‘What unifies nuclear medicine in Europe is the dissimilarity of models and practice in the European countries’.Footnote 1 This observation, made in a 1998 interview with Peter Ell, head of the Institute of Nuclear Medicine at University College London, highlights the heterogeneity of the field. This was due not only to the diversity of scientific, clinical and industrial stakeholders involved, but also to the different methodologies and roots of nuclear medicine, such as physics, radiology and internal medicine. Moreover, diagnostic and therapeutic needs, as well as the availability of radioisotopes – initially subject to international agreements – varied considerably. The search for common ground in nuclear medicine was therefore challenging and required diplomatic skills to overcome both disciplinary and political boundaries.
This article investigates the emergence of nuclear medicine in Cold War Europe as an outcome of epistemic and methodological negotiations, driven by cross-border exchanges of experts and materials, shared social values and the equal importance of clinical practice and research. It analyses the significance of physicians and facilities in Austria in this process and their contribution to unifying and disseminating medical technologies. To what extent did they make use of the International Atomic Energy Agency (IAEA), established in Vienna in 1957 as an intergovernmental organization to promote the peaceful use of nuclear energy? Did pre-Second World War expertise in radioactive tracer techniques – developed in 1913 by George de Hevesy at the Vienna Institute for Radium Research and the basis of nuclear medicine to this day – provide a foothold for catching up with leading US and UK institutions?Footnote 2 We will explore the challenges and benefits of the field’s heterogeneity for the formation of a professional identity and the interaction with other specialities such as radiology.Footnote 3
Despite the almost simultaneous discovery of X-rays and nuclear radiation in 1895–6, their clinical applicability differed considerably. By the turn of the century, affordable and easy-to-operate X-ray machines, along with their popular and credible images, had already been introduced to patient care.Footnote 4 Treatment with X-rays brought rapid success in cancer and skin diseases, followed by the use of naturally occurring radium as a radiation emitter, which was promoted by American physicians in particular.Footnote 5 In contrast, artificial radioisotopes applicable to humans were first produced in the 1930s with a few high-cost cyclotrons, supplemented from 1946 or 1947 onwards by supplies from the nuclear reactors at the Oak Ridge National Laboratory, Tennessee, and the British Atomic Energy Research Establishment at Harwell, Oxfordshire.Footnote 6 Moreover, it took years of research before nuclear radiation could be used in medical imaging. Initially, cyclotron-produced radioisotopes were applied to patients for therapeutic, not diagnostic, purposes, first by Berkeley haematologist John Lawrence (1904–91) in his work on leukaemia in 1937.Footnote 7 While researchers originally referred to this new field as ‘medical applications of radioisotopes’ or ‘atomic medicine’, which included treating the harmful effects of excessive radiation, the term ‘nuclear medicine’ only gradually gained acceptance.Footnote 8 An important step was its inclusion in the title of the American Journal of Roentgenology, Radium Therapy and Nuclear Medicine in 1952, to be followed by similar changes in European periodicals.Footnote 9
Focusing on multilateral interactions, this article spans the period from the first widespread clinical application of radioisotopes after the Second World War to the founding of the European Nuclear Medicine Society in 1974. In the early years, specialists working in hospitals across Europe were dependent on transdisciplinary and transnational collaboration. Physicists supervised the production of radioisotopes, either in reactors or in the few existing clinical cyclotrons. After transporting the radioisotopes to the individual hospitals, physicians administered them directly or had chemists synthesize the radiopharmaceuticals. However, it was not until the late 1960s that nuclear medicine claimed recognition as ‘a discipline in its own right’, once the first departments, imaging devices and textbooks had emerged.Footnote 10 We argue that the focus on metabolic functions, the integration of diagnosis and therapy, and roots in laboratory science set this emerging area apart from more established specialities, and made it a legitimate and authoritative field of inquiry in both clinical research and practice.
Recent scholarship has explored the role of researchers in international affairs and the influence of science and technology on global governance.Footnote 11 A dynamic field of research on the historical dimensions of science diplomacy has emerged, which draws on foundational studies of scientists as policymakers and post-1945 US–European relations.Footnote 12 Thus far, this has primarily dealt with big-science initiatives, multilateral bodies and transatlantic exchange, with an emphasis on the Cold War.Footnote 13 For example, Angela Creager’s work on the production and medical application of radioisotopes in the United States underscores the complexities of scientific governance, while Maria Rentetzi explores the IAEA’s role in standard-setting and the material culture of nuclear diplomacy, illustrating how policy and practice shape global regulatory frameworks.Footnote 14 Case studies, such as the use of radioiodine in Spain and medical knowledge transfer in the Baltic region, show the engagement of local contexts with broader scientific trends.Footnote 15 In Inspectors for Peace, Elisabeth Röhrlich analyses the IAEA’s ‘paradoxical mission’ of sharing nuclear technologies while seeking to deter the development of weapon programmes.Footnote 16 Further investigations into the post-war bilateral radioisotope supply and its evolution into a global market reveal the economic foundations of scientific exchange.Footnote 17 Notably, Alison Kraft’s examination of medical physics and the clinical use of isotopes in Britain highlights the practical implications of these developments.Footnote 18
Before this diplomatic turn, research into the history of nuclear medicine had mainly been undertaken by practitioners.Footnote 19 These were often written and published to commemorate institutional anniversaries, with the overwhelming majority covering the Anglo-American world and the period before 1950.Footnote 20 In recent years, research on medical imaging and nuclear regulation, notably by Regula Valérie Burri on visualization technologies and boundary work in radiology, has furnished insights into overlapping methodologies.Footnote 21 Historians of science and technology have also produced comprehensive surveys of nuclear physics in Austria, tracing its shifting development in response to regime changes.Footnote 22 In particular, Florian Bayer’s study of the Isotope Station at Vienna University Hospital illustrates its role as an interface within the East–West conflict.Footnote 23 Analyses of neutrality in Austria and other European countries show how scientific cooperation was pursued amid political tensions, cultivating a notion of impartiality later embraced by multilateral organizations.Footnote 24
Building on these contributions, our article sets out to further disentangle the roles of under-explored actors, sites and fields in transnational scientific exchange: physicians, hospitals and medical specialities, all of which required and relied upon consistent methods and regulations. By focusing on small-sized laboratories and local practitioners, we uncover new facets of science diplomacy, highlighting the significant role these actors play in the broader dynamics of Cold War knowledge production. In this context, the booming clinical use of radioisotopes in post-1945 Europe – which was marked by an intricate interplay of geopolitics, technological innovations and ethical issues – is a useful case study both for the HSTM community and for scholars in international relations and health studies. Drawing on (archival) sources from eminent nuclear physicians and (inter)national bodies, we will examine the power-based dynamics and individual strategies around the consolidation of this emerging field. Using the example of how these stakeholders engaged in community building, we ask under which changing conditions, goals and profit expectations did early practitioners of nuclear medicine collaborate? Which resources did they tap? And which ‘standards’ did they favour (local, national, European)? What does this mean for the transdisciplinary realities and claims of nuclear medicine, including in competition with other disciplines? In this context, we understand transdisciplinarity as an approach that goes beyond interdisciplinary collaboration, integrating practical clinical expertise and shared values into a theoretical and methodological framework that connects scientific inquiry with societal challenges.Footnote 25 Rather than concentrating on the (inter)national policies involved in specialization, we will analyse it as a multifaceted negotiation process on three levels: diplomatically, through exchange programmes and multilateral bodies; practice-oriented, within clinical laboratories and their instrumentation; and professionally, in nuclear-medicine societies and conference series.
Diplomatic arenas: exchange programmes and multilateral bodies
There is not one single branch of medicine where isotopes have not found some application … However valuable a diagnostic test may appear to be when it is tested on an experimental basis, the fact remains that its ultimate acceptance must depend on extensive practical trials by the clinicians concerned … A prerequisite to the acquisition of the necessary clinical experience with these new methods is that they should be more generally available than is the case at present.Footnote 26
When the British physicist Norman Veall (1919–91) and the Austrian physician Herbert Vetter (1920–2009) published the first European textbook on Radioisotope Techniques in Clinical Research and Diagnosis (1958), tracer technologies were not a medical speciality, but a method.Footnote 27 Radioisotopes were already used for therapy in many specialities, and their diagnostic applications had become routine in a few hospitals, such as the Massachusetts General Hospital (Boston) and the Hammersmith Hospital (London).Footnote 28 In continental Europe, however, nuclear medicine had less well-equipped facilities and less government or industrial support on which to rely. Many young clinicians devoted themselves to this novel method on their own initiative and in addition to their routine duties. The backgrounds of these practitioners were very different: in West Germany and Spain (as well as the United States), they were primarily radiologists and radiotherapists; in the United Kingdom and the Soviet Union, nuclear physicists and engineers; in France and Scandinavia, biophysicists and physiologists; and in Austria and several Eastern bloc countries, internists and surgeons.
