GBG – Contact Details
Country
Germany
Chairs
Professor Dr med. G. von Minckwitz, J.W. Goethe University Frankfurt, Department of Obstetrics and Gynaecology, Theodor-Stern-Kai 7, 60590 Frankfurt, GERMANY. Tel: +49 69 6301 7024 Fax: +49 69 6301 7938 Email: [email protected]
Professor Dr med. M. Kaufmann, J.W. Goethe University Frankfurt, Department of Obstetrics and Gynaecology, Theodor-Stern-Kai 7, D-60590 Frankfurt, GERMANY. Tel: +49 69 6301 5115 Fax: +49 69 6301 4717 Email: [email protected]
Project Management, Monitoring and Data Center
GBG Forschungs GmbH, Schleussnerstrasse 42, 63263 Neu-Isenburg (Frankfurt), Germany. Tel: +49 6102 798 74 0 Fax: +49 6102 798 74 40 Email: [email protected]
Website
GBG – Study Details
Title
A multi-center randomized Phase III study evaluating 4 cycles of docetaxel, doxorubicin and cyclophosphamide (TAC) versus 4 cycles of vinorelbine and capecitabine (NX) in patients not sufficiently responding to 2 cycles of TAC and 4 cycles of TAC versus 6 cycles of TAC in patients sufficiently responding to 2 cycles of TAC as preoperative treatment of locally advanced (T4 a-d, N0-3,M0) or operable (T ≥ 2 cm, N0-2,M0) primary breast cancer/GBG 24.
Coordinator(s)
Principal Investigator
Professor Dr med. G. von Minckwitz, University of Frankfurt, GERMANY. Tel: +49 6102 79874 10 Fax: +49 6102 79874 40 Email: [email protected]
Project Management
Dr O. Schickling, GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg (Frankfurt), GERMANY. Tel: +49 6102 79874 14 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, randomized Phase III trial including an internal Phase II trial opened in September 2001, Phase III trial opened in July 2002
Study Population
Locally advanced (T4 a-d, N0-3,M0) or operable (T ≥ 2 cm, N0-2,M0) primary breast cancer
Primary Objectives
- To determine the pCR rate of 4 cycles of TAC and of 4 cycles of NX (TAC versus NX) as a salvage treatment in patients not sufficiently responding (i.e. cNC) to 2 cycles of TAC as preoperative treatment of operable (T ≥ 2 cm, N0-2, M0) primary breast cancer.
- To determine the pCR rate of 6 cycles versus 8 cycles of docetaxel, doxorubicin and cyclophosphamide (TAC × 6 versus TAC × 8) in patients sufficiently responding (i.e. cPR or cCR) to the first 2 cycles of TAC as preoperative treatment of operable (T ≥ 2 cm, N0-2,M0) primary breast cancer.
Scheme
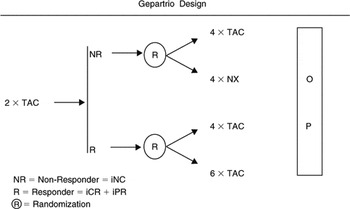
Update
- 2104 patients enrolled until enrolment ended in May 2005.
Related Publications
von Minckwitz G, Blohmer JU, Raab G, et al. German Breast Group. In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 2005; 16(1): 56–63.
Topics
- Anthracyclines
- Capecitabine
- Locally advanced breast cancer
- Taxanes
- Vinorelbine
Keywords
Preoperative chemotherapy, breast cancer
***************************************************
Title
A multi-center randomized Phase III study to compare capecitabine alone or in combination with trastuzumab in patients with HER-2 positive metastatic breast cancer and progression after previous treatment with trastuzumab (Treatment Beyond Progression, TBP).
