Plasminogen Activator Inhibitor Type 1 (PAI-1) is a protein that reduces plasma fibrinolytic capacity. Its production is encoded by a gene with several polymorphisms. The presence of the 4G allele at a common insertion–deletion polymorphism in the promoter of the PAI-1 gene has been associated with elevated plasma PAI-1 concentration and activityReference Ye, Green and Scarabin1. Carriers of the 4G allele (4G/4G and 4G/5G) may be at increased risk of IHDReference Fu, Jin, Song, Zhang, Shen and Huang2, Reference Boekholdt, Bijsterveld, Moons, Levi, Buller and Peter3, but this could depend on interactions with other environmental factors, which may explain why results obtained from Western populations have been ambiguous. On the other hand, previous studies have shown that the presence of the 5G allele in the -675 4G/5G polymorphism decreases gene expression, which predisposes to lower plasma PAI-1 concentrationsReference Eriksson, Kallin, van't Hooft, Bavenholm and Hamsten5. A recent meta-analysis suggests the importance of this polymorphism because of a per-allele relative risk of about 1·06 of coronary disease in individuals with the -675 4G variant of the PAI-1 geneReference Ye, Liu, Higgins, Keavney, Lowe, Collins and Danesh6.
However, it is known that plasma PAI-1 concentration is influenced by many other factors, including diet, smoking, exercise, obesity, fasting plasma TAG concentrations and the insulin resistance syndromeReference Cesari, Sartori, Patrassi, Vettore and Rossi7, Reference Kohler and Grant8. In this context, Sanders et al. have demonstrated in a group of middle-age men that postprandial variations in fibrinolytic activity are modulated by the PAI-1 -675 4G/5G genotype but not by the fat content of a mealReference Sanders, de Grassi, Acharya, Miller and Humphries9. Some data show that the substitution of dietary SFA by MUFA causes a decrease in plasma PAI-1 concentrationsReference Avellone, Di Garbo and Cordova10, Reference Junker, Pieke, Schulte, Nofer, Neufeld, Assmann and Wahrburg11. In this respect, nutrigenetics is emerging as a multidisciplinary field that focuses on studying the interactions between nutritional and genetic factors, and health outcomesReference Junker, Pieke, Schulte, Nofer, Neufeld, Assmann and Wahrburg11. However, it has not been determined whether the presence of the -675 4G/5G polymorphism modifies the response to different diets. In view of the previous evidence, the aim was to determine whether this polymorphism is related to the response of functional plasma PAI-1 concentrations to changes in the amount and quality of dietary fat in healthy subjects.
Experimental methods
Subjects and diet
Fifty-nine healthy Spaniard normolipaemic volunteers (thirty men and twenty-nine women) participated in the study. All subjects were less than 30 years of age, with no evidence of any chronic illness or unusually high levels of physical activity. Mean initial BMI was 21 and remained constant throughout the experimental period. They were encouraged to maintain their regular physical activity and lifestyle, and were asked to record in a diary any event that could affect the outcome of the study. They consumed < 30 g/d alcohol during the study.
The study design included an initial 28 d period during which all subjects consumed a SFA-enriched diet containing 15 % protein, 47 % CHO and 38 % fat (20 % SFA, 12 % MUFA, 6 % PUFA). After this period, thirty subjects received a MUFA-enriched diet for 28 d in a randomized, crossover design. This diet contained 15 % protein, 47 % CHO and 38 % fat ( < 10 % SFA, 6 % PUFA, 22 % MUFA). The MUFA-enriched diet was followed for 28 d by a high-CHO diet containing 15 % protein, 55 % CHO and < 30 % fat ( < 10 % SFA, 6 % PUFA, 12 % MUFA). The other twenty-nine subjects received the CHO diet before the MUFA diet. Cholesterol content remained constant (under 300 mg/d) during the three periods. Virgin olive oil, used for cooking, salad dressing and as a spread, provided 80 % of the MUFA diet. The CHO component of the high-CHO diet was based on the consumption of biscuits, jam and bread. Butter and palm oil were used during the SFA dietary period.
The Human Investigation Review Committee at Reina Sofia University Hospital approved the study. The compositions of the experimental diets have been described previouslyReference López-Segura, Velasco and López-Miranda12. Dietary compliance was verified by analysing the fatty acids in LDL-cholesterol esters at the end of each dietary periodReference Ruiz-Gutiérrez, Prada and Pérez-Jiménez13.