For this pioneering generation, particularly in Central Europe, war damage, isolation and the emigration of outstanding scientists during the Second World War made the re-establishment of international relations a necessary part of scientific research. On one level, this concerned the procurement of hands-on medical knowledge from the United States. There, the radioiodine therapy established by Saul Hertz (1905–50) for the treatment of hyperthyroidism had opened up a fresh field of application, promising efficacy and – if extended to other thyroid disorders – a large number of patients.Footnote 29 In addition, many European countries relied on the import of radioisotopes due to a lack of production facilities. Before these compounds became available from industrial suppliers in the 1960s, US and UK distribution networks dominated the growing isotope market. Their development into a global business was closely linked to the establishment of training programmes at Oak Ridge and Harwell, where (inter)national participants were schooled in handling these technologies and their potential for civilian research.Footnote 30
This transfer of radioisotopes and specialist knowledge was based on political agreements granting friendly nations access to the technologies available at US and UK facilities. As a means of ‘diplomacy’, exchanges with allied and neutral countries were intended to convince their stakeholders of the technological leadership of the ‘Western world’ and its way of life. Newly created fellowships, such as the US Fulbright and the British Council programmes, strengthened the ties of promising foreign researchers to the Anglo-American communities.Footnote 31 For some nations, such as Sweden, stays abroad by individual researchers preceded the development of nuclear-medicine research within the country; in others, such as France (which launched its own radioisotope distribution programme in the mid-1950s), fellowships provided career support and access to infrastructure not yet available at home.
The US Atomic Energy Act of 1946, resulting from a debate between proponents of Marshall Plan-style scientific internationalism and national monopoly, allowed civilian purchase of reactor-produced isotopes but restricted cross-border sharing, including with Britain.Footnote 32 In the long run, the Act had the opposite effect to its intent: the United Kingdom developed its own government-sponsored isotope industry, which surpassed the capacity of Oak Ridge as early as 1949, with five thousand shipments per year.Footnote 33 Commercial interest in clinically useable radioisotopes and the significance of continental Europe as a sales market for British compounds increased in the following years. In London hospitals, alliances between industrialists and medical physicists such as William Mayneord (1902–88) sought new therapeutic applications in oncology and developed measuring equipment for diagnosis.Footnote 34 By 1956, no less than 178 researchers from Western Europe, including many future nuclear physicians, were attending the Isotope School at Harwell, which also offered clinical placements.Footnote 35
With the exception of non-aligned Yugoslavia, the communist countries of Eastern Europe were excluded from these exchange programmes. However, World Health Organisation (WHO, founded in 1948) fellowships provided their physicians with access to ‘Western’ technologies.Footnote 36 Moreover, the French isotope distribution programme supplied individual countries such as Poland, until some of their needs could be met by their national nuclear industries in the 1960s. The Soviet Union also provided radioisotopes to the Eastern bloc, but due to the overlap between its medical research and weapons programmes, Soviet scientists focused more on radiation biology and protection from the harmful effects of radioactivity.Footnote 37 Since local production and Soviet imports fell well short of medical demand in many communist countries, national authorities responsible for supplying radioisotopes had to buy them from the ‘West’ using foreign currency.
Austria occupied a special position in this post-war political landscape. Until the signing of the State Treaty (1955) during the Cold War thaw (on the condition that the new state committed to neutrality), Austria’s territory and capital were divided between the four Second World War Allies.Footnote 38 Research at the Vienna University Clinics, once highly regarded in fields such as internal medicine, had fallen behind major European hospitals: there was a lack of equipment and pharmaceuticals, the infrastructure had been damaged and a significant proportion of the staff had been displaced or murdered. The Academy Institute for Radium Research, which before the Second World War was among the leading institutions for studying radioactive compounds, was similarly affected – although contacts with British physicists enabled the institute to arrange the import of Harwell radioisotopes from 1949. From then on, it served as a national distribution centre.Footnote 39 Despite little interest from industry, by 1955 about thirty medical facilities in Austria had become customers, attracted by the promising technology and the initial funding from the Rockefeller Foundation (New York). Clinicians of various backgrounds experimented with therapeutic and diagnostic procedures for heart, spine and tumour diseases by in vivo injection or superficial application of iodine-131, cobalt-60, gold-198, phosphorus-32 and sodium-24. Overall, however, the supply of radioisotopes did not keep pace with the transfer of expertise on their proper use, and the therapeutic benefits hardly justified the logistical efforts involved:
The isotopes landed at the British-occupied Schwechat airport and were transported to Vienna in army vehicles [unchecked by Soviet officials – from the Radium Institute, they were carried to the hospital on foot]. To get them through customs, they had to be declared as ‘rare earths’ … Deliveries were infrequent, irregular, and with little warning … The doses administered amounted to several millicurie, because the measuring apparatus was very insensitive … Equipment failures were frequent at first; by the time they were fixed, the sodium-24 had often degenerated.Footnote 40
The Zweite Medizinische Universitätsklinik (Second Medical University Clinic) in Vienna emerged as a hotspot for radioisotope applications. The initiative stemmed from the young internist Herbert Vetter, who had survived the war in Vienna despite his Jewish origins. Physicists at the Radium Institute built the instrumentation for his first studies, including a gamma ray counter. Soon, Rudolf Höfer (1923–2023) joined Vetter in setting up the clinic’s isotope laboratory. By 1955, its staff had already performed up to four thousand examinations, including radioiodine uptake tests (to examine thyroid function), liver blood flow tests and tumour localizations (Figure 1).Footnote 41 The novel method found an influential advocate in clinic director Karl Fellinger (1904–2000), later president of the University of Vienna. Excluded from teaching under the Nazi regime, he pursued the goal of ‘bringing Viennese medicine up to the level of Western countries’.Footnote 42 For this purpose, he promoted international contacts and introduced into clinical practice techniques from other disciplines within the natural sciences, such as radioactive tracers, dialysis machines and electron microscopes.Footnote 43 To secure public funding, Fellinger touted to the press the non-invasiveness and broad applicability of radioisotope techniques, as ‘an important tool, especially in the fight against cancer’.Footnote 44
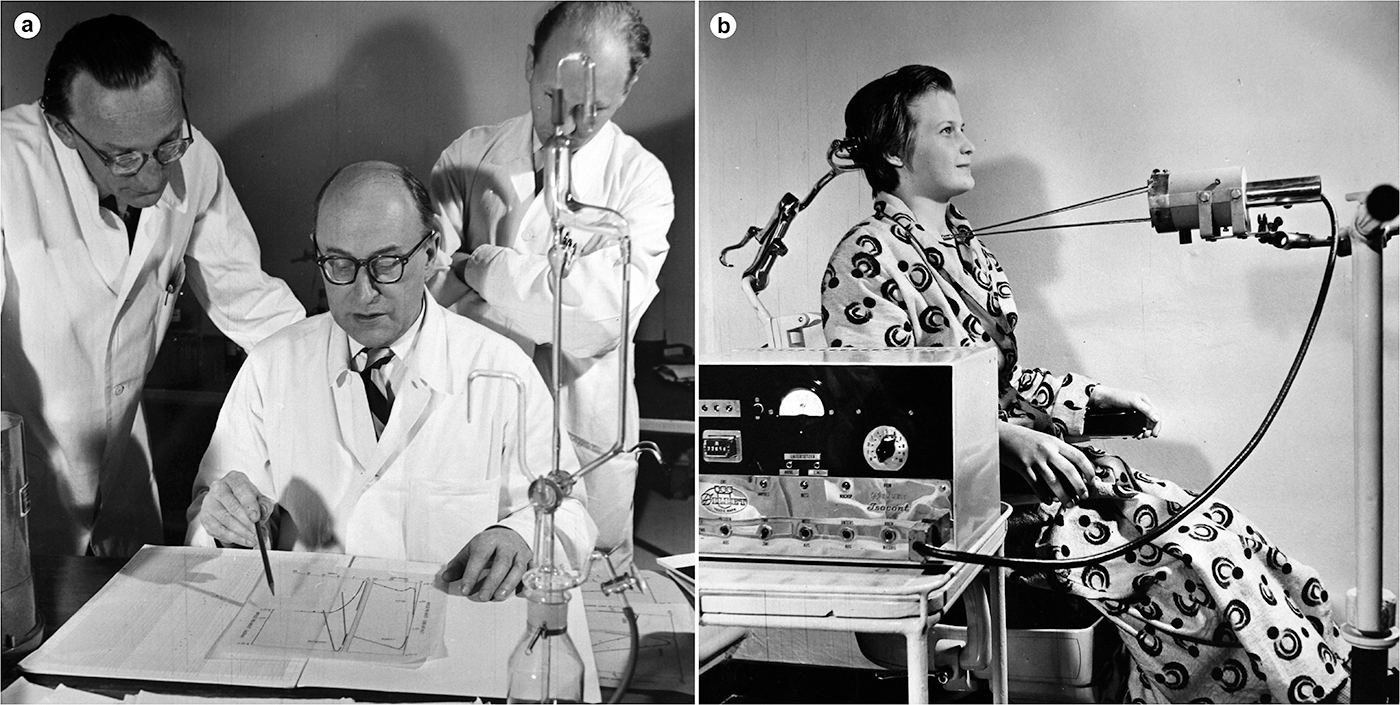
Figure 1. Discussion (a) (left to right: Herbert Vetter, Karl Fellinger, Rudolf Höfer) and operation (b) of a thyroid function test in the isotope laboratory of the Second Medical University Clinic, 1955. The measuring device (Geiger–Müller tube) is housed in the cylindrical lead shield, with the rods resting against the patient to ensure the correct distance to the device. Medical University of Vienna, Nuclear Medicine Division, Archive.
