Second-generation antipsychotics (SGAs) are a group of medications that include clozapine, olanzapine, risperidone, quetiapine, ziprasidone, paliperidone, lurasidone, asenapine and aripiprazole. SGAs were developed in the 1990s and are approved in Canada for the treatment of adolescents and adults with schizophrenia and bipolar disorder, and in adults as an adjunctive treatment for depression unresponsive to antidepressants (quetiapine and aripiprazole). In addition, SGAs are often prescribed off-label, for insomnia and generalised anxiety disorders in adults,Reference Pringsheim and Gardner1 as well as to children with aggressive behaviour and conduct disorders.Reference Pringsheim, Lam and Patten2 The development of SGAs was met with high expectations, as they appeared to lack many of the adverse extrapyramidal side-effects associated with first-generation antipsychotics.Reference Divac, Prostran, Jakovcevski and Cerovac3 However, in randomised controlled trials (RCTs), SGAs have been shown to be associated with adverse metabolic side-effects such as weight gain, dyslipidaemia, hyperglycaemia, hypertension and type 2 diabetes,Reference Chaplain and Taylor4 leading to treatment discontinuationReference McEvoy, Meyer, Goff, Nasrallah, Davis and Sullivan5 and severe long-term health consequences in many patients.Reference Kurzthaler and Fleischhacker6
Although RCTs have established a relationship between SGAs and metabolic dysfunction in clinical trial populations, research has been generally limited to short-term trials that explore incident cases, and therefore cannot account for other factors that influence prevalence in the population, such as disease severity and duration. The relationship between SGAs and metabolic side-effects is especially important because the underlying diseases that SGAs are used to treat carry risks of metabolic dysfunction as well. Several studies have shown that metabolic abnormalities and coronary disease are more prevalent in people with schizophrenia.Reference Hagg, Lindblom, Mjorndal and Adolfsoon7–Reference Mortensen and Juel9 Further, people with mental illnesses are significantly more likely to develop and die prematurely as a result of cardiovascular disease, regardless of antipsychotic treatment.Reference Harris and Barraclough10, Reference Laursen, Munk-Olsen and Vestergaard11 A solid understanding of the population-level associations between SGAs and health can add to the understanding of the effect of these medications, an important consideration for both clinicians and patients in managing treatment. Here, we aimed to quantify the association between SGAs and metabolic dysfunction in the population, using a large and nationally representative sample.
Method
Objectives
This research aimed to address two objectives. The primary objective was to quantify the association between SGAs and evidence of metabolic dysfunction in the Canadian population. To assess this association, three separate cycles of the Canadian Health Measures Survey (CHMS), a cross-sectional Canadian population health survey, were examined. To understand the overall effect of these associations, an understanding of the frequency of SGA use in Canada is also necessary. Other studies have shown that SGA use has recently increased in Canada and as such, a second objective was to identify the number of Canadians taking SGAs and assess how the prevalence of use has changed over the three cycles of the CHMS.
Data source and participants
The CHMS is a cross-sectional survey of Canadians aged 3–79 years.12 Launched in 2007, the purpose of the CHMS is to directly collect nationally representative data to enable the study of disease, health status and emerging public health issues in the Canadian population.12 To produce a nationally representative sample, the CHMS sample is stratified by 11 age–gender groups, with approximately 500–600 units per group.12 The nationwide sampling frame was stratified across five regions based on census data, and grouped with respect to provincial boundaries, health regions and population-density data.12 The first phase of CHMS data collection involved a household visit, where a Statistics Canada interviewer obtained information from selected respondents by computer-assisted personal interview.Reference Tremblay, Wolfson and Gorber13 Content of the household visit questionnaires includes variables related to health status, nutrition and food, medication use, health behaviours, environmental factors and socioeconomic information.Reference Tremblay, Wolfson and Gorber13 The mobile clinic portion of the CHMS involves screening, blood and urine collection, cardiorespiratory measurements, anthropomorphic measurements and muscular strength assessments.Reference Bryan, St-Denis and Wojtas14 The survey does not include approximately 4% of the Canadian population, as persons living on reserves, in aboriginal settlements, in the three territories, members of the armed forces, institutionalised persons and residents of certain remote regions were not sampled.12 Detailed reports of the sampling strategy have been previously published.12
The first three cycles of the CHMS, covering the period 2007–2013, were used in this research. Prevalence estimates were obtained using all CHMS respondents aged 3–79 years, whereas only those aged 18 years and older were included in our analysis of metabolic dysfunction because of changing cut-off values for metabolic indicators over the life cycle. Permission to use the CHMS data was obtained through an application to Statistics Canada, and an ethics waiver was also obtained from the Conjoint Health Research Ethics Board at the University of Calgary, consistent with the standards of the Tri-Council Policy Statement 2 (2014). Furthermore, data was accessible only through the Statistics Canada Prairie Regional Data Centre, located at the main campus of the University of Calgary. Statistics Canada analysts vetted all research outputs gathered in the Prairie Regional Data Centre to ensure Statistics Canada research and confidentiality guidelines were upheld. Sample size restrictions laid out by Statistics Canada were followed in this analysis; some associations that were examined were not reportable under these guidelines.
