids without a sugar molecule, so-called aglycones, were considered to be able to pass across the gut wall (Hollman & Katan, Reference Hollman and Katan1997). However, the absorption of quercetin glycosides from onions in human subjects (Hollman et al. Reference Hollman, Devries, Vanleeuwen, Mengelers and Katan1995) and the presence of the flavanone glycoside naringin in plasma and urine after oral administration (Ishii et al. Reference Ishii, Furuta and Kasuya2000; Fang et al. Reference Fang, Wang, Ma, Su, Bai and Zhao2006) have now been demonstrated.
Liquid chromatography (LC)-MS/MS has emerged as the preferred technology for the quantitative determination of metabolites in different biomatrices, due to its sensitivity and selectivity through MS/MS experiments and the fact that it enables structural identification (Murphy et al. Reference Murphy, Bonate, Kasper, Gillespie and Delong1994). Ionspray ionization, together with tandem MS for structural characterization, has become a popular and versatile method for flavonoid analysis (Roura et al. Reference Roura, Andres-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos2005; Urpí-Sardà et al. Reference Urpí-Sardà, Jáuregui, Lamuela-Raventós, Jaeger, Miksits, Covas and Andrés-Lacueva2005; Fang et al. Reference Fang, Wang, Ma, Su, Bai and Zhao2006).
The use of dogs as a model has been shown to be helpful in evaluating the absorption of flavonoids from green tea (Swezey et al. Reference Swezey, Aldridge, Le Valley, Crowell, Hara and Green2003). Thus, the present study aims to assess the major flavanone forms in plasma after the oral administration of a grapefruit extract; and to evaluate the kinetics of these metabolic forms in the plasma by considering biotransformation, thus providing a general model that can be used for studies on flavonoid bioavailability.
Methods
Chemicals
Naringin (naringenin-7-O-rhamnoglucoside) and blank dog plasma were purchased from Sigma-Aldrich (St Louis, MO, USA). Naringenin (4′,5,7-trihydroxyflavanone), narirutin (naringenin-7-O-rutinoside) and the internal standard taxifolin were purchased from Extrasynthese (Genay, France). Methanol and acetonitrile, HPLC grade and formic acid were purchased from Scharlau Chemie S. A. (Barcelona, Spain). Ultrapure water (Milli-Q) was obtained from a Millipore system (Millipore, Bedford, MA, USA).
Animals and study design
Animals
Ten healthy adult beagle dogs were randomly chosen. The dogs had a mean weight of 13·97 (sd 2·96) kg and were deprived of food overnight before the experiment. Two capsules containing 200 mg grapefruit extract (70 mg flavanones) were orally administered to the dogs; two additional dogs were chosen as controls and were given an excipient containing talc. The grapefruit extract contained naringin, narirutin and naringenin as citrus flavanones. Blood was drawn before capsule administration and at the following times after administrations: 10 min, 20 min, 30 min, 40 min, 80 min, 2 h, 4 h, 6 h, 8 h and 24 h. The dogs were fed with a polyphenol-free diet 2 h after the capsules were given. Blood samples (5 ml) were collected in vacutainer tubes containing EDTA as anticoagulant (Becton, Dickinson, Franklin Lakes, NJ, USA). Plasma was obtained after blood centrifugation at 13 000 g for 15 min and stored in Eppendorf tubes at − 80°C until analysis.
The study was carried out at Isoquimen S. L. (Barcelona), in accordance with the Guide for the Care and Use of Laboratory Animals (Committee on Care & use of Laboratory Animals, 1985). The study protocol was approved by the Isoquimen S. L. Ethics Committee.
Sample extraction procedure for grapefruit flavanone and flavanone metabolites
Flavanone compounds in plasma were extracted by solid-phase extraction as previously described (Roura et al. Reference Roura, Andres-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos2005).