Visits by practitioners to leading US and UK clinics, emphasizing the orientation of the emerging field towards the ‘West’, were instrumental in realizing these ambitions. In 1951–2, as a British Council fellow, Vetter conducted research with Veall at the London Medical School and returned to Vienna with instruments to introduce in vitro analysis of blood and urine samples. In 1956–7, Höfer was invited as a Fulbright fellow to collaborate with John Lawrence at the Donner Laboratory, Berkeley, followed by a research stay in London.Footnote 45 In 1962–3, the Tyrolean surgeon Georg Riccabona (1933–2019) travelled to the Massachusetts General Hospital to work with John Stanbury (1915–2015), a specialist in goitre caused by iodine deficiency in Patagonia. Back at Innsbruck, Riccabona set up a laboratory based on the Boston model and initiated research on goitre in remote alpine valleys.Footnote 46
Research visits to UK institutions developed into bilateral collaborations, such as joint publications, benefiting both British physicists and Austrian physicians. From a clinical point of view, physicists relied on practitioners to accredit and disseminate isotope techniques, and in the United Kingdom there was little interest in this high-effort medicine outside a few major hospitals.Footnote 47 In Austria, on the other hand, physicians were treating large numbers of patients, had their costs covered by the national health insurance, and were using their clinical data to improve the feasibility of new techniques. From a scientific point of view, the treatment of thyroid disorders, which progressed methodologically in Britain and clinically in Austria, showed early success and promised application in countries throughout the global South.Footnote 48 From a geopolitical point of view, British scientists were able to strengthen their presence in continental Europe and gain influence in the German-speaking communities, which maintained good relations with colleagues behind the Iron Curtain. Austrian stakeholders, in contrast, sought partners to reclaim a role on the international stage.
In the early 1950s, the superpowers’ arms race and US president Eisenhower’s ‘Atoms for Peace’ speech before the United Nations, in which he announced the supply of nuclear information to friendly nations for civilian purposes, brought atomic research into the public eye.Footnote 49 Opening a wide-ranging campaign, Eisenhower advocated using international relations to turn these technologies into a tool for peace, health and prosperity. In Austria, alliances among political, industrial and (medical) science stakeholders, which were intended to strengthen the country’s economy and link it to the ‘West’, also supported the construction of national research reactors.Footnote 50 Shortly after regaining sovereignty from the Allies, the government accepted a cooperation offer from the United States, which in turn intended to expand its influence in the new buffer state between the superpowers.Footnote 51 Previously, nuclear physicians had advised on the process of deciding on Austria’s resumption of nuclear research. As delegates to two United Nations conferences on the peaceful uses of atomic energy in Geneva (1955 and 1958), they promoted their clinical studies as a significant national contribution to worldwide nuclear research.Footnote 52 In doing so, they benefited from Austrian foreign policy, using its geopolitical position to host multilateral organizations such as the IAEA.
Established in 1957 under United Nations auspices, the IAEA regulated the hitherto bilateral exchange of fellows and technologies, becoming a driving force for the global dissemination of radioisotope techniques. The choice of a neutral country for the headquarters, which until 1979 were located in a former grand hotel next to the Vienna Opera House, marked Austria’s re-entry onto the diplomatic stage.Footnote 53 In its founding years, the IAEA, staffed by 250 researchers and diplomats, mostly of Austrian, American, British and Soviet citizenship, viewed itself as a ‘scientific and technical organization’.Footnote 54 Henry Seligman (1909–93), previously head of the Isotope Division at Harwell, became the agency’s ‘chief scientist’ and deputy director general for research and isotopes. His friendship with Vetter led to the latter’s appointment as head of the Medical Section. Based on his experience as a clinician, Vetter interpreted nuclear medicine narrowly as a speciality encompassing scientific and technical knowledge, but to be practised by medical doctors autonomously and at their own risk. This understanding influenced the section’s initial work programme, placing the emphasis on sharing clinical practice, rather than telling physicians which radiation ‘doses … should be considered permissible’ for a specific treatment.Footnote 55
By 1962, the section already had an impressive track record: the award of two hundred fellowships to physicians and physicists from low- and middle-income countries for residencies in renowned hospitals; the organization of training courses globally; and the secondment of experts from industrialized countries on twelve technical-assistance missions to East Asia, Latin America and the Middle East to establish isotope laboratories.Footnote 56 Additionally, the section awarded contracts either to hospitals in middle-income countries to conduct on-site studies of common diseases, or to leading facilities to explore new applications. Its workshops became a hub for novel methods, starting with the Vienna Symposium on Medical Radioisotope Scanning (1959), the IAEA’s first scientific conference (Figure 2).Footnote 57 One of the section’s most notable impacts was its pivotal role in advancing the quality assurance of clinical procedures. Working groups compiled manuals with representative radiation dose distributions, a significant asset for radioiodine uptake testing. Even though this was the ‘most common medical use’ of radioisotopes, ‘hardly two laboratories in the world’ applied ‘exactly the same technique’.Footnote 58 To reduce such inconsistencies and to calibrate local equipment, an employee of the section travelled the world in the 1960s with mannequins containing an artificial thyroid gland.Footnote 59 In some of these projects, the IAEA cooperated with the WHO. However, the latter was more concerned with the negative effects of radiation, such as contamination of food, and so did not become substantially involved in joint ventures before the 1970s.Footnote 60

Figure 2. Vienna Symposium on Medical Radioisotope Scanning, 1959. Front row, left to right: Harold Johns (Toronto), Rudolf Höfer (Vienna), Luigi Donato (Pisa), Gordon Brownell (Boston), Merrill Bender (Buffalo), Franz Bauer (Los Angeles). Around forty experts from twenty-one countries took part. IAEA Archives, IAEA-ARC-AV-PH-01-01-C0327-001.