SGA status
The Anatomical Therapeutic Chemical Classification System was used to code medication data in the CHMS (https://www.whocc.no/atc_ddd_index/). Metabolic dysfunction was assessed in all participants aged >18 years that took SGAs and those that did not. Prevalence estimates were obtained for individual SGAs when possible and for SGA use as a group. For analysis of metabolic outcomes, respondents taking multiple SGAs were not viewed differently than respondents taking only one SGA. Participants that took multiple SGAs could not be analysed separately from those that took one SGA because of sample size restrictions.
Outcomes
Several indicators of metabolic dysfunction were assessed, including weight, body mass index (BMI), waist circumference and systolic and diastolic blood pressure. Derived variables were created to group CHMS respondents based on their metabolic characteristics. Definitions for these variables can be found in Table 1. We also used two definitions of metabolic syndrome, outlined by the International Diabetes Federation (IDF) and the National Cholesterol Education Program–Adult Treatment panel III (NCEP–ATP III), to assess for metabolic dysfunction in CHMS respondents. These definitions can also be found in Table 1.15, Reference Alberti, Zimmet and Shaw16
Table 1 Derived variable definitions
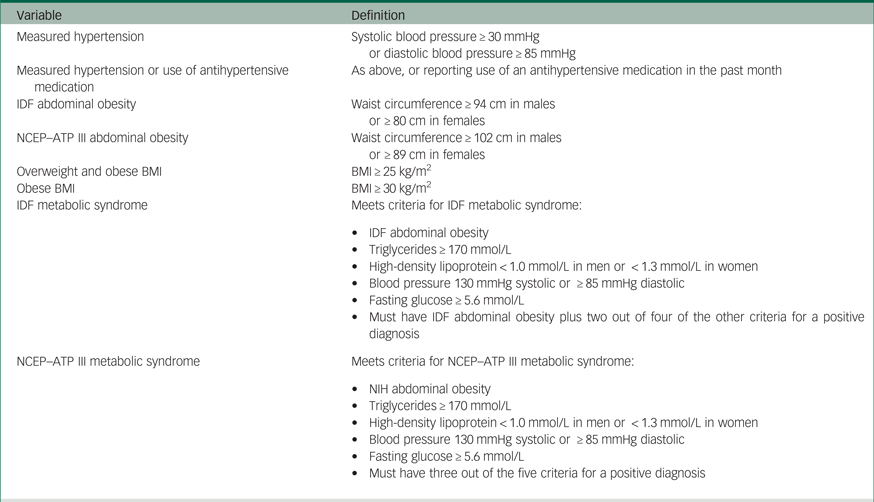
BMI, body mass index; IDF, International Diabetes Federation; NCEP–ATP III, National Cholesterol Education Program-Adult Treatment Panel III; NIH, National Institutes of Health.
Analysis
Logistic and linear regression models were used to compare respondents taking SGAs with those that did not, on all metabolic outcomes. An assessment of effect measure modification and confounding by age and gender was conducted by nested models. Effect measure modification was examined for statistical significance with a likelihood ratio test. Confounding was examined through manual backwards elimination of interaction terms. Interaction terms were removed from the model individually and a subjective assessment of any change in the association was performed to determine if individual variables acted as confounders in the model. A non-parametric sign test was performed to test the expected directionality of our associations. Sample estimates were calculated with survey weights provided by Statistics Canada to create population representative estimates. A bootstrap variance estimation procedure was used to adjust the variance and confidence intervals of our prevalence estimates to account for the complex clustered sampling design. STATA 13.1 (StataCorp LP, College Station, Texas) was used as the statistical software for all data analysis presented here. When P-values were obtained, an a priori α significance level of 0.05 was used.