Blood sampling and biochemical determinations
Venous blood for analysis of insulin, glucose, lipid and lipoprotein was collected from the subjects in tubes containing EDTA after a 12 h overnight fast at the end of each dietary period. Each analysis was performed in triplicate. Total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG were assayed by procedures described previouslyReference Allain, Poon, Chan, Richmond and Fu14–Reference Friedewald, Levy and Fredickson17
Plasminogen Activator Inhibitor Type 1 activity
For analysis, blood samples anticoagulated with sodium citrate were collected and PAI-1 activity (control range 3–15 IU/ml) was determined according to Chemielewska et al. using a commercial kit (Spectrolyse (fibrin); Bio-Pool, Umea, Sweden)Reference Chemielewska, Ranby and Wiman18.
Genetic analysis
DNA extraction was performed using standard procedures. The PAI-1 genotypes of the -675 4G/5G polymorphism were determined by means of an allele-specific PCR, which was performed for each allele determination according to conditions previously describedReference Morange, Henry, Tregouet, Granel, Aillaud, Alessi and Juhan-Vague19.
Statistical analyses
Statistical analyses were carried out using the SPSS statistical package version 13 (SPSS Inc., Chicago, IL, USA). ANOVA for repeated measures was used to analyse the differences in plasma lipid and PAI-1 activity between dietary phases. When statistically significant effects were demonstrated, Tukey's post hoc test was used to identify between-group differences. Correlation analysis was performed with Pearson's coefficient of correlation. A value of P < 0·05 was considered significant. All data are presented as means and standard deviations.
Results
The study sample comprises a group of fifty-nine volunteers. Of these, ten were 4G/4G homozygotes, twenty-eight were 4G/5G heterozygotes and twenty-one were 5G/5G homozygotes. Fatty acid composition during each dietary period was analysed on the cholesterol ester fraction of plasma LDL, as we have previously publishedReference Perez-Martinez, Gomez, Paz, Marin, Gavilan-Moral, Lopez-Miranda, Ordovas, Fernandez de la Puebla and Perez-Jimenez20. An enrichment in palmitic acid was observed following the SFA diet, and in oleic acid after the MUFA diet, suggesting that there had been good adherence to the dietary protocol. No differences were recorded between genotypes with respect to age, BMI, smoking status, TAG, HDL-cholesterol and apo A-1 plasma concentration at baseline. However, 4G/4G homozygotes showed higher concentrations of total cholesterol (P = 0·03), LDL-cholesterol (P = 0·01) and apo B (P = 0·01) than did 4G/5G and 5G/5G subjects.
Total cholesterol, LDL-cholesterol and HDL-cholesterol were significantly reduced (P < 0·001) following the consumption of the CHO and MUFA diets as compared with the SFA-enriched diet (Table 1). An effect of the interaction of genotype and diet was observed, because the consumption of a MUFA diet decreases plasma PAI-1 concentrations in subjects carrying the 4G allele (4G/4G, 4G/5G) more than the SFA and CHO diets (P = 0·028; Fig. 1). An effect associated with genotype was also noted, the 5G/5G homozygote subjects showed the lowest plasma PAI-1 concentrations, without any changes as a result of the amount and quality of the dietary fat (P = 0·002; Fig. 1).
Table 1 Plasma lipid, lipoprotein and Plasminogen Activator Inhibitor Type 1 (PAI-1) activity values after each dietary period
(Mean values and standard deviations)
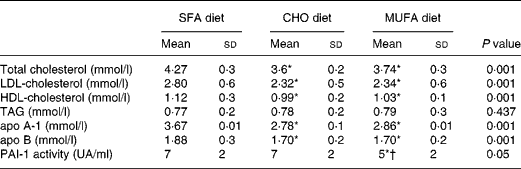
CHO, carbohydrate.
Mean values were significantly different from those of the SFA diet (ANOVA test for repeated measures): *P < 0·05.
Mean value was significantly different from that of the CHO diet (ANOVA test for repeated measures): †P < 0·05.

Fig. 1 Functional Plasminogen Activator Inhibitor Type 1 (PAI-1) levels (UA/ml) during the three dietary periods (■, SFA; □, carbohydrate (CHO); □, MUFA) according to genotype (4G/4G, n 10; 4G/5G, n 28; 5G/5G, n 21). Values are means with their standard deviations depicted by vertical bars. Mean values were significantly different from those of the SFA and CHO diet: *P < 0·05. P genotype = 0·002; P diet = 0·003; P genotype × diet interaction = 0·028.
Discussion
The present results show that the consumption of a MUFA-rich diet for 4 weeks decreases plasma PAI-1 concentrations in subjects carrying the 4G allele (4G/4G, 4G/5G) in the PAI-1 gene promoter, compared with the SFA- and CHO-rich diets, while the 5G/5G homozygotes showed the lowest plasma PAI-1 concentrations, without any changes resulting from the amount and quality of the dietary fat.