BIG 3-05 – GBG 26
Coordinator(s)
Principal Investigator
Professor Dr med. G. von Minckwitz, University of Frankfurt, GERMANY. Tel: +49 6102 79874 10 Fax: +49 6102 79874 40 Email: [email protected]
Project Management
Dr M. Zerm, GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 27 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, randomized Phase III trial
Study Population
Patients with HER-2 positive metastatic breast cancer and progression during or after previous chemotherapy and trastuzumab treatment as follows:
- Taxanes + trastuzumab given as adjuvant therapy
- Taxanes + trastuzumab given as first line therapy for palliation
- Trastuzumab given as first line therapy for palliation alone or in combination with chemotherapeutic agents other than capecitabine or taxanes
- Trastuzumab has to be given previously for at least 12 weeks, treatment-free interval of trastuzumab for a maximum of 6 weeks. No more than one chemotherapy for palliation
Aims
The primary aim is to compare the time to disease progression in patients with HER 2 positive metastatic breast cancer and progression after previous treatment with trastuzumab randomized to capecitabine alone or in combination with trastuzumab.
Secondary Aims/Endpoints
- To compare the objective response rate between the two arms.
- To compare the duration of response.
- To compare the clinical benefit defined as CR, PR, or stable disease >24 weeks between the two arms.
- To evaluate the safety of the capecitabine + trastuzumab combination.
- To compare overall survival between the two arms.
Treatment
- Capecitabine 2500 mg/m2 orally day 1–14 q day 22 until progression or unacceptable toxicity, patient’s request or withdrawal from study, and discontinuation of Trastuzumab.
- Capecitabine and Trastuzumab Capecitabine 2500 mg/m2orally day 1–14 q day 22 until progression or unacceptable toxicity, patient’s request or withdrawal from study.
- Trastuzumab 6 mg/kg body weight every 3 weeks intravenous (i.v.) as a 90 minutes infusion until progression or unacceptable toxicity, patient's request or withdrawal from study.
Scheme
None available
Update
- 135 enrolled as of September 2006.
Related Publications
None available
Topics
- Capecitabine
- HER-2 positive patients
- Metastatic breast cancer
- Trastuzumab
Keywords
Trastuzumab beyond progression, HER-2 positive breast cancer
***************************************************
Title
A Phase III multi-centre double blind randomized trial of celecoxib versus placebo in primary breast cancer patients.
BIG 1–03 – ICCG / C/20/01 – GBG 27
(see also description under ICCG)
Coordinator(s)
Management
Professor R. C. Coombes, London (ICCG)
Dr P. Hupperets, Maastricht (ICCG)
Professor Dr G. von Minckwitz, Frankfurt (GBG)
International Collaborative Cancer Group (ICCG) and the German Breast Group (GBG) Intergroup Study
Summary
- A multi-centre, Phase III, placebo controlled randomized trial. Patients are randomized between 2 years celecoxib and placebo in a 2:1 ratio in favour of celecoxib
Chemotherapy
Prior to randomisation, patients may have received chemotherapy (all HR negative patients MUST have received chemotherapy). All patients who have received chemotherapy should have finished their treatment, which will have consisted of at least 4 cycles. The schedule of preference is FEC 3-weekly for 6 courses, however, other dose schedules of FEC or FAC plus combinations that contain EC/AC followed by a taxane, or Epirubicin/Doxorubicin plus a taxane are permitted. CMF may be substituted for patients where Epirubicin is contraindicated.
Aims
The primary aim is to assess the disease-free survival (DFS) benefit of 2 years adjuvant therapy with the COX-2 inhibitor celecoxib compared with placebo in primary breast cancer patients.
Secondary Aims/Endpoints
- To compare overall survival.
- To define the safety of adjuvant therapy with celecoxib in this patient population.
- To compare the incidence of second primary cancers.
- In postmenopausal hormone receptor (HR) positive patients, to assess tolerability of celecoxib with tamoxifen.
- To assess DFS benefit of 2 years adjuvant celecoxib compared with placebo in HR positive (i.e. ER positive and/or PR positive) and in HR negative (i.e. ER negative/PR negative) disease.
Scheme
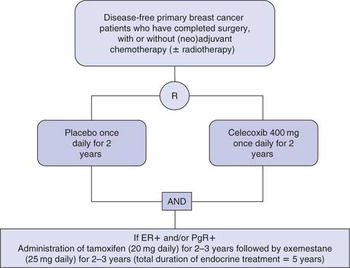
Update
- Planned study start: November 2006.