Between 1960 and 1966, the share of medical research in the IAEA’s project expenditure increased from 5.5 per cent to 27 per cent, turning the section into the most significant multilateral promoter of clinical radioisotope applications.Footnote 61 Through the provision of fellowships and contracts to the same individuals in sequence, the section created groups of experts in countries throughout the global South, but also gave practitioners from industrialized countries the opportunity to share their ‘standards’ globally as envoys of IAEA missions. Since political neutrality could be an advantage in such science diplomacy activities, especially in communist countries, and since Vetter remained affiliated with the University of Vienna despite his IAEA duties, the section often chose Austrian physicians for its missions. For example, by 1970, Höfer alone had participated in missions to Egypt, Latin America, Romania, Sudan, Syria and Tunisia. In addition, the IAEA awarded research contracts to Austrian facilities, some of which developed into individual collaborations after the completion of the IAEA laboratories (1963) in Seibersdorf (near Vienna). By 1966, Austrian facilities had received more IAEA project funds than those of Britain, France, West Germany, the Soviet Union or even the United States.Footnote 62
This leads us to two preliminary conclusions. First, post-1945 power politics, commercial interests and progress expectations associated with nuclear research played a significant role in shaping radioisotope applications. Science diplomacy programmes steered bilateral and multilateral exchanges, using these as ‘political instruments’ to gain influence over stakeholders in target countries and to open export markets for new technologies.Footnote 63 These collaborations bridged tensions and created a common reference to the same bodies of knowledge and practice. They thus became a means of acculturation, enabling physicians at lesser-known institutes to adopt the methods, tools and values of a few leading institutions. Second, as clinicians and officers of multilateral bodies, Austrian physicians shared responsibility for positioning radioisotope techniques as a medical field. They drew on the resources of the IAEA in Vienna and on on-site traditions in atomic research. Acting as intermediaries, they sought cooperation with renowned facilities in the ‘West’, but from the 1960s onwards they also involved colleagues from Eastern bloc countries. As we will argue in the following section, hospital laboratories became an important site of community building, where medical and scientific claims met and competed with other specialities at the level of routine practice.
Clinical practices: laboratories and instrumentation
Hospital laboratories and their devices are vital to how nuclear-medicine professionals position themselves today. Their expertise in the deployment of technologies for non-invasive procedures, together with standardized workflows and quantitative models that support the reliability of their methods, enables them to navigate hierarchies within and beyond the field while collaborating with (other) clinicians, scientists and technicians. When the first studies with radioisotopes began in individual hospitals across Europe around 1950, however, practitioners ‘did not have an army of highly skilled technicians [and] palatial laboratories’ at their disposal.Footnote 64 Nor were there any guidelines for running these facilities properly and protecting patients and staff. As Zdeněk Dienstbier (1926–2012), head of the first isotope laboratory in Czechoslovakia, put it, ‘What did those poor physicians know about terms like millicurie or integrator?’Footnote 65
In contrast to other branches of nuclear science such as energy research or particle physics, where Cold War competition also mobilized public funds to construct large-scale research centres and accelerators, nuclear medicine in Europe developed in small-size laboratories under what might now be considered amateurish conditions.Footnote 66 Vacant basement rooms or former animal stalls often served as laboratory spaces. These were divided into patient-accessible areas for in vivo administration and detection, and staff-only sections for data analysis and in vitro studies. Since the equipment could not be obtained from industrial suppliers at first, physicists and engineers built or modified it in workshops attached to these laboratories:
[At the London University College Hospital], our earliest linear scanners, replying [sic] on tape measures, plastic tables, and hand-held lead collimators could be designed to their rather recondite research purposes years before commercially available equipment was thought of … Even the basic unit, the Geiger–Müller counters, involved journeys by bus into South London to a small, skilful workshop to watch the necessary tubes being glass blown, and to select 3 or 4 of these … in the hope that one would count reliably.Footnote 67
In these pioneering years, innovation and translation into hospital practice went hand in hand. The treatment of large patient numbers made applications into full-scale clinical trials, and their outcomes, although sometimes less promising, became evidence for the mechanism of new tools or inspired further research. Although many of the experimental techniques developed in individual hospitals across Europe did not gain (inter)national acceptance, they served as a basis for negotiating standards at the local level. Early textbooks such as Atomic Medicine (1949) and The Practice of Nuclear Medicine (1958) contributed to the spread of applications developed mainly in the United States.Footnote 68 The aforementioned textbook Radioisotope Techniques (1958), on the other hand, placed more emphasis on the clinical context and lesser-known procedures from European institutions, making it a resource for teams seeking to test novel methods. Isotope laboratories not only made hospital practice more ‘objective’ by shifting from physicians’ experience to the results of laboratory techniques, but also explored their ‘practical value and limitations’ and implemented quality assurance measures.Footnote 69 In this regard, on collaboration within a laboratory at the Royal Marsden Hospital (Sutton), the physicist Nigel Trott (1919–2004) stated,
We set about devising new therapy procedures … teaching ourselves how to prepare and maintain standards and exploring new areas of clinical investigation. A major effect arose from attempts to localise brain tumours … Although this did not lead to an established diagnostic procedure, it did give us some hope for the future.Footnote 70
Between 1955 and 1965, innovations on several levels turned radioisotope techniques from a laboratory method into a booming diagnostic field. In terms of radiopharmaceuticals, the use of radiolabelled compounds rather than pure isotopes broadened applicability and reduced radiation exposure to patients.Footnote 71 The introduction of technetium-99m as a versatile diagnostic tracer, along with portable technetium generators, made regional hospitals eligible for isotope techniques and less dependent on daily supply.Footnote 72 In terms of analytical methods, the radioimmunoassay developed in 1959–60 by the later Nobel laureate Rosalyn Yalow (1921–2011) laid the foundation for many in vitro tests that soon outnumbered those performed on patients.Footnote 73 Mathematical models, like compartmental analysis, provided information on circulatory and metabolic functions such as blood volume and renal excretion. Finally, in terms of instrumentation, the construction of one of the first rectilinear scanners in 1950 by Benedict Cassen (1902–72) and the gamma (or scintillation) camera in 1957 by Hal Anger (1920–2005) made it possible to visualize organs such as the liver, heart and brain, and to locate tumours by measuring tracer accumulation.Footnote 74 Whereas previously (handheld) Geiger–Müller counters were used to detect administered compounds and record them in the form of graphs (Figure 1), scanners enabled nuclear emissions to be captured in two-dimensional images by moving a detector over a patient (Figure 3). Automation led to the emergence of whole-body scanners in the mid-1950s, along with computer systems for data processing and storing.
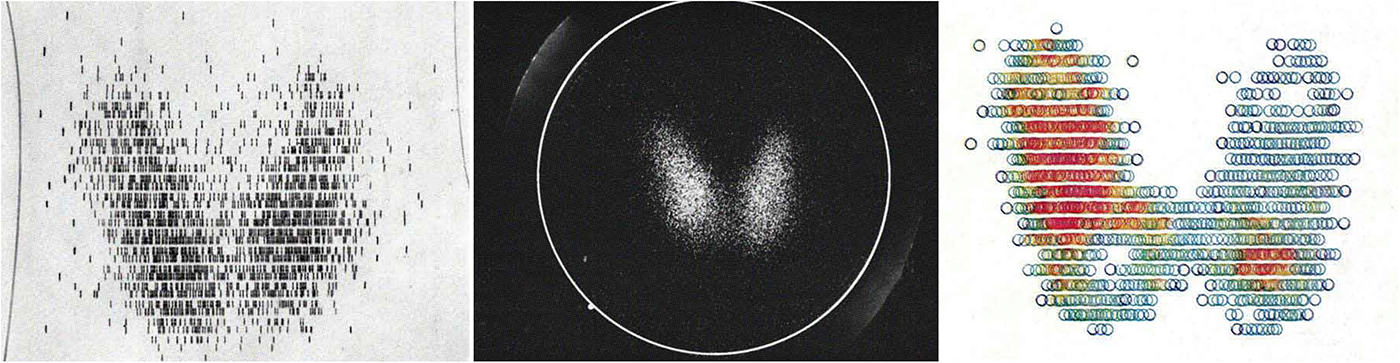
Figure 3. Comparison of scanning techniques available around 1970. Left to right: line, gamma camera and colour scintigrams of the thyroid. Based on the image, it is possible to determine the form and function of the butterfly-shaped thyroid as well as to detect dystopias of iodine-storing tissues. Unlike radiographs, scintigrams are more sensitive to physiological processes but difficult to interpret due to their low spatial resolution. In Heinz Oeser, Werner Schumacher, Helmut Ernst et al., Atlas der Szintigraphie, Berlin: De Gruyter, 1970, pp. 9–13.