Dog plasma samples were treated as follows: 24 μl of IS (8224 nmol/l) were added to 1 ml of plasma and then was mixed with 370 μl of antioxidant solution (containing 0·2 g/ml ascorbic acid, 1 mg/ml EDTA). After 2 min of vortex-mixing, samples were diluted with 3 ml water. Solid-phase extraction with Waters Oasis HLB 3 cm3 (60 mg) cartridges (Waters Oasis, Mildford, MA, USA) was applied to the mixture. Cartridge activation was achieved by adding 1 ml methanol and 1·5 M formic acid in water (mol/l), respectively. Sample clean-up was performed with 2 ml 1·5 M formic acid in water (mol/l) and 2 ml water–methanol solution (95 : 5 v/v). Flavonoid metabolites were eluted with 1 ml acidulated methanol (0·1 % formic acid). The eluted fraction was evaporated in a sample concentrator (Techne, Duxford, Cambridge, UK) at 25°C under a stream of N gas and reconstituted with 300 ml mobile phase, before being filtered through a 4 mm, 0·45 μm PTFE filter (Waters) into an amber vial insert for LC-MS/MS analysis.
Preparation of the standards and sample treatments were performed in a darkened room with a red safety light to avoid the oxidation of the analytes.
LC-diode array detection
The grapefruit extract was analysed in an HP 1050 (Hewlett-Packard, Palo Alto, CA, USA) liquid chromatograph equipped with an automatic injector (HP1050) and an HP diode array (1050 M) at 280 nm. The conditions for HPLC corresponded to those previously described by Mata et al. (Reference Mata-Bilbao, Andrés-Lacueva, Jáuregui and Lamuela-Raventós2007).
LC-MS/MS
Grapefruit metabolites were identified and quantified by LC-MS/MS plasma analysis. LC analysis was performed using a Perkin-Elmer series 200 (Perkin-Elmer, Norwalk, CT, USA) equipped with a quaternary pump and an autosampler. A Luna C18 column (50 × 2·0 mm internal diameter, 5 μm; Phenomenex, Torrance, CA, USA) was used at room temperature, and the injected volume was 20 μl. Gradient elution was carried out with water (0·1 % formic acid) and acetonitrile (0·1 % formic acid) at a constant flow of 600 μl/min. A gradient profile with the following proportions (v/v) of acetonitrile (0·1 % formic acid) was applied (time in min, % acetonitrile): (0, 5), (2, 25), (7, 90), (9, 100) and (12, 100). The column was equilibrated for 10 min between runs.
A triple quadrupole mass spectrometer (API 3000; Applied Biosystems, PE Sciex, Concord, Ontario, Canada), equipped with a turbo IonSpray source was used to obtain the MS and MS/MS data. TurboIonspray source settings were as follows: capillary voltage, − 3500 V; nebulizer gas (N2), 10 (arbitrary units); curtain gas (N2), 12 (arbitrary units); collision gas (N2), 10 (arbitrary units); focusing potential, − 200 V; entrance potential, 10 V; drying gas (N2), heated to 400°C and introduced at a flow rate of 8000 cm3/min. The declustering potential and collision energy were optimized for each compound with infusion experiments: individual standard solutions (10 ppm) dissolved in 80 : 20 (v/v) mobile phase were infused at a constant flow rate of 5 μl/min into the mass spectrometer using a model syringe pump (Harvard Apparatus, Holliston, MA, USA).