Related Publications
None available
Topics
- Aromatase inhibitors
- Celecoxib
- Tamoxifen
Keywords
Breast cancer, aromatase inhibitors, celecoxib, tamoxifen
***************************************************
Title
Prospective Register Study of the German Adjuvant Breast Cancer Study Group (GABG) or Diagnosis and Treatment of Breast Cancer in Pregnancy / BIG 2–03, GBG 29.
Coordinator(s)
Principal Investigator
Dr S. Loibl, University of Frankfurt, Klinik für Gynäkologie und Geburtshilfe, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, GERMANY. Tel: +49 69 630170 24 Fax: +49 69 630179 38
Project Management
GBG Forschungs GmbH, Dr C. Hanne, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: + 49 6102 79874 20 Fax: + 49 6102 79874 40 Email: [email protected]
Funding guaranteed by the BANSS-Foundation.
Summary
- Breast cancer complicating pregnancy is a rare coexistence. Breast cancer is after the age of 25 the most common malignancy in pregnancy. Little is known about the incidence in Germany and Western Europe. We therefore want to initiate a trial collecting prospectively as much data as possible for the diagnostic, treatment, and maternal and foetal outcome during pregnancy. The primary endpoint is the foetal outcome 4 weeks after delivery. Secondary endpoints will include maternal outcome, pregnancy outcome, diagnostic procedures used and the biology of the tumour. A flow sheet for the treatment is given and the acceptance of these guidelines will be evaluated.
Primary Endpoint
- Foetal outcome 4 weeks after delivery.
Secondary Endpoints
- Maternal outcome of pregnancy.
- Stage of and biological characteristics of breast cancer.
- Breast cancer therapy (treatment, response to chemotherapy, type of surgery).
- Sensitivity and specificity of diagnostic procedures (palpation, US, mammogram).
- Outcome of the newborn after 5 years of therapy.
- Outcome of breast cancer 5 years after diagnosis.
Scheme
Prospective and retrospective registration of patients who have been diagnosed with breast cancer during pregnancy
Update
- Accrual as of March 2006: 61.
Related Publications
Loibl, et al. Breast cancer during pregnancy – recommendations from an international expert panel. Cancer 2006; 106: 237–246.
Topics
- Premenopausal patients
Keywords
Breast cancer, pregnancy, foetal outcome
***************************************************
Title
Ibandronate with or without capecitabine in elderly patients with early breast cancer – ICE – Study / BIG 4-04, GBG 32. (see also description under WSG)
Coordinator(s)
Principal Investigator
Professor Dr med. U. Nitz, Frauenklinik der Universitäts-Klinikum Düsseldorf, Mohrenstraβe 5, 40225, DÜSSELDORF, GERMANY. Tel: +49 211 81175 50 Fax: +49 211 312283 Email: [email protected]
Project Management
GBG Forschungs GmbH, Dr C. Hanne, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 20 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
- Prospective, multi-center, controlled, open-label, randomized Phase III
Study Population
Age ≥65 years, N+ or N– (high risk: T ≥ 2 cm or G II/III or receptor negative)
Primary Objective
- To compare the event-free survival in elderly patients after local treatment for primary breast cancer treated with either ibandronate alone or ibandronate and capecitabine as adjuvant treatment.
Secondary Objectives
- To compare the overall survival between the two arms.
- To determine the compliance in both arms.
- To determine the toxicity in both arms.
- To determine the rate of bone-related events in hormonsensitive and insensitive disease (with or without anastrozole).
- To determine the preference to oral or intravenous application of ibandronate.
- To assess quality of life.
- To compare a geriatric assessment by Charlson versus VES 13 score.
Tertiary Objectives
- To determine prognostic factors on tumour tissue collected from primary surgery and to correlate them with study treatment effect.
- To evaluate the prognostic impact of age, serum albumin, hemoglobin level, creatinine clearance, Charlson score, VES-score in a multivariate analysis for the prediction of treatment associated adverse events and limited life time expectancy.
Scheme

Update
- Planned patient No: 1397
- Accrual as of March 2006: 580.
Related Publications
O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracyclinepretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol 2002; 20: 2812–2823.