Results
Trends in SGA use
The estimated prevalence of all SGA use and the estimated prevalence of quetiapine use were determined for each cycle of the CHMS. To maintain survey respondent confidentiality, Statistics Canada requires minimum cell counts to be met when estimates are released to the public. Because of these requirements, separate prevalence estimates for other SGAs could not be published. The estimated percentage of Canadians taking any SGA or quetiapine during each cycle of the CHMS is displayed in Table 2 and Fig. 1. Both total SGA and quetiapine use increased over the three cycles of the CHMS; proportions of both approximately tripled from cycle one to cycle three.
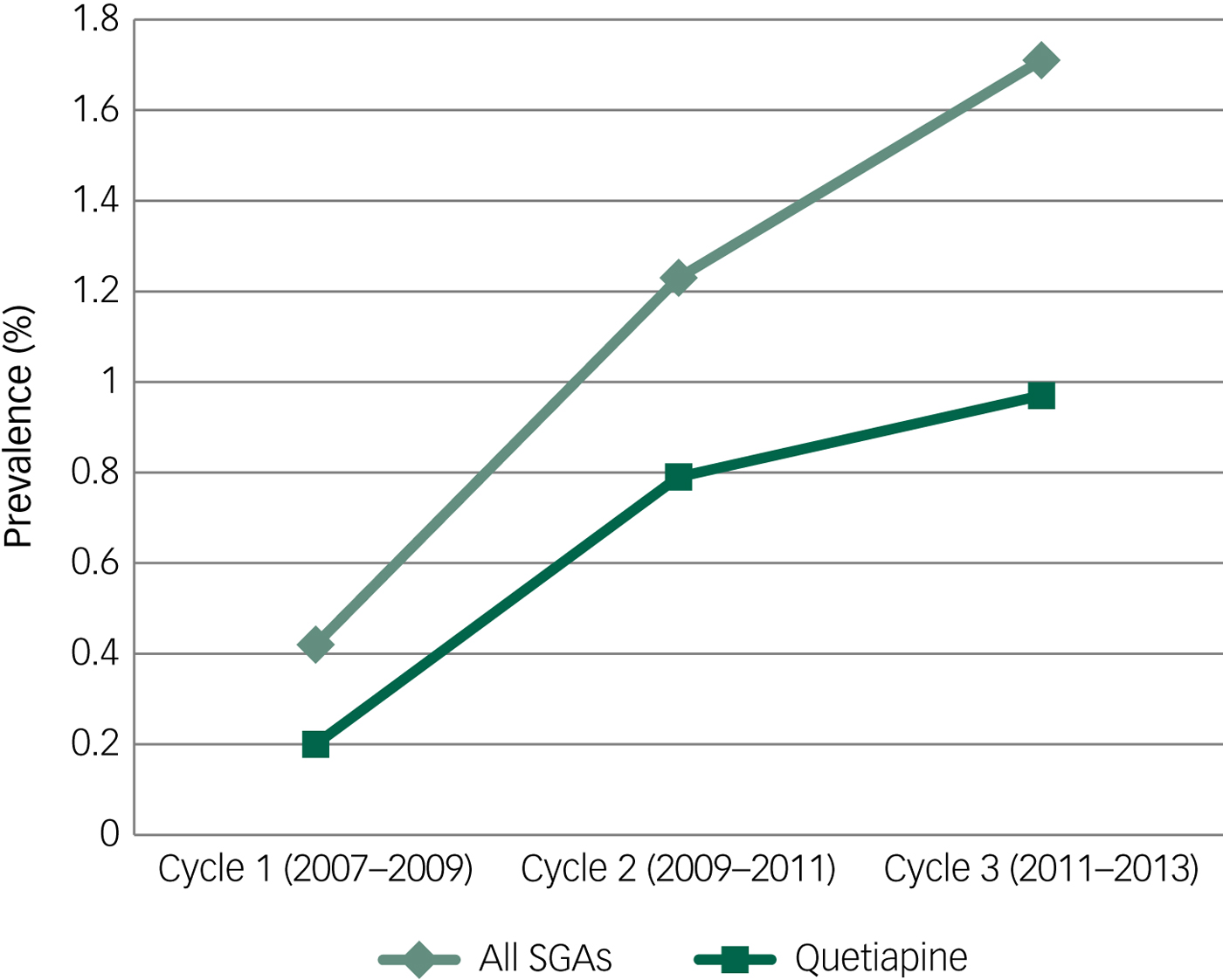
Fig. 1 Prevalence of second-generation antipsychotics (SGAs) and quetiapine use in each cycle of the Canadian Health Measures Survey.