Full scan data were acquired by scanning the mass-to-charge ratio (m/z) from 100 to 600 in profile mode, using a cycle time of 2 s. For MS/MS, a product ion scan utilising a cycle time of 2 s was used. MS/MS product ions were produced by the collision-activated dissociation of selected precursor ions in the collision cell of the triple quadrupole mass spectrometer and the mass analysed using the second analyser of the instrument. Multiple reaction monitoring, the method of choice because of having the highest selectivity and sensitivity in quantitative LC-MS/MS, was used to monitor five transitions for each analysis: naringin, m/z 579 → 271; narirutin m/z 579 → 271; naringenin, m/z 271 → 151; naringenin glucuronide m/z 447 → 271; naringenin sulphate m/z 351 → 271; taxifolin, m/z 303 → 285. Both quadrupoles (Q1 and Q3) were operated at unit resolution. The criteria for identifying grapefruit metabolites, such as retention time, multiple reaction monitoring transition as mentioned above and transitions 579 → 271 and 271 → 151 (at a higher declustering potential value), were chosen to confirm the multiple reaction monitoring trace for each metabolite in collisionally induced dissociation-MS/MS experiments (Roura et al. Reference Roura, Andres-Lacueva, Jauregui, Badia, Estruch, Izquierdo-Pulido and Lamuela-Raventos2005; Urpí-Sardà et al. Reference Urpí-Sardà, Jáuregui, Lamuela-Raventós, Jaeger, Miksits, Covas and Andrés-Lacueva2005).
Pharmacokinetic analysis
Pharmacokinetic parameters were determined by means of a non-compartmental analysis using the WinNonlin Professional software version 3.3 (Pharsight® Corporation, USA). The linear trapezoidal method was used to calculate the area under the plasma concentration curve (AUC0–t) from time 0 until the last detectable concentration. The total area under the curve (AUC0–∞) was calculated by the expression: AUC0–t+ AUCextr, where AUCextr is the extrapolated area under the curve. The maximum plasma concentration (C max) and the time needed to reach C max were determined by visual inspection of the experimental data. Mean residence time (MRT) was estimated by means of the ratio AUMC/AUC, where AUMC is the first moment curve. The parameter C max/AUC was also calculated.
Statistical analysis
The pharmacokinetic parameters for naringin, naringenin and naringenin glucuronide were compared by one-way ANOVA on ranks followed by a Scheffe's multiple comparison test. P < 0·05 was considered significant. The statistical analysis was performed using SPSS software (Version 11.5; Japan Inc., Tokyo, Japan).
Quality parameters relating to the determination of citrus flavanone metabolites in dog plasma
To determine selectivity, dog plasma without any placebo or extract was analysed to discard any endogenous peaks at the same analyte retention time. The linearity of the method was investigated by spiking commercial blank dog plasma with known concentrations of naringin and naringenin at seven concentration levels ranging from 8·62 to 1724·14 nmol/l for naringin, and from 9·19 to 367·65 nmol/l for naringenin. The sample concentration was determined by weighted (1/X2) linear regression of the standard line (Kiser & Dolan, Reference Kiser and Dolan2004). Extraction efficiency (%), as the recovery, was investigated by spiking blank dog plasma with known quantities of naringin and naringenin at different concentration levels within the linear range of the calibration curve (naringin, 8·62–1724·14 nmol/l; naringenin, 9·19–367·65 nmol/l). Replicate analysis of samples containing known amounts of naringin, naringenin and taxifolin prepared in blank dog plasma were conducted to determine precision and accuracy. Repeatability and reproducibility for retention time were also calculated.
Results
Flavanone composition of grapefruit extract
The quantiative results of LC-diode array detection for grapefruit extract flavonoids were as follows: naringin (21·1 %), narirutin (12 %) and naringenin (2·1 %). A quantity of grapefruit extract measuring 200 mg was administered; this contained 42·1 mg naringin, 24 mg narirutin and 4·3 mg naringenin.
Identification and confirmation of citrus flavanones and flavanone metabolites in plasma
The flavanones and their metabolites quantified in dog plasma after the oral administration of a grapefruit extract were naringin (m/z 579 → 271), naringenin glucuronide (m/z 447 → 271) and naringenin (m/z 271 → 151). The chromatograms of these compounds, along with their retention times, are shown in Fig. 2. Although the extract administered contained narirutin as a flavanone glycoside, only the flavanone glycoside naringin could be quantified in its native form in all samples.