In agreement with previous studies which used different experimental designsReference Avellone, Di Garbo and Cordova10–Reference López-Segura, Velasco and López-Miranda12, Reference Perez-Jimenez, Castro, Lopez-Miranda, Paz-Rojas, Blanco, Lopez-Segura, Velasco, Marin, Fuentes and Ordovas21, the present findings confirm that the isoenergetic substitution of dietary SFA by MUFA is associated with lower plasma levels of the fibrinolysis inhibitor. To the best of our knowledge, the current study is the first to examine the association between the -675 4G/5G polymorphism at the PAI-1 gene and functional plasma PAI-1 concentrations in response to dietary intervention. Our most relevant finding, which has not been described before, is the observation that there exists an interaction between the intake of dietary fat and the presence of the 4G allele. Thus, the expected reduction in plasma PAI-1 concentrations was observed only in subjects carrying the 4G allele but not in the 5G/5G homozygotes, although these subjects showed the lowest plasma levels. The lack of a differential response to different dietary fats which we observed in 5G/5G subjects is in agreement with a previous study that tested the response after a test meal that was very high in butter fatReference Byrne, Wareham, Martensz, Humphries, Metcalfe and Grainger22. In that study, subjects carrying the 4G allele also showed an increase in PAI-1 activity. These data confirm the findings of previous reports that PAI-1 activity in 5G/5G homozygotes is lower than in subjects who carry one or more 4G allelesReference Vaisanen, Humphries, Luong, Penttila, Bouchard and Rauramaa23, Reference van der Bom, Bots, Haverkate, Kluft and Grobbee24. In a previous study, Sanders et al. have demonstrated a lower plasma TAG concentration in the subjects homozygous for the 5G alleleReference Sanders, de Grassi, Acharya, Miller and Humphries25. All these data suggest that the PAI-1 promoter -675 4G variant is associated with a higher risk of myocardial infarction. However, subjects are more likely to hyper-respond to the presence of MUFA in the diet because of a greater decrease in PAI-1 concentrations.
A potential mechanism to explain the observed changes in PAI-1 concentrations resulting from different fat intakes lies in the identification by Eriksson et al. Reference Eriksson, Nilsson, Karpe and Hamsten26 of a VLDL-inducible factor that binds a putative VLDL response element located to residues -672 to -657. The VLDL-induced factor was found to bind to the region adjacent to and partly overlapping the binding site of the 5G allele. Competition between the 5G allele-specific transcriptional repressor proteinReference Eriksson, Kallin, van't Hooft, Bavenholm and Hamsten5 and the VLDL-induced factor could explain the 4G/5G allele-specific relations between VLDL TAG and PAI-1 activity levels in plasma. In addition, competitive binding between the 5G allele-specific repressor and the common transcriptional activator could explain the differences in basal transcriptional activityReference Eriksson, Nilsson, Karpe and Hamsten26. This competition between the activator and the repressor will not be present or decreased in 4G/4G and 4G/5G subjects, supporting the lower overall levels of circulating PAI-1 observed in the preesnt study for 5G/5G homozygotes. Alternatively, Chen et al. Reference Chen, Billadello and Schneider27 have identified a fatty acid response element, distinct from the VLDL response elementReference Eriksson, Nilsson, Karpe and Hamsten26, and shown a NEFA-induced increase in the expression of PAI-1 independent of previously reported effects mediated by TAG or lipoproteins. This could mediate the differential responses observed for the various dietary fatty acids in the current study. However, PAI-1 regulation appears to be highly complex and probably tissue dependent and this hypothesis needs to be further tested in vitro and in vivo.
In summary, the presence of the 4G allele at the PAI-1 gene promoter is associated with higher levels of PAI-1. This group, which makes up 40 % of the general population, is sensitive to reductions in SFA or CHO in the diet intake, and responds with a reduction in plasma PAI-1 concentrations when the recommendation to consume a MUFA-enriched diet is followed. In conclusion, the present study suggests that the cardioprotective effect of a chronic intake of a MUFA diet enriched in olive oil could be due, at least in part, to its protective effect on fibrinolytic activity.
Acknowledgements
This work was supported by research grants from CIBER (CBO/6/03), Instituto de Salud Carlos III; CICYT (SAF 01/2466-C05 04 to F. P.-J., SAF 01/0366 to J. L.-M.), the Spanish Ministry of Health (FIS 01/0449); Fundación Cultural “Hospital Reina Sofía-Cajasur” (to C. M. and P. G.); Consejería de Salud, Servicio Andaluz de Salud (00/212, 00/39, 01/239, 01/243, 02/64, 02/65, 02/78); Consejería de Educación, Plan Andaluz de Investigación, Universidad de Córdoba and by NIH/NHLBI grant no. HL54776 and contracts 53-K06-5-10 and 58-1950-9-001 from the US Department of Agriculture Research Service.