O'Shaughnessy JA, Blum J, Moiseyenko V, et al. Randomized, open-label, phase II trial of oral capecitabine (Xeloda) versus a reference arm of intravenous CMF (Cyclophosphamide, methotrexate and 5–fluorouracil) Ann Oncol 2001; 12: 1247–1254.
Topics
- Bisphosphonates
- Capecitabine
- Elderly patients
Keywords
Elderly patients, chemotherapy, bisphosphonates, breast cancer
***************************************************
Title
GAIN: German Adjuvant Intergroup Node-positive Study.
A Phase III trial to compare ETC versus EC-TX and ibandronate versus observation in patients with node-positive primary breast cancer / GBG 33.
Coordinator(s)
Principal Investigator
Professor Dr med. V. Möbus, Städtische Kliniken Frankfurt a.M.-Höchst, Gotenstr. 6–8 D-65929 Frankfurt, GERMANY. Tel: +49 69310623 55 Fax: +49 69310625 55 Email: [email protected]
Project Management
Dr P. Segura-Eicke, GBG Forschungs GmbH. Tel: +49 6102 79874 13 Fax: +49 6102 79874 40 Email:[email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
- A German Intergroup Study of the Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) German Breast Group (GBG) and Nordostdeutsche Gesellschaft für Gynäkologische Onkologie (NOGGO)
Design
Prospective, multi-center, controlled, non-blinded, randomized Phase III with a 2 ×2 factorial design
Primary Objectives
- To compare the disease-free survival after adjuvant chemotherapy with “ETC” (Arm A1) or “EC-TX” (Arm A2) in patients with primary node-positive breast cancer.
- To compare the disease-free survival with (Arm B1) or without ibandronate (Arm B2) treatment for 2 years in patients with primary node-positive breast cancer.
Secondary Objectives
- To compare overall survival between Arms A1 versus A2 and B1 versus B2.
- To evaluate the compliance in Arms A1 versus A2 and in B1.
- To compare the safety between Arms A1 versus A2 and B1 versus B2.
- To assess the rate of responders to erythropoesis stimulating factors in Arms A1 and A2.
- To compare the incidence of secondary primaries between Arms A1 versus A2.
- To compare the event-free survival in subgroups of hormone sensitive and insensitive disease and in groups with 1–3, 4–9 or 10 + involved nodes between Arms A1 versus A2 and B1 versus B2.
Tertiary Objective
- To determine prognostic factors like TS or TP and others on tumour tissue collected from primary surgery and to correlate them with study treatment effect.
Related Publications
V.J. Möbus, M. Untch, A. du Bois, et al. Dose-dense sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high-risk breast cancer patients (≥4 + LN). First results of an AGO-trial. ASCO oral presentation, 5.6.2004.
Topics
- Node positive disease
- Dose dense therapy
- Capecitabine
- Paclitaxel
- Bisphosphonate
Keywords
Breast cancer, dose-dense, bisphosphonates
***************************************************
Title
A randomized, multi-center, open Phase III study comparing the postoperative use of zoledronic acid versus no treatment in patients with histological tumour residuals after preoperative anthracycline and taxane-containing chemotherapy for primary breast cancer (Neo-Adjuvant Trial Add-On) / GBG 36.
Coordinator(s)
Principal Investigator
Prof Dr med. Gunter von Minckwitz, University of Frankfurt, GERMANY. Tel: +49 6102 79874 10 Fax: +49 6102 79874 40 Email: [email protected]
Project Management
Dr O. Schickling, GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 14 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, randomized, open Phase III trial
Study Population
Completely resected unilateral or bilateral primary carcinoma of the breast with histologically detectable tumour residuals (ypT2-4) and or histologically confirmed involvement of axillary nodes (ypN1-3) after prior preoperative chemotherapy for at least 4 cycles, of which at least two must contain a taxane and an anthracycline.
Primary Objective
To determine the event-free survival (EFS) after zoledronic acid for 5 years versus no postoperative treatment in patients with “chemo-insensitive” breast cancer (ypT2-4 and/or ypN1–3) after preoperative anthracycline/taxane-containing chemotherapy.