Fig. 2 Multiple reaction monitoring chromatogram of dog plasma after an intake of grapefruit extract. (A) naringin (Rt 7·28 min); (B) naringenin glucuronide (Rt 7·49 min); (C) naringenin (Rt 8·24 min).
Product ion scan mode was also applied as a second experiment in order to confirm the identity of the naringenin glucuronide peak, selecting m/z 447 as the parent ion; product ion scan spectra for 447 produced an ion at m/z 271 due to the loss of 176 units, which corresponded to a glucuronic acid. The position of the glucuronide group could not be determined owing to the lack of a reference standard. Nevertheless, naringenin has three possible sites for conjugation: 7-, 4′- and 5-OH, with 5-OH being the least reactive owing to its low acidity (Zhang & Brodbelt, Reference Zhang and Brodbelt2004). Analysis was also undertaken for sulphate metabolites, but these were not detected.
Citrus flavanones and their metabolites were not present in dog plasma at time 0, prior to consumption of the grapefruit extract, or in the control subjects that had been given an excipient.
Quality parameters relating to the determination of citrus flavanone metabolites in dog plasma
The seven-point calibrator concentration showed a linear and reproducible curve with correlation coefficients of 0·9975 and 0·995, respectively. Limits of detection and limits of quantification for naringin were 0·74 and 2·48 nmol/l, respectively, whereas the values for naringenin were 2·06 and 6·91 nmol/l. Recovery (%) of known amounts of naringin and naringenin were 80 (sd 0·11) % and 89 (sd 0·14) %, respectively. The precision and accuracy of the method were determined and have been accepted at all concentration levels (US Department of Health & Human Services, 2001). The repeatability and reproducibility of the retention time were also calculated. Within-day precision (n 10) was 0·9, 1·1 and 6·6 % for naringin, naringenin and taxifolin respectively. Between-day precision, evaluated over a period of 3 d (n 30), was 7·7, 9·5 and 7·8 %, respectively.
Pharmacokinetics of citrus flavonoids after oral intake of grapefruit extract
A flavanone in its native form and two flavanone metabolites were identified in canine plasma after the oral intake of a grapefruit extract. Fig. 3 represents the plasma concentration curves for naringin, naringenin glucuronide and unconjugated naringenin aglycone. Values were expressed as means and standard deviations.

Fig. 3 Time v. plasma concentration curves for naringin (●), naringenin glucuronide (Δ) and unconjugated naringenin aglycone (○) for ten beagles receiving 70 mg grapefruit flavanones. Data were expressed as mean values and standard deviations.
The following pharmacokinetic parameters corresponding to each of the flavanone metabolites (C max, AUC0 → 24, MRT0 → t, time to C max and C max/AUC0 → 24) are summarised in Table 1. Values are expressed as median and range, together with the results of the statistical analysis carried out. There were no significant statistical differences between most of the pharmacokinetic parameters corresponding to naringin, naringenin and naringenin glucuronide. This was not the case, however, with AUC0 → 24, whose values showed significant differences between naringenin and naringin, and between naringenin and naringenin glucuronide. Naringenin had the lowest extended exposure (AUC0 → 24) in plasma, whereas naringin in its native form presented the highest maximum plasma concentration (C max) values, as well as the highest extended exposure (AUC0 → 24). As shown in Table 1, naringenin glucuronide presented the highest MRT (MRT0 → 24; 8 h), followed by naringin (3.3 h) and naringenin (2.8 h). However, interindividual variations in the pharmacokinetic parameters values were observed.
Table 1 Pharmacokinetic parameters of the grapefruit flavanone naringin and its metabolites (naringenin and naringenin glucuronide) in beagles after an oral intake of grapefruit extract (Median values and ranges for ten determinations)
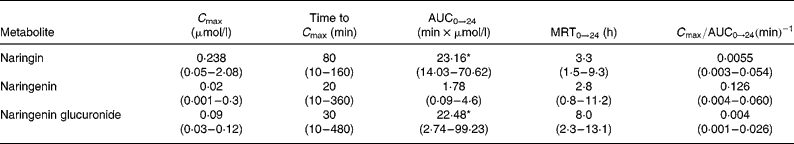
MRT, mean residence time.