With the subsequent transition from state-controlled supply of tracers and devices to privately managed commercial providers, laboratories, particularly in Western Europe, shifted from the creation of local solutions to the wider adoption of existing technologies. Rising acquisition costs and the availability of the same products at different hospitals tended to homogenize quality demands and workflows, as well as to ensure economical and safe use. This was especially evident in the government-regulated trade in radiopharmaceuticals. Because of costly production facilities, this business remained the preserve of a few public–private partnerships such as that between the British Radiochemical Centre (Amersham) and the Buchler Company (Brunswick), which expanded their market presence through mergers.Footnote 75
The situation was different for technical devices. Due to the presence of many manufacturers, these devices and the skills needed to handle them varied widely, as did the reliability and comparability of their measurements. Although important inventions originated in the United States and the United Kingdom, a nuclear-instrument industry also emerged early on in continental Europe, albeit with less participation from public and clinical bodies. Private enterprises such as Frieseke & Höpfner (Erlangen), Mecaserto (Paris) and SELO (Milan) competed with the US-based Nuclear Chicago company, established in Europe with a branch in Amsterdam after 1965.Footnote 76 Devices constructed in clinical laboratories continued to play a role, either as prototypes or as temporary replacements for commercial units. Delivery delays were particularly common in the Eastern bloc, where the state-run Gamma company (Budapest) manufactured equipment for communist countries and, to generate foreign currency, for Western export.Footnote 77 For example, nine years passed between the commissioning of the first commercial gamma camera in Britain (1963) and in Hungary (1972).
High equipment and safety costs, especially for waste disposal facilities, favoured the centralization of radioisotope services. Staff composition in these first major laboratories, such as those at Middlesex Hospital (London), the Service hospitalier Frédéric-Joliot (Orsay), or Bonn University Hospital, was highly individual. It was based on local research interests, disease prevalence and the specific department (e.g. internal medicine, oncology, orthopaedics) for whose patients a given laboratory provided services. In addition to physicians and physicists, the teams included chemists, pharmacists and/or engineers, turning isotope laboratories into multifaceted working environments. While radiologists and internists with physical–technical training were usually in charge, in Britain, France and Scandinavia instead it was mainly medical (bio)physicists, reflecting the early development of this profession in those countries.Footnote 78 In regional hospitals, radiotherapy and radiology departments often took over radioisotope services. Created as separate or joint clinical units from 1900 onwards, these departments were already using solid and sealed radioisotopes in therapy (for internal or external irradiation) and were familiar with imaging techniques, albeit focused on anatomical features.
In the early days of laboratory work, particularly in continental Europe, separate responsibilities for tracer preparation, treatment and data analysis had not yet been developed, with scientists relying on physicians to administer radioisotopes. Collaboration tended to be experimental in the sense that different experts cooperated in the widespread absence of role models from other specialities. By the 1960s, more sophisticated equipment and the availability of radiolabelled compounds produced on site required the division of workflows into tasks and more discipline-specific expertise, such as in chemistry.Footnote 79 The workshop character of isotope laboratories, with ‘tinkerers’ exploring potential applications, gave way to more futuristic sites, where specialists combined scanners and tracers to detect multiple body functions of their patients simultaneously. The growing separation between research and routine duties, with each involving both physicians and non-medical staff in different capacities, reinforced the work hierarchy. Laboratory technicians, mostly young women who had been hired to perform routine tests, played a special role in this process.Footnote 80 As the profile of nuclear physicians evolved, technicians took over many time-consuming and undervalued tasks such as apparatus operation and in vitro sampling. This, as with other specialisms, created the conditions in which physicians could focus on scientific and supervisory activities.
According to medical historian George Weisz, since the 1950s clinical practice has witnessed an explosion of specialities, driven by new technologies, societal needs and intensified international exchange.Footnote 81 In the case of radioisotope techniques, individual institutional origins and local medical needs led to diverging concepts of professionalism. At the heart of this was the challenge of how to integrate this novel field and its fast-growing body of knowledge within hospital life. Overlapping competencies and decisions to be made about the management of units and their budgets made the question whether nuclear medicine was a speciality in its own right a practical one.Footnote 82 By the early 1960s, two communities of thought had emerged among European practitioners about the (trans)disciplinary framework of this new area of expertise. On the one hand, many radiologists and physicists argued for considering radioisotope techniques a tool of their own domain, or an integrative field in between established specialities. Having contributed substantially to this method but now facing a loss of influence, they criticized the limited pathological experience of future practitioners, their few therapeutic procedures and – from the perspective of non-physicians – the priority given to clinical tasks.Footnote 83 On the other hand, many internists, influenced by subspecializations and methodological diversity within their area of expertise, argued for the pooling of nuclear-medicine knowledge in the formation of dedicated practitioners familiar with all aspects of radioisotope applications. In their view, this allowed nuclear physicians to collaborate as equals with other clinicians and avoid being treated as service providers. One major advocate for the field’s autonomy was the IAEA’s Medical Section, notably Vetter, who promoted the ‘new discipline’ at numerous conferences and eventually in the agency’s bulletin:
Is there such an animal as a specialist in nuclear medicine? Many people, particularly in England and the United States, deny this vehemently. They argue that radioisotopes are just a tool, and that they should be used by the clinical specialist along with other tools of his profession … Certainly, X-rays are also only a tool but their most efficient use soon required a specialist physician who made radiology his main business … Personally, I would prefer to see a physician in charge of an isotope laboratory … He should understand the pathology, diagnosis, and therapy of the disorders … Otherwise, his role will soon be reduced to that of a technician.Footnote 84
Behind the section were internists from continental Europe who drove the accreditation of this recent area of expertise as a transdisciplinary speciality. Their understanding of nuclear medicine as distinct from fields not exclusively concerned with hospital care, such as radiation biology, in turn increased competition with other technology-based clinical specialities.Footnote 85 In particular, boundary work with radiology and radiotherapy necessitated the development of distinctions in treatment procedures. At the 1963 UN Geneva conference on technologies for less-developed areas, IAEA scientists, notably Seligman, had announced that radioisotope applications as a ‘cheaper, safer, and considerably more versatile’ method would soon replace radiotherapy, with its solid and sealed compounds.Footnote 86 Following the introduction of external beam radiotherapy and chemotherapy in the late 1950s, however, only a few of the many radioisotope applications remained standard therapies, including those for the treatment of thyroid and blood cancers.Footnote 87
Innovations in scanning technologies enabled nuclear physicians to turn to in vivo diagnostics and the assay of metabolic body functions.Footnote 88 The primary emphasis was on the study of pathophysiology: visualizing the functioning of one or several organs and their pathological changes. These methods overshadowed radiological techniques, which were limited to anatomical imaging. Consequently, isotope laboratories experienced a surge in patient numbers. However, this boom was short-lived. With the emergence of new morphological visualization techniques, such as sonography and X-ray computed tomography (CT), nuclear medicine lost its key role in organ diagnostics as early as the 1970s.Footnote 89 Moreover, regular laboratory physicians took over in vitro testing with radioisotopes and gradually replaced them with non-radioactive methods. This meant that the main task of nuclear physicians remained ‘only’ the examination of metabolic body functions.
These multiple changes in treatment routines made it challenging for practitioners to gain autonomy and recognition in clinical work. Rather more resilient to these shifts was the study of the thyroid, which had emerged as a dedicated area of expertise in landlocked countries or areas, including Austria, East Germany, Spain and Switzerland.Footnote 90 The lack of iodine-rich seafood meant that the population there was at elevated risk of developing hypothyroidism (underactivity of the iodine-dependent thyroid), leading to congenital intellectual disability, physical deformity and other disorders.Footnote 91 In its turn, the thyroid – usually cared for by endocrinology-trained internists, or by surgeons in the case of pathological enlargement – was particularly suitable to radioiodine diagnostics and therapy. This was helped by the fact that Harwell and other distribution programmes provided hospitals with iodine-131 early on. The implementation of comprehensive treatment solutions and the ability to fully replace or limit surgical interventions were meant to counteract the understanding of radioisotope techniques as a ‘service for’ or ‘methodology within another specialty’.Footnote 92
To take an example, radioiodine applications made it possible to differentiate between hyperthyroidism and hypothyroidism and to treat the former disorder and thyroid cancer non-invasively. As the efficiency of these procedures at university hospitals specializing in thyroid disorders gained recognition, their isotope laboratories were expanded into nuclear-medicine departments, serving as models for facilities in regional hospitals. These institutional developments, especially the founding of the first dedicated university chairs in countries such as France (1962), Switzerland (1962) and Italy (1963), occurred throughout Europe in the subsequent years, spanning both sides of the Iron Curtain.Footnote 93 However, in countries like Britain, where isotope laboratories were headed by physicists, radiologists or pathologists, nuclear-medicine facilities had more difficulty competing with other specialities, often forming units with related fields or remaining confined to a few central hospitals.