Treatment
Zoledronic acid 4mg (or adjusted dose based on renal function) will be given i.v.:
- Every 4 weeks for the first 6 doses (year 0–0.5)
- Every 3 months for 8 doses (year 0.5–2.5)
- Every 6 months for 5 doses (year 2.5–5)
Patients with hormone sensitive tumours (ER and/or PgR positive) should receive simultaneous endocrine treatment according to current treatment guidelines. In case an aromatase inhibitor is indicated for postmenopausal patients >50 years, letrozole should be used. Letrozole will be provided free of charge.
Scheme
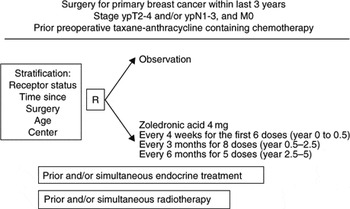
Update
- Enrolment period: December 2004–December 2007.
- Planned patients: 904.
- Enrolled patients: 130.
Related Publications
Diel IJ, Solomayer EF, Gollan C. Bisphosphonates in the reduction of metastases in breast cancer – extended follow-up results [Abstract 314]. Proc Am Soc Clin Oncol 2000.
Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A, et al. Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 2002; 20: 3219–3224.
Topics
- Aromatase inhibitors
- Bisphosphonates
- Hormonal therapy
Keywords
Breast cancer, adjuvant, bisphosphonates
***************************************************
Title
Prospective randomized multi-center study to prevent chemotherapy-induced ovarian failure with the GnRH-agonist goserelin in young hormone-insensitive breast cancer patients receiving anthracycline-containing (neo-)adjuvant chemotherapy / GBG 37, ZORO-Study
Coordinator(s)
Principal Investigator
Professor Dr med. B. Gerber, Klinikum Südstadt, Universitätsfrauenklinik, Südring 81, 18075 Rostock, GERMANY. Tel: +49 0381 4401 4500 Email: [email protected]
Project Management
GBG Forschungs GmbH, Dr C. Hanne, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 20 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, randomized, open Phase II trial
Study Population
Age < 45 years, spontaneous and regular menstrual periods before study entry with FSH below 15mIU/mL in follicular phase; histologically confirmed primary breast cancer with the need for anthracycline-based chemotherapy; steroid receptor (estrogen and progesterone) negative tumour (diagnosis according to hospital standard procedures).
Primary Objective
To increase the percentage of patients with normal ovarian function at 6 months after application of (neo)adjuvant, anthracycline-containing polychemotherapy in parallel with goserelin compared to chemotherapy alone.
Secondary Objectives
To compare the two treatment groups regarding:
- Compliance to treatment
- Toxicity
- Quality of life
- Menopausal symptoms score
- Ovarian function at 6, 12, 18 and 24 months
- Duration until recovery of regular menstrual period
- Pregnancy rate
Scheme

Update
- Planned Patient No.: 62.
- Patients recruited as of October 2006: 40.
Related Publications
Recchia, et al. Cancer 2006; 106: 514–523.
Topics
- Fertility and chemotherapy
- HR negative breast cancer
- Premenopausal patients
Keywords
Ovarian function, goserelin, breast cancer
***************************************************
Title
A multi-centre Phase I–II study to investigate the combination of bendamustine with weekly paclitaxel as first or second line therapy in patients with anthracycline-pretreated metastatic breast cancer / GBG 38, Rita – Study.
Coordinator(s)
Principal Investigator
Dr med. S. Loibl, Klinik für Gynäkologie und Geburtshilfe, Theodor-Stern-Kai 7, 60590 Frankfurt am Main. Tel: +49 696301 7024 4117 Fax: +49 696301 7938 83469
Project Management
GBG Forschungs GmbH, Dr C. Hanne, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 20 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, multi-centre, sequential Phase I–II
Primary Objectives
- To determine the maximum tolerated dose of the combination of bendamustine and weekly paclitaxel (Phase I part).
- To determine the objective response rate achievable with the recommended dose of this combination (Phase II part).
Secondary Objectives
- To determine the time to progression (Phase II part).