* Values were significantly different from those for naringenin: P < 0·05.
Discussion
The metabolic forms that reach the peripheral circulation and tissues may be different from those present in foods, and their biological activity is consequently likely to be different (Kroon et al. Reference Kroon, Clifford, Crozier, Day, Donovan, Manach and Williamson2004). The identification and measurement of flavonoid conjugates are key prerequisites to understand the role of these compounds, since these are the forms that will reach tissues and exert their biological effect. Previous studies of naringin (naringenin-7-O-rhamnoglucoside) metabolism have suggested that sugar moiety cleavage, by gut microflora α-rhamonosidases, is the first step of this pathway, leading to the formation of naringenin, which undergoes rapid glucuronidation or sulphatation in the intestine or liver (Fuhr & Kummert, Reference Fuhr and Kummert1995; Felgines et al. Reference Felgines, Texier, Morand, Manach, Scalbert, Régerat and Rémésy2000; Scalbert & Williamson, Reference Scalbert and Williamson2000). Most studies have applied enzymatic hydrolysis with sulphatase and glucuronidase in order to identify the aglycone naringenin, and thus the individual metabolic profiles are lost during the hydrolysis procedure (Fuhr & Kummert, Reference Fuhr and Kummert1995; Ishii et al. Reference Ishii, Furuta and Kasuya1996, Reference Ishii, Furuta and Kasuya1997; Hollman et al. Reference Hollman, Bijsman, van Gameren, Cnossen, de Vries and Katan1999; Erlund et al. Reference Erlund, Meririnne, Alfthan and Aro2001; Bugianesi et al. Reference Bugianesi, Catasta, Spigno, D'Uva and Maiani2002; Manach et al. Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Damange and Remesy2003; Zhang & Brodbelt, Reference Zhang and Brodbelt2004).
In the present study, a method without prior sample hydrolysis and based on LC-MS/MS technique has been developed. The method is capable of identifying non-transformed naringin and flavanone metabolites in dog plasma after the oral administration of 70 mg citrus flavanone contained in a grapefruit extract. The results of this study corroborate the suggestion that both flavonols and flavanone glycosides can be absorbed as glycosides. However, narirutin (naringenin-7-O-rutinoside), a flavonoid rutinoside also present in the grapefruit extract, could not be detected, probably because of its sugar moiety, which, as previously reported, affects flavonoid absorption (Erlund et al. Reference Erlund, Kosonen, Alfthan, Mäenpää, Perttunen, Kenraali, Parantainen and Aro2000; Olthof et al. Reference Olthof, Hollman, Vree and Katan2000; Rowland et al. Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey2000; Arts et al. Reference Arts, Sesink, Faassen-Peters and Hollman2004; Manach et al. Reference Manach, Scalbert, Morand, Remesy and Jimenez2004; Nielsen et al. Reference Nielsen, Chee, Poulsen, Offord-Cavin, Rasmussen, Frederiksen, Enslen, Barron, Horcajada and Williamson2006).
In recent years, a greater understanding of flavonoid absorption and metabolism has been achieved. Flavonoid glycosides are thought to reach the small intestine intact, and it is believed that they may require deglycosidation for absorption across the intestine (Scalbert & Williamson, Reference Scalbert and Williamson2000; Manach et al. Reference Manach, Scalbert, Morand, Remesy and Jimenez2004). The presence of naringin in the plasma demonstrates that the deglycosidation of naringin is not always necessary for its absorption. Previous studies (Fang et al. Reference Fang, Wang, Ma, Su, Bai and Zhao2006) have administered naringin as a pure compound, whereas in the present study citrus flavanones were administered in the form of a grapefruit extract (as it occurs in nature), in a dose equivalent to that of half a grapefruit. The influence of food matrices must always be considered when interpreting results; it should be borne in mind that they could have been different had pure compounds been used. Other compounds present in the extract could affect the mechanisms involved in the absorption, distribution and elimination of the flavanones studied.