In consequence, competing views on the epistemic framework of nuclear medicine – a transdisciplinary speciality, or a field lying in between established specialities – emerged from differences in the composition of practitioners and their favoured treatment domains. Technological advances and their medical application tended to precede clinical evidence of efficacy. This had a lasting impact on the way norms for this diverse field were created among European practitioners. First, local staff had greater influence over which procedures they tested for practical value and adopted if found beneficial. Despite the IAEA’s efforts to standardize a few procedures, particularly in exchange with developing countries, ongoing differences in instrumentation and calibration delayed the implementation of international guidelines.Footnote 94 Second, the lack of clinical evidence strengthened the position of physicians, with one prominent example being radiation dosimetry, which until the 1960s remained (in the words of Veall and Vetter’s textbook) ‘uncertain at best and sometimes largely guesswork’.Footnote 95 In this context, it was ultimately the physicians’ professional experience that determined whether a technique or dose was deemed useful in diagnosis and therapy. Physicists continued to play a significant role, particularly in improving devices, although often under the supervision of physicians. Third, in landlocked countries or areas, the transition from a technology-based to a more organ-specific field brought to the fore the question of who, or which specialities, should determine clinical routines.Footnote 96 Unlike radiation biology, where dose limits were less tied to local conditions such as the (residual) radioactivity of a given isotope shipment, disparities in nuclear-medicine practice persisted longer and contributed to the field’s differing institutionalization across Europe. As we will show in the following section, the initiatives carried out at conferences and in professional societies were all the more important for consensus building.
Professional identities: conferences and societies
The first symposia on radioisotope applications were held in Europe in the early 1950s, some fifteen years before the establishment of national nuclear-medicine societies. Since journals were not widely available in the aftermath of the Second World War, face-to-face meetings provided the opportunity to socialize and discuss the latest findings with a wider specialist audience. What these symposia had in common was that they covered a broad spectrum, including biochemical, agricultural and industrial applications. Of the ninety-eight papers presented at the Isotope Techniques Conference (1951) in Oxford, sixty-two were on medical and physiological topics, with a focus on experimental rather than routine uses.Footnote 97 Sponsored by officials from Harwell, this symposium brought together five hundred scientists and physicians; mainly from Britain and France, but also from other Western European countries and Canada. The fact that few researchers from the United States attended the meeting was due both to the difficulty of travel and to disagreements between American and British communities resulting from the US Atomic Energy Act of 1946. This, in turn, spurred initiatives among European stakeholders. As one American commentator noted, ‘we were probably ahead of our British and Continental colleagues in instrumentation [and] in breadth of exploration of the overall field … They, on the other hand, were well into the basic aspects of many problems’, including clinical applications.Footnote 98
Thriving research on both sides of the Atlantic soon led to international conference series devoted exclusively to radioisotopes in medicine. The first was organized in Bad Gastein, a historic Austrian resort town with radium springs, in early 1954 under the title Radioactive Isotopes in Clinic and Research; it was held there on a biannual basis from then on.Footnote 99 A few months later, Seattle, WA hosted the inaugural conference of the Pacific Northwest Society of Nuclear Medicine, which in 1956 became an annual US meeting with changing locations as the society evolved into a nationwide organization.Footnote 100 With only twenty-seven and seventeen lectures respectively, the two conference series initially had a rather informal atmosphere, despite the high profile of many speakers, but their development took different courses. Launched by the two internists Vetter and Höfer with clinic director Fellinger as its first president, the Bad Gastein Symposia had a high level of continuity, with the same organizer(s) in charge for forty years; it attracted clinicians mainly from Western and Eastern Europe. In contrast, the Society of Nuclear Medicine meetings, attended by physicians, physicists and technicians from North America and abroad, were organized by a rotating multidisciplinary committee. Despite their role as the pivotal gathering in the field, these meetings lost their intimate feel as the society and the number of exhibitors grew.Footnote 101
The open format of a symposium and the casual atmosphere of the Bad Gastein meetings proved advantageous in Europe’s politically and scientifically diverse context. This enabled the organizers to better use their diplomatic skills to meet the individual needs of the participants, especially in arranging social activities to complement the scholarly agenda. Held just a few hours’ drive from the Iron Curtain, the symposia were the only international nuclear-medicine meetings that delegates from communist countries could attend. The venue of the conference series, which took place during low season in January, was the imposing Grand Hotel de l’Europe, once one of the most elegant addresses of the Habsburg monarchy.Footnote 102 Participants at the first symposia came from a wide variety of clinical backgrounds. Over champagne and cake, they not only explored the latest trends in radioisotope techniques, but also made lifelong friends, forged plans and balanced disciplinary and ideological tensions. By transforming ‘the scientific contact into a friendly and personal one, too’, the organizers strengthened collegiality among early European practitioners, without entering into exhausting discussions about the boundaries of the emerging field or the duties of future specialists:Footnote 103
Hardly any other area of medical research is as dependent on the cooperation of different branches of knowledge as this one … [At this first Symposium] clinicians critically evaluated what had already been tried and tested, and the younger generation presented what had just been discovered … Clinicians and theorists, diagnosticians and therapists, speakers and listeners met in question-and-answer sessions, in serious as well as cheerful discussions.Footnote 104
In this way, the Bad Gastein Symposia contributed to the development of a professional identity in nuclear medicine. However, this ‘professionalism’ was initially based less on discipline-specific knowledge than on shared social behaviours and values, such as cosmopolitanism, medical ethics and the equal importance of clinical research and practice.Footnote 105 Working with medical radioisotope applications meant contributing to their joint transnational exploration, which was intended to serve the good of many and – at the first conferences, at least – left little room for competition. Within a few years, participants already considered the symposia a ‘permanent institution’ and a ‘real family reunion’, ‘where one could … talk things over, and move closer together … even in these tense times’.Footnote 106 Rather than establish original policies, these meetings promoted exchange and coordination, paving the way for shared initiatives later formalized in multilateral bodies like the IAEA. By defining topical conference themes, such as computational data processing – while rejecting off-topic presentations – the organizers succeeded in becoming a driving force for the developing field at European level. An important factor in this regard was the publication of the papers and discussions in comprehensive proceedings, even before journals dedicated to nuclear medicine emerged.
The success of the symposia was also due to their international character. Table 1 provides an overview of the participants’ citizenship between 1954 and 1990. While the symposia began as a gathering of German-speaking and British communities, European and North American participation increased in the late 1950s as the event grew in prestige and the organizers’ transatlantic contacts improved.Footnote 107 By contrast, other conference series, such as the French Colloque national sur l’utilisation des isotopes radioactifs (1959) or the meetings of the Society for Nuclear Medicine founded in West Germany (1963), were more national in character and did not match Bad Gastein’s cosmopolitan atmosphere. Moreover, participants from West Germany were a vital part of the Bad Gastein community, so initially there was no competition between these conference formats. By the 1960s, with participants from the Middle East, Latin America and North Africa, the symposia became a global hub for knowledge and methods, which the conference community increasingly subsumed into the single category of ‘nuclear medicine’. This was also due to the addition of an industry exhibition to the programme, in response to the interest of equipment manufacturers in marketing their products. Exhibitors included not only large US and West German enterprises, but also suppliers from communist countries.Footnote 108 For example, Isocommerz, the company responsible for supplying East Germany, used the conference to negotiate contracts for importing imaging devices.
Table 1. Nationality of symposia participants, 1954–90. Calculation based on the lists of participants in the proceedings between 1962 and 1990. Data up to 1960 refer only to the named speakers and discussants.
