Rationale
The accepted approach to breast conservation therapy (BCT) for early-stage breast cancer is the surgical removal of the primary breast lesion followed by whole-breast radiotherapy. With this approach, in-breast control rates exceeding 90% can be expected [1–4]. Standard of care presently dictates that all women should receive radiation after breast conservation surgery to optimize local control rates regardless of age or tumor size, however, the protracted course of whole-breast radiotherapy can present a logistical problem for many patients.
Review of both clinical and pathologic evidence finds that there are scarce data to support the concept that the entire breast requires treatment. In fact, review of pathologic and clinical failure patterns suggest that the primary target requiring treatment is likely limited to a 1–2 cm margin around the edge of the lumpectomy cavity [5–14]. If indeed the target volume can be restricted to a portion of the breast, then this reduction in volume provides the opportunity to accelerate the dose delivery while avoiding an increase in normal tissue toxicity.
Patient selection
Patients with a significant risk of harboring microscopic disease within the breast, but located outside of the stated treatment target (1–2 cm beyond the lumpectomy cavity), are not optimal candidates for accelerated partial breast irradiation (APBI). Two societies have endorsed conservative patient selection criteria and avoided the use of APBI in patients with a risk of disease remote from the lumpectomy cavity [15,16]. The American Brachytherapy Society patient selection criteria include: patients≥45 years of age, invasive ductal carcinoma only, tumor size of ≤3 cm, negative resection margins (no tumor on ink), and a negative axillary nodal status. Similarly, the American Society of Breast Surgeons selection criteria include: patients ≥50 years of age, invasive ductal carcinoma or ductal carcinoma-in-situ, tumor size of≤2 cm, negative resection margins (defined as at least 2 mm in all directions), and a negative axillary nodal status. An extensive intra-ductal component, limited positive-nodal status, infiltrating lobular histology, ductal carcinoma-in-situ, and young age have been used as exclusion criteria based on the successful early APBI treatment experiences.
Treatment technique
The multi-catheter interstitial brachytherapy approach is the APBI technique that has been in use the longest and has the most extensive follow-up [17]. With this approach, after-loading catheters are placed through the breast tissue surrounding the lumpectomy cavity. In general, catheters are placed in two to three planes, with an inter-catheter spacing of 1.0–1.5 cm, and an inter-planar separation not exceeding 3 cm. These implants generally require 14–20 catheters to assure proper dose coverage. The exact number of planes and catheters is determined by the size and shape of the target using established brachytherapy dosimetric guidelines [18,19]. Dosimetric treatment planning is then completed.
To assure that the goals of target coverage and dose homogeneity are achieved, advances in placement technique have been necessary to reduce the degree of operator dependence and improve the reproducibility of the procedure. The incorporation of image-guidance and computed tomography (CT)-based 3D planning has made a significant impact on the quality of multi-catheter brachytherapy implants. Several approaches have been established that allow physicians to adjust a technique to their specific clinical practice. Kuske [20] has described a method of closed-cavity implantation that is performed under local anesthesia. A biologically compatible contrast material is injected into the lumpectomy cavity under ultrasound guidance, revealing its shape and extent. A template system is used to place catheters under real-time fluoroscopic or mammographic guidance. Accurate coverage of the cavity is verified before the completion of the procedure.
At Virginia Commonwealth University, catheters are placed under real-time CT guidance [21]. The procedure is performed in the radiation oncology CT-simulation suite, under local anesthesia with conscious sedation. Initial guide catheters are placed based on the appearance of the cavity on a pre-brachytherapy CT scan. An intra-operative CT scan is then obtained to evaluate the position of the guide catheters in relation to the lumpectomy cavity, and adjustments are made as necessary. Catheters are placed free-hand, although a template could be incorporated with this approach. The implant is completed, and a final CT scan is obtained and transferred to the 3D planning system. The entire procedure, from the initial catheter placement to image acquisition for treatment planning, is completed in 1.5–2 h.
Another important development is the creation of conceptual tools to allow the quality of the implant to be assessed for both target coverage and dose homogeneity so that treatment experiences can be compared, local control optimized, and toxicity avoided. The incorporation of CT-based 3D planning has replaced two-dimensional (2D) planning as the standard of care. These planning systems allow for the calculation of dose–volume parameters (such as the volume of breast tissue receiving 100%, 150%, and 200% of the prescription dose) which appear to correlate with the incidence of fat necrosis, skin toxicity, and the development of fibrosis [4,22,23].
With the incorporation of image-guided catheter placement techniques (stereotactic mammography, ultrasound or CT-guided) and 3D dosimetric planning, the multi-catheter approach has evolved into a reliable and reproducible technique. This approach is the most adaptable APBI technique and can be used in a variety of treatment situations, regardless of lumpectomy cavity size, shape, or location within the breast.
Treatment experience
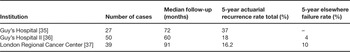
The number of published ABPI experiences continues to increase yearly. The majority of the patients treated with the longest follow-up in these reports have been treated with the multi-catheter interstitial technique. The successful published interstitial brachytherapy experiences are summarized in Table 1 [22,24–34]. Collectively, these trials represent an experience of hundreds of patients and demonstrate in-breast failure rates of less than 5%. These treatment experiences share in common conservative selection criteria and treatment delivery techniques that assured the coverage of an appropriately defined target. In three reports [35–37], unacceptable in-breast disease control rates were observed (Table 2). These higher rates of in-breast failure appear to be directly related to the lack of patient selection criteria and/or treatment quality assurance. For example, microscopic margin assessment was not employed in the earlier Guy's Hospital experience and it is not clear whether the patients treated were appropriate for breast conservation at all. Additionally, the authors themselves question the methods of target delineation and the ability to confirm dosimetric coverage of the target [35]. At the London Regional Cancer Center, the target appears to have been limited to the cavity only, without surrounding tissue at risk included, questioning the validity of their target delineation and coverage [37]. These publications further validate that the success of APBI is dependent on proper patient selection and quality assurance of treatment delivery.
Table 1. Successful multi-catheter APBI series.
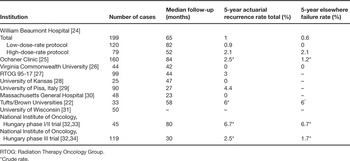
Table 2. Multi-catheter APBI series with unfavorable results.
Future directions
With the emergence of simplified brachytherapy techniques (balloon catheter) and non-invasive approaches (3D-CRT), the use of multi-catheter brachytherapy is likely to be limited to specific centers in selected patients. Continued studies are necessary to address questions regarding patient selection criteria and details of treatment technique. With the continued reporting of the initial trials and the initiation of additional single and multi-institutional phase I/II trials and phase III prospective randomized trials, these questions will be appropriately addressed and further define the role of APBI in the management of early-stage breast cancer.