Previous studies using enzymatic hydrolysis have reported plasma concentrations of 1·3–2·2 μmol/l hesperitin metabolites with an intake of 130–220 mg given as orange juice (Manach et al. Reference Manach, Morand, Gil-Izquierdo, Bouteloup-Damange and Remesy2003) and up to 6 μmol/l naringenin metabolites with 200 mg ingested as grapefruit juice (Erlund et al. Reference Erlund, Meririnne, Alfthan and Aro2001). Fang et al. (Reference Fang, Wang, Ma, Su, Bai and Zhao2006) have reported plasma concentrations of 3·8, 0·23 and 43·58 μg/ml for naringin, naringenin and naringenin glucuronide respectively after an oral administration of 746·7 mg/kg naringin as a pure compound. In the present study, 70 mg flavanones given as a grapefruit extract were orally administered, and several pharmacokinetic parameters were calculated for naringin and for each of the flavanone metabolites that have been found in dog plasma. The AUC parameter until the final experimental time (AUC0 → 24) was used to compare the pharmacokinetic parameters because AUCext values were not less than 20 % in all cases.
The differences between naringenin and naringin and between naringenin and naringenin glucuronide in terms of AUC0 → 24 values suggest that the aglycone naringenin had the lower extended exposure. As shown in Table 1, naringenin glucuronide presented the highest MRT (MRT0 → 24), which indicates that this metabolite remains in the body for a longer period of time.
Similar interindividual variations have previously been reported, suggesting that these variations were caused by differences in the gastrointestinal microbiota responsible for the hydrolysis of naringin (Erlund et al. Reference Erlund, Kosonen, Alfthan, Mäenpää, Perttunen, Kenraali, Parantainen and Aro2000; Rowland et al. Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey2000). Interindividual variation is an important factor that must always be taken into consideration when performing dietary assessment studies.
The ratio C max/AUC0 → 24 represents the rate of absorption, and, as expected, the aglycone naringenin was the most rapidly absorbed, probably owing to its greater ability to cross the lipid cell membrane (Mohsen et al. Reference Mohsen, Marks, Kuhnle, Rice-Evans, Moore, Gibson, Debnam and Srai2004). In contrast, naringin and naringenin glucuronide reached their peak concentration at 80 and 30 min, respectively, whereas naringenin reached its C max at 20 min. Although aglycones are known to be absorbed more rapidly, the aglycone absorption was detected here at a much earlier time (20 min) than that reported by Bugianesi et al. (Reference Bugianesi, Catasta, Spigno, D'Uva and Maiani2002), who found that C max was reached 2 h after the ingestion of tomato paste (which contains naringenin aglycone) in men. This result could be due to differences in the species and to the influence of the food matrix.
Three different flavanone forms were found in dog plasma, thus demonstrating grapefruit flavanone absorption after an oral intake of grapefruit extract: naringin in its native form, naringenin and naringenin glucuronide. These results confirm the bioavailability of grapefruit flavanones and their metabolites in beagles after the oral administration of 70 mg grapefruit flavanone. The aglycone naringenin showed the highest rate of absorption but the lowest extended exposure and lowest MRT in the body. Both naringin and naringenin glucuronide showed high extended exposure values, whereas naringenin glucuronide presented the highest values for MRT, remaining in the body for approximately 8 h. This study demonstrates the presence of grapefruit flavanone and its metabolites in dog plasma, and the data could provide a model for further studies, although a greater number of subjects would be necessary to support these results.
Acknowledgements
The authors thank the R&D Department, Affinity Pet-care, Barcelona, without whose support this project would not have been possible. M. L. M. B. is also grateful to the Danone Institute for its partial contribution to the study through her pre-doctoral fellowship.