- To determine the safety and tolerability of the combination.
- To determine the dose-limiting toxicity (DLT) (Grade IV haematological toxicities or Grade III non-haematological toxicities).
Scheme

Update
- Accrual October 2006 : 11 patients, Dosis-Level III filled, no DLTs.
Related Publications
None available
Topics
- Metastatic breast cancer
Keywords
Metastatic breast cancer, bendamustine, Phase I
***************************************************
Title
A multi-center Phase II study to determine the efficacy of capecitabine as first line monochemotherapy in patients with HER-2 negative metastatic breast cancer / GBG 39, Monica – Study.
Coordinator(s)
Principal Investigator
Professor Dr med. M. Kaufmann, Universitätsfrauenklinik, Klinikum der J. W. Goethe Universität, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, GERMANY. Tel: +49 696301 5115 Fax: +49 696301 6317 Email: [email protected]
Project Management
GBG Forschungs GmbH, Dr C. Hanne, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 20 Fax: +49 6102 79874 40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, open Phase II trial
Primary Objective
- To determine the time to disease progression in patients with HER-2 negative metastatic breast cancer after 1st line monochemotherapy with capecitabine.
Secondary Objectives
- To determine the objective response rate.
- To determine the duration of response.
- To determine the clinical benefit defined as CR, PR, or stable disease 24 weeks.
- To evaluate the safety and toxicity of capecitabine.
- To assess quality of life within 1 year after start of capecitabine treatment.
- To determine overall survival.
- To determine the objective response rate in male patients.
- To evaluate QoL the modified Brunner Score.
Tertiary Objective
- To determine the DPD and proteomics in serum.
Scheme
Capecitabine 2000 mg/m2 orally day 1–14 q day 22 until progression
Update
- Planned patients: 200.
- Accrual October 2006: 88.
Related Publications
None available
Topics
- Capecitabine
- HER-2 negative patients
- Metastatic breast cancer
Keywords
Capecitabine, HER-2 negative patients, metastatic breast cancer
***************************************************
Title
GeparQuattro: A randomized Phase III study exploring the efficacy of capecitabine given concomitantly or in sequence to EC – Doc with or without trastuzumab as neoadjuvant treatment of primary breast cancer. A joint study of GBG and AGO / GBG 40.
Coordinator(s)
Chairmen
Prof Dr med. G. von Minckwitz, University of Frankfurt, GERMANY. Tel: +49 6102 79874 10 Fax: +49 6102 79874 40 Email: [email protected]
Professor Dr med. M. Untch, Helios-Kliniken, Berlin Buch, Frauenklinik, Wiltbergstr. 50, 13125 Berlin, GERMANY. Tel: +49 30 9401 2270 Fax: +49 30 9401 4326 Email: [email protected]
Project Management
Dr C. Hanne / Dr O. Schickling, GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874-14 Fax: +49 6102 79874-40 Email: [email protected]
Sponsor
GBG Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY.
Summary
Design
Prospective, randomized, Phase III trial
Study Population
Locally advanced (T4 a–d, N0-3, M0) or operable (T ≥2 cm, N0-2, M0) primary breast cancer. Tumour lesion in the breast with a palpable size of ≥2cm or a sonographically size of ≥1cm in maximum diameter. Stage of disease in which also adjuvant chemotherapy would be considered.
Primary Objectives
- To compare the pCR rates of NACT with versus without capecitabine and 8 versus 12 cycles in patients with primary breast cancer.
- To compare the pCR rates in patients with HER-2/neu positive tumours receiving trastuzumab simultaneously to NACT to patients with HER-2/neu negative tumours receiving NACT only.