Table 2 Prevalence of second-generation antipsychotics (SGAs) and quetiapine use in each cycle of the Canadian Health Measures Survey
The estimated proportions of total SGA use attributed to quetiapine, risperidone and other SGAs were calculated and are shown in Table 3 and Fig. 2. There were no significant differences in the estimated proportions of total SGA use attributed to quetiapine, risperidone or other SGAs when calculated separately for each cycle, therefore the separate cycle estimates were combined to increase precision. Based on the CHMS data, quetiapine use accounts for approximately half of all SGA use in Canada. Risperidone is the second most commonly used SGA in Canada, with approximately one-quarter of respondents who use SGAs reported as taking risperidone. Less than one-quarter of respondents taking SGAs in Canada use an SGA other than quetiapine or risperidone.
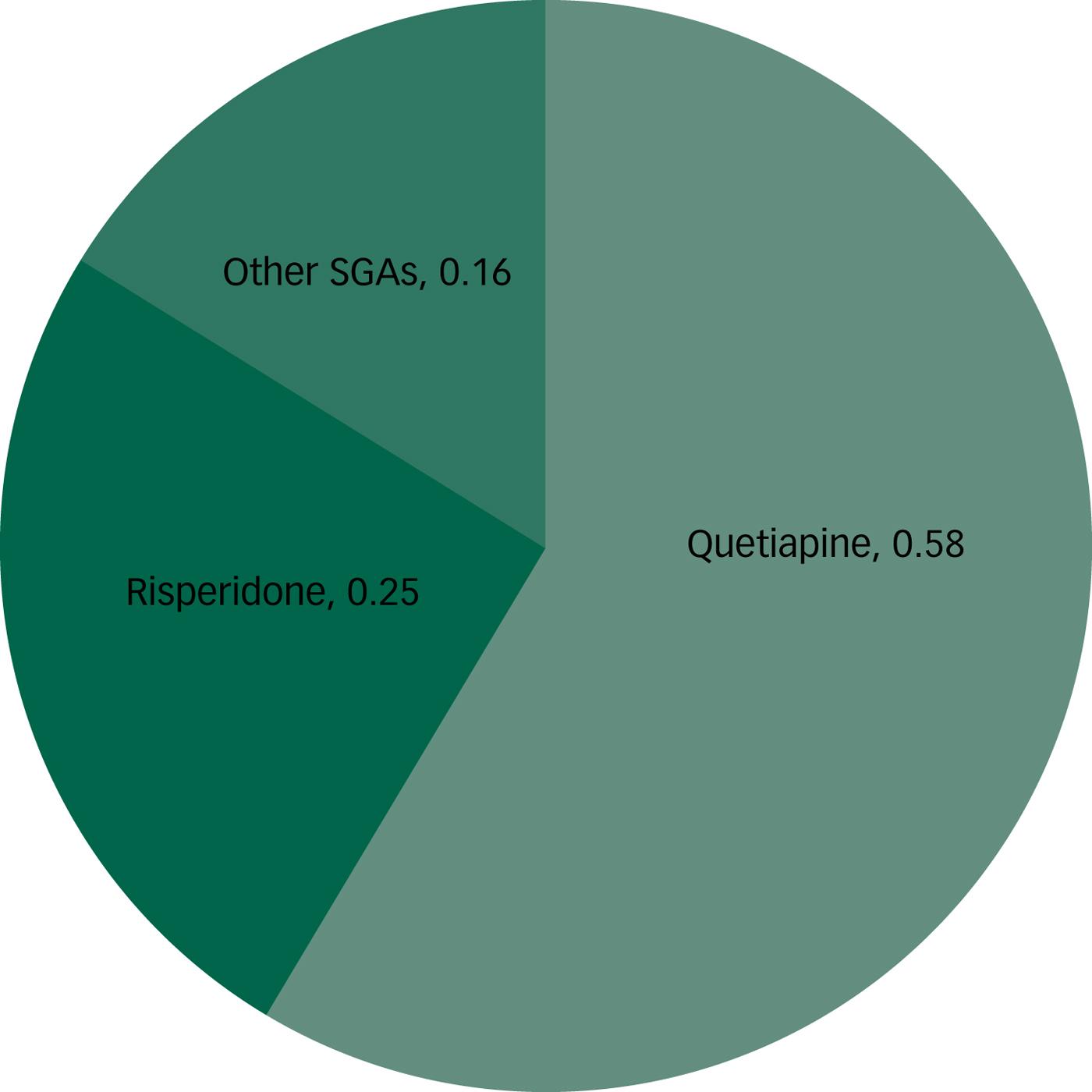
Fig. 2 Proportion of individuals taking quetiapine, risperidone or other second-generation antipsychotic (SGA). Please note the remaining 0.01 can be considered ‘decimal dust’.