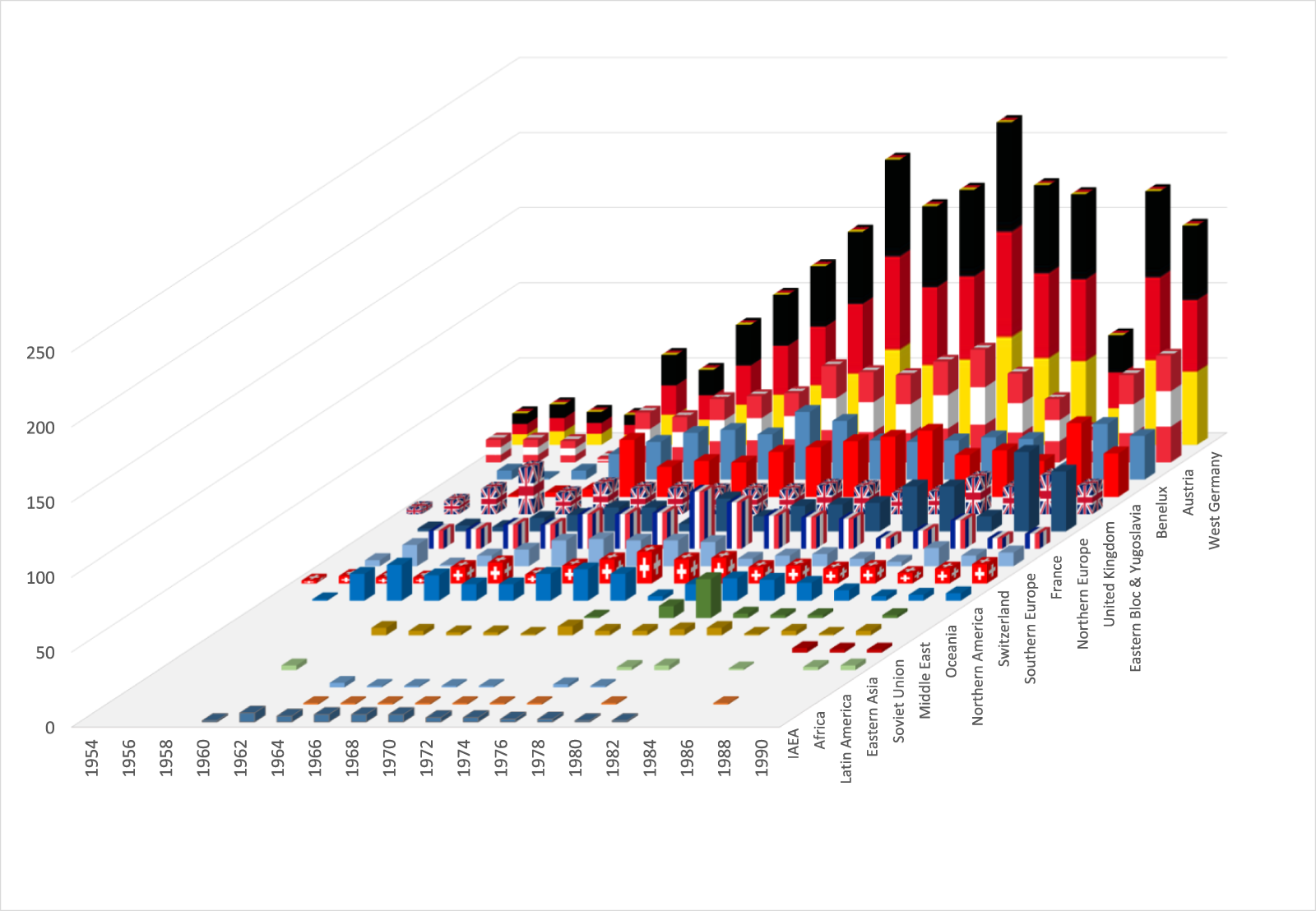
For physicians from the Eastern bloc, the Bad Gastein Symposia were of special significance. Despite making up just 8 per cent of all participants, these physicians attended regularly from the early 1960s to network and learn about recent innovations.Footnote 109 In some cases – such as Hungary, where the government aimed to export its nuclear-medicine products – participation in the symposia was explicitly encouraged.Footnote 110 However, because some communist countries did not have enough foreign currency to send delegations, the Vienna organizers negotiated an agreement that allowed East German participants (for example) to attend for free. In return, the latter agreed to invite Austrian physicians on a regular basis to their annual meetings in Reinhardsbrunn (Thuringia).Footnote 111 Growing trust between Austrian and Eastern European stakeholders made the former into advocates for their colleagues in communist countries. In turn, the conference series’ role as a platform for East–West knowledge exchange strengthened the organizers’ position within the European community. As Austrian chancellor Bruno Kreisky (1911–90) expressed it in a 1974 speech before the Soviet Academy of Sciences, the mediating role between the two power blocs made his country a ‘clearing house’ and ‘place of constant encounter’.Footnote 112 Similarly, the symposia, which peaked in the early 1980s with some 450 participants from fifty nations, including Australia, Japan and the Soviet Union, served as a vehicle for building a transnational nuclear-medicine community.
With the growth of clinical facilities and the training of a second ‘generation’ of practitioners beginning in the mid-1960s, the lack of professional organization and the disparities within the field became apparent. Unlike many of the pioneers, who had begun their research as individualists with diverse medical residency titles, their assistants, some with no certified training, had nothing less than their future as professionals at stake.Footnote 113 On the one hand, there were hardly any curricula or quality standards for the training and continuing education of practitioners of nuclear medicine; on the other, there was little prospect of what one could do after graduating, since radioisotope applications were still the responsibility of the respective specialities. West German interns, representing the largest portion of prospective nuclear physicians in Europe, complained that after training they would be ‘neither radiologists nor internists’.Footnote 114 Although the community agreed on the transdisciplinary skills and clinical expertise of future professionals, there was a lack of consensus about the speciality-specific components of a training programme. In France, for example, training (albeit without a specialist title) was centrally regulated as early as 1965. In addition to a university degree in biophysics, a six-month clinical internship and a three-month course at the Institut national des sciences et techniques nucleaires (National Institute for Nuclear Science and Technology) were required.Footnote 115 In West Germany, on the other hand, eminent practitioners took the initiative to form an association, the Deutsche Akademie für Nuklearmedizin (German Academy for Nuclear Medicine), with the goal of providing certified training courses for their staff.Footnote 116
To better coordinate these efforts, the first nuclear-medicine societies were formed in Europe in the 1960s, initially at the national level and often as spin-offs of larger speciality-based societies. Their goal was to integrate the broad spectrum of practitioners involved in radioisotope applications and, driven by younger members, to transform their work profile into a profession: a recognized medical speciality with well-defined career paths. In the following decades, many of these societies lobbied at the national level to establish nuclear-medicine specialist titles, often with limited success. The first transnational association to take this approach was the Gesellschaft für Nuklearmedizin (Society of Nuclear Medicine, SNM), founded in Freiburg (Germany) in 1963, which added ‘Europe’ to its name in 1980.Footnote 117 Unlike later initiatives linked to the Bad Gastein Symposia, the SNM emerged independently from a West German working group of internists and haematologists. Its goal, similar to that of its namesake Society of Nuclear Medicine in the United States, was to create ‘a forum where especially representatives of internal medicine and radiology can collaborate’.Footnote 118 Accordingly, many SNM members advocated for better recognition of nuclear medicine among the medical specialities, but in association with radiology. In the early years, the internist Ludwig Heilmeyer (1899–1969) and the radiotherapist Josef Becker (1905–83) took turns at the helm of the SNM, while the physicist and Nobel laureate George de Hevesy (1855–1966) served as honorary president. Equipment suppliers also joined the SNM and supported its activities, such as publishing a journal and inviting guest speakers, especially from North America. Despite being established as a European organization, its predominantly West German membership fell short of this vision. Furthermore, its committee structure, based on majority voting by individual members, ensured a ‘pronounced national element in governance’.Footnote 119
Concerns about this dominance within the SNM, its transatlantic orientation and the involvement of radiologists arose among representatives of smaller countries, especially from Eastern Europe. For them, membership was also impossible for political reasons alone. At the end of the 1960s, nuclear physicians from communist countries approached Höfer, one of the organizers of the Bad Gastein Symposia, with the proposal to initiate a second European society as a ‘common platform for East and West’.Footnote 120 Such intra-European cooperation was intended to ‘bring the disciplines closer together’, redress national imbalances and promote the autonomy of the field through representation to international medical bodies.Footnote 121
Although Höfer initially resisted, reluctant to burden the symposia with bureaucracy or risk conflicts with the SNM, he soon recognized the initiative’s potential. Eventually taking the reins, he headed the founding committee and sought support from the French-speaking community. The biggest hurdle, however, was defining a ‘professional’ profile for members, and, building on that, integrating national communities without over-representing any one group. For example, UK representatives expressed concern that restricting membership to ‘professional’ nuclear physicians would exclude physicists, as was already the case with the British Nuclear Medicine Society.Footnote 122 At the same time, new frontier areas such as radiopharmacy had to be considered without opening up too much to other specialities, as the Swiss physician Bernard Delaloye (1928–98) pointed out:
Does this mean we are going to accept all the haematologists, all the oncologists, all the radiologists, and all the internists who are remotely involved in nuclear medicine? … It seems we are in danger of hypertrophying, on the one hand, and risking a dilution effect on the other … I think we should avoid our society becoming a kind of refuge … We have to be careful, because the radiologists will most certainly get in amongst our ranks, and nuclear medicine will become nothing more than scintigraphy.Footnote 123
The search for a common position between all European partners took years, and ultimately ended with a compromise: the recognition and valorization of the field’s diversity as an important resource for meeting future challenges such as multispeciality disease patterns. Consequently, the founding committee avoided defining the field, also because of its rapid development. In the new society, anyone with an ‘academic or professional degree’ who works full-time in nuclear medicine could become a member.Footnote 124 The designation thus became an umbrella term for all practitioners involved in clinical radioisotope applications, but at the same time excluded other specialists unless they devoted themselves primarily to nuclear medicine. Another difference from the SNM was that motions were voted on by a delegates’ assembly, to which national societies (in Britain, also the Hospital Physicists Association) nominated a total of two delegates per country. As a result, the interests of smaller states received greater consideration in decision making. In November 1973, representatives from fifteen countries across the continent ratified the statutes of the future European Nuclear Medicine Society (ENMS) at the Hotel de France in Vienna. As a sign of the integration of Eastern European countries, Claude Kellershohn (Orsay) and Zdeněk Dienstbier (Prague) became president and vice president, and the society’s permanent office was established in Vienna with Höfer as first secretary–treasurer.Footnote 125 Although the ENMS never reached the membership numbers of the SNM, the two societies competed for influence for years.