Treatment
All patients will receive 4 cycles of EC:
- Epirubicin 90mg/m2 given simultaneously with
- Cyclophosphamide 600mg/m2, all day 1 q day 21
Thereafter they will be randomized to:
- Arm A: Docetaxel 100mg/m2day 1 q day 21 for 4 cycles (EC-Doc)
- Arm B: Docetaxel 75mg/m2day 1 q day 21 for 4 cycles concomitantly given with Capecitabine 1800mg/m2day 1–14 q day 21 for 4 cycles (EC-DocX)
- Arm C: Docetaxel 75mg/m2day 1 q day 21 for 4 cycles followed by Capecitabine 1800mg/m2day 1–14 q day 21 for 4 cycles (EC-Doc-X)
Patients with HER-2/neu positive tumors will receive trastuzumab 6mg/kg intravenous (i.v.) every 3 weeks concomitantly to cytotoxic treatment, starting with a loading dose of 8mg/kg i.v. on day 1 of the first EC-cycle. A total number of 8 (in the EC-Doc and EC-DocX arm) or 12 (in the EC-Doc-X arm) infusions will be given preoperatively.
Scheme

Update
- Enrolment period: August 2005–December 2006.
- Planned patients: 1042.
- Enrolled patients: 510.
Related Publications
None available
Topics
- Anthracyclines
- Capecitabine
- Locally advanced breast cancer
- Taxanes
- Trastuzumab
Keywords
Preoperative chemotherapy
***************************************************
Title
Randomized study comparing 6 × FEC with 3 × FEC followed by 3 × docetaxel in high-risk node-negative patients with operable breast cancer: comparison of efficacy and evaluation of clinico-pathological and biochemical markers as risk selection criteria.
A joint study of AGO, the EORTC Receptor and Biomarker Group, and the GBG (GBG 42 / NNBC 3-Europe).
Coordinator(s)
Principal Investigators
Professor Dr med. C. Thomssen, University of Halle, Frauenklinik Ernst-Grube-Strasse 40, 06047 Halle an der Saale, GERMANY. Tel: +49 345 557 1847 Fax: +49 345 557 1501 Email: [email protected]
Professor Dr med. N. Harbeck, Klinikum Rechts der Isar, Frauenklinik, Ismaninger St. 22, 81675 München, GERMANY. Tel: +49 894140 2420 Fax: +49 894140 4965 Email: [email protected]
Project Management
Dr M. Zerm, German Breast Group Forschungs GmbH, Schleussner St. 42, 63263 Neu-Isenburg, GERMANY. Tel: +49 6102 79874 27 Fax: +49 6102 79874 40 Email: [email protected]
Summary
Design
The trial is a prospective, randomized multi-centre open-label Phase III trial that is designed to detect a difference in efficacy between two chemotherapy regimens (FE100C*6 versus FE100C*3 followed by Docetaxel100*3) in high-risk node-negative breast cancer patients. Additionally, risk assessment by the traditional clinico-pathological factors and by tumour-biological factors (aPA/PAI-1) will be compared.
Study Population
Primary breast cancer, tumour size ≥0.5 cm and ≤5cm (pT1b-pT2, pN0, M0), age ≥18 years, ≤70 years
Aims/Endpoints
- Amongst the chemotherapy treated population:
– The primary endpoint of the study is Disease-Free Survival (DFS).
– The secondary endpoints are: Overall Survival (OS) and safety.
- Amongst the entire population of registered patients:
– DFS in each low-risk group (or of each patient group stratified for type of risk selection, respectively).
– The proportion of node-negative breast cancer patients grouped into each low-risk group.
– Side-effects of chemotherapy in each patient group.
Scheme

Update
- 999 patients enrolled as of October 2006.
Related Publications
Jänicke F, Prechtl A, Thomssen C, et al. for the German “Chemo-N0” Study Group. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J Natl Cancer Inst 2001; 93(12): 913–920.
Look MP, van Putten WLJ, Duffy MJ, et al. Pooled analysis of prognostic impact of uPA and PAI–1 in 8377 breast cancer patients. J Natl Cancer Inst 2002; 94(2): 116–128.
Harbeck N, Kates RE, Gauger K, et al. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I: novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thromb Haemost 2004; 91(3): 450–456. Review.
Hayes DF. Prognostic and predictive factors revisited. Breast 2005; 14(6): 493–499. Epub 2005 Oct 18. Review.
Topics
- Taxanes
- Prognostic factors
- Node-negative breast cancer
- UPA
- PAI-1
Keywords
Taxanes, prognostic factors, node-negative breast cancer, uPA, PAI-1