Table 3 Proportion of individuals taking quetiapine, risperidone or other second-generation antipsychotics (SGAs)

Metabolic dysfunction
SGA use was significantly associated with hypertension (as defined by measured hypertension or use of an antihypertensive medication) and the NCEP–ATP III definition of abdominal obesity in adults (Table 4). Other indicators of metabolic dysfunction (measured hypertension, IDF-defined abdominal obesity, overweight and obese BMI, obese BMI) and both definitions of metabolic syndrome (NCEP–ATP III and IDF) had estimated odds ratios >1, but the 95% confidence intervals crossed the null value of 1. All eight of the associations examined in adult CHMS respondents have an estimated odds ratio of >1, leading to a significant sign test (P = 0.0039). This shows that, when taken in aggregate, indicators of metabolic dysfunction are associated with SGA use in adults.
Table 4 Association between indicators of metabolic dysfunction and second-generation antipsychotic use in cycles 1–3 of the Canadian Health Measures Survey

BMI, body mass index; IDF, International Diabetes Federation; NCEP–ATP III, National Cholesterol Education Program-Adult Treatment Panel III.
a. Significantly different from 1 (P < 0.05).
Age was not found to be an effect measure modifier or confounder. Some variables included gender in the coding, such as abdominal obesity, because different cut-off values were considered for males and females. For measures where this was not the case (measured hypertension, measured hypertension/use of an antihypertensive medication and both BMI variables), gender was considered in the model as a potential covariate. Gender was not found to be an effect measure modifier or confounder, but gender-adjusted odds ratios are still presented when possible. Odds ratios and 95% confidence intervals for indicators of metabolic dysfunction and both definitions of metabolic syndrome can be found in Table 4.
Continuous variables were further examined by linear regression to calculate estimated mean differences between respondents taking SGAs and those not taking SGAs in the combined/adults file. All variables were examined in all adults, and in males and females separately. Estimated mean differences can be found in Table 5. When examined as continuous variables, those taking SGAs had significantly higher estimated waist circumference and higher estimated BMI; however, a significant difference in estimated BMI was not seen when stratified by gender. All other ratings were higher in respondents taking SGAs, but not significantly so. Estimated differences in systolic and diastolic blood pressure were clinically negligible, however, these ratings were not adjusted for antihypertensive medication use.
Table 5 Association between indicators of metabolic dysfunction and second-generation antipsychotic use in cycles 1–3 of the Canadian Health Measures Survey
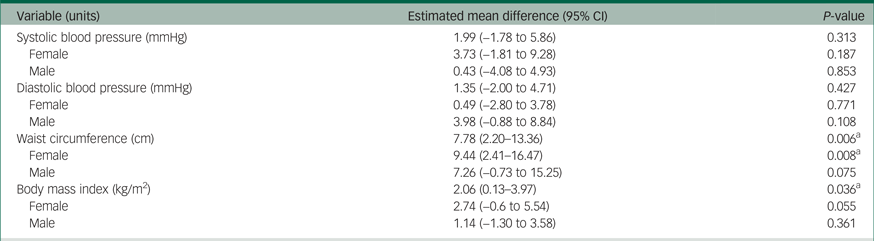
a. Significantly different from 0 (P < 0.05).