The parallelism of these (overlapping) communities and their differing concepts of professionalization had serious consequences. Rather than standardizing clinical practice, the societies concentrated on their roles as professional representatives. This involved the creation or harmonization of training programmes, speciality titles and career opportunities. In 1982, a Linking Committee was established between the two European societies to promote cooperation.Footnote 126 With many suppliers unwilling to sponsor two annual European conferences per year, joint conferences began in 1984. Two years later, the ENMS and SNM merged into the European Association of Nuclear Medicine (EANM), while continuing the journal of the former and maintaining its office in Austria. After years of negotiations, the European Union of Medical Specialists finally recognized nuclear medicine as a separate speciality in 1989, followed by countries that did not yet have a speciality title, such as Britain and Sweden.Footnote 127
Overall, the field’s transdisciplinary orientation between medicine and technology, basic research and clinical application, made it difficult to unite most practitioners and their diverse work patterns into a single (medical) profession. Initially, thematic conferences and professional societies, designed for exchange rather than collaboration, created a sense of community. They celebrated social togetherness and provided a common space within Europe’s fragmented research landscape. In a second step, with increasing clinical responsibilities and the need to train qualified staff, many societies and conferences began to address professional issues alongside scientific ones. Because the former often concerned national healthcare legislation, their consideration tended to overlook the intra-European diversity of the field and narrow the self-image of practitioners to the profile of a medical specialist. Although led by physicians, nuclear-medicine societies usually remained committed to the field’s diversity and sought to integrate the majority of practitioners involved. However, through their expert opinions on the tightening of legislation, they helped to restrict the use of radioisotope techniques by (non-specialist) researchers without appropriate infrastructure.
Conclusion
This article has shed light on the rise of nuclear medicine in post-1945 Europe as a clinical laboratory science that had to navigate its own inconsistencies and overlaps with other specialities. Thus far, historians have primarily examined Cold War science diplomacy and transatlantic knowledge exchange through the lens of major research facilities and multilateral organizations. Our article, however, shifts the focus to lesser-studied clinician–scientists and hospital laboratories throughout Europe, exploring their transnational networks and strategies for gaining authority. In the context of East–West tensions, medical applications emerged as a compelling testament to the peaceful use of atomic energy. Their (public) standing derived not only from multilateral cooperation, but also from the integration of natural-science methods into clinical research and the promise of curing serious diseases. Innovative devices, imaging technologies and quantitative models all proved crucial to establishing credibility, even though the data behind them sometimes lacked clinical evidence.Footnote 128
As we have shown here, the process of speciality formation in nuclear medicine unfolded within similar frameworks as in other scientific medical fields, involving the establishment of university chairs, curricula and periodicals. However, its practitioners, drawn from diverse backgrounds, encountered a paradoxical situation. Despite the field’s transnational orientation, consistent treatment routines were limited, and there was no consensus on how to effectively organize the accumulated knowledge. In the immediate post-war period, several factors contributed to a fragmented research landscape in Europe: Anglo-American leadership in nuclear research, political restrictions on technology supply, and the uneven availability of experts for collaboration. Moreover, varying clinical needs and the levels of public and industry support in different countries fostered competing notions of professionalism.
In European countries, where thyroid disease was prevalent and nuclear medicine found extensive application, advocates of medical autonomy gained the upper hand early on, advancing their agenda to the EU level. Conversely, in nations with a lower incidence of thyroid disease, such as Britain, the role of a specialist providing nuclear-medicine services emerged comparatively late. Until the 1980s, nuclear medicine in these countries primarily remained a scientist-driven methodology for applying radioactive tracers in medical measurements. Meanwhile, in the United States, where numerous radioisotope applications had been developed, clinicians from various specialities integrated them into their practices. There, too, an official body was established in 1972 after lengthy negotiations: the American Board of Nuclear Medicine. Composed of radiologists, internists, pathologists, and Society of Nuclear Medicine delegates, the board provided formal certification to nuclear-medicine practitioners upon completion of a three-year residency.Footnote 129 However, in exchange, it had to allow certifying bodies from other specialities to offer equivalent (but shorter) nuclear-medicine training programmes. This means that a wide range of medical professionals in the United States now uses tracer technologies.
We contend that in post-war Europe, the diversity of experts and practices around medical radioisotope applications fostered community building rather than constraining it. It is true that professional societies and conferences were not free of tensions or boundary work, with discussions about the lack of consistency and clinical evidence sometimes arising at an early stage. Nevertheless, practitioners in Europe demonstrated remarkable resilience through integrative efforts across specialities and political divides. Given the transdisciplinary nature of nuclear medicine and the few (inter)national benchmarks, these early experts could more readily adapt to local conditions and technology-driven shifts in treatment applications. Another factor was the policy of individual IAEA officials, who set out to make medical applications available to low- and middle-income countries worldwide. Focusing on radioiodine uptake tests and training courses, their initial efforts cautiously homogenized clinical practice while preserving the autonomy and standing of physicians vis-à-vis physicists.Footnote 130
In this context, the key to professionalizing nuclear medicine was to effectively manage its diversity across epistemic, political and social levels, and to use this breadth to enhance its status as a distinct speciality. To return to the words of Peter Ell, quoted in the introduction, the fact that this was achieved in Europe without a common framework of ‘minimum standards of care and practice’ is the result of a transnational and transdisciplinary integration process that might well have gone differently had other actors or institutions been involved.Footnote 131
Acknowledgements
The authors gratefully acknowledge the Austrian Academy of Sciences for awarding them the Bader Award for the History of the Natural Sciences, which initiated this article. They are deeply indebted to the late Rudolf Höfer (d. 2023) for his invaluable insights and for granting access to his extensive source material. Heartfelt thanks go to Sandra Klos (Friedrich-Alexander-University of Erlangen-Nuremberg) for her significant contributions to the research, as well as for her review and feedback on the manuscript. The unwavering support of Mitchell G. Ash (University of Vienna) and Marcus Hacker (Medical University of Vienna) has been instrumental, and is sincerely acknowledged. Special appreciation is extended to Helmar Bergmann and Ingrid Leitinger of the Medical University of Vienna; Barbara Braunsperger, Clemens Decristoforo and Wolfgang Zechmann of the Medical University of Innsbruck; Christian Noe of the University of Vienna; and Isolde Füger and Dorothea Felicitas Riccabona for generously sharing their perspectives through insightful interviews. Moreover, the authors would like to express their sincere thanks to Ralph McCready (Royal Sussex County Hospital), Maria Rentetzi (Friedrich-Alexander-University of Erlangen-Nuremberg), Wolfgang Wadsak (European Association of Nuclear Medicine) and the Austrian Society of Nuclear Medicine and Theranostics (OGNT) for their support and collaboration. The constructive feedback from the editor and two anonymous reviewers is greatly appreciated. Last but not least, the authors especially thank Kirsty Jane Falconer (Prague) for her language editing of this paper.