Discussion
This study was designed to investigate SGA use in the Canadian population. SGAs are a well-studied group of medications; however, much of the knowledge about SGAs is limited to clinical populations. This study contributed to the overall knowledge of these medications by expanding that information to the household population, an especially important consideration given the frequent off-label use of these medications.
Data from the CHMS confirmed and expanded on results from previous studies showing increasing SGA use in the Canadian population.Reference Pringsheim and Gardner1, Reference Alessi-Severini, Biscontri, Collins, Kozyrskyj, Sareen and Enns17–Reference Murphy, Gardner, Cooke, Kisely, Hughes and Kutcher20 This study is the first to confirm these results with data from a source other than a prescription drug database. The data presented here illustrate that the number of people taking SGAs is increasing, with an approximately threefold increase in the proportion of Canadians taking SGAs in general and the proportion of Canadians taking quetiapine between cycle one (2007–2009) and cycle three (2011–2013). This is consistent with the reported 300% increase in quetiapine prescriptions dispensed in a similar time period (2005–2012).Reference Pringsheim and Gardner1 Furthermore, our reported quetiapine prevalence in cycle three (0.97%, 95% CI 0.36–1.56%) is in line with findings from a provincial study in Alberta showing a quetiapine use prevalence of 1.3%.Reference Duncan, Cooke, Symonds, Gardner and Pringsheim21 Our reported prevalence estimates include children and adults, as separate proportions for the adult and child populations could not be released. However, based on previous studies that have examined SGA prescription rates for these age groups separately, it is thought that the increase in proportions is valid as an all ages measure, as use has been increasing in Canada for all age groups.
Studies have shown that the estimated prevalence of bipolar type 1 disorder and major depressive disorder have not changed in recent years in Canada.Reference McDonald, Bulloch, Duffy, Bresee, Williams and Lavorato22–Reference McDonald, Bulloch, Duffy, Bresee, Williams and Lavorato24 It is unlikely, then, that the increase in SGA use can be attributed to any increase in the prevalence of the underlying conditions. Therefore, the observed increase is most likely attributable to other factors, including the approval of quetiapine for the adjunctive treatment of major depressive disorder in 2009, increased off-label prescribingReference Pringsheim and Gardner1, Reference Duncan, Cooke, Symonds, Gardner and Pringsheim21 or a switch in preference to SGAs by clinicians and patients. The reason for this increase could not be directly studied in this data-set, nor could assumptions about off-label usage suggested by other studies be examined by dosage information. Some studiesReference Herbeck, West, Ruditis, Duffy, Fitek and Bell25–Reference Connolly and Taylor27 have examined factors that influence providers' choice in SGA prescribing patterns, but have found inconsistent results regarding the role of patient characteristics on medication choice. In summary, the increase in SGA use in the Canadian population has been confirmed by this study, yet SGA prescribing patterns in general are still inadequately described.
We found the prevalence of hypertension, diagnosed by direct measurement plus use of an antihypertensive medication, and abdominal obesity defined by the NCEP–ATP III criteria were significantly higher in respondents taking SGAs than those that did not. The prevalence of abdominal obesity defined by the IDF criteria, obese BMI and overweight BMI were higher, but not statistically significantly higher, in respondents taking SGAs than those that did not. The prevalence of obese BMI and overweight/obese BMI in adult respondents that did not take SGAs in all three cycles of the CHMS are in line with other studies reporting on increasing and elevated weight in Canadians. We found an estimated 61% (95% CI 58.4–63.5%) of adult respondents that did not take SGAs had a BMI ≥25 and an estimated 25.4% (95% CI 23.2–27.6%) had a BMI ≥30; previous reports of all adult Canadians have shown the prevalence of BMI ≥25 to be approximately 45% and BMI ≥30 to be approximately 23%.28 These results show that increased weight, as demonstrated by elevated BMI, is a problem for the general Canadian population regardless of SGA use. Furthermore, the IDF definition of abdominal obesity uses relatively low cut-off values, and thus nearly 60% of Canadians have abdominal obesity based on these guidelines. On the other hand, the NCEP–ATP III criteria for abdominal obesity are higher, thus only very abdominally obese persons would qualify based on these criteria. As a significantly higher proportion of respondents taking SGAs fall into this category, it is possible that SGA use is associated with extremely elevated abdominal obesity, as opposed to the more moderate abdominal obesity reflected in the IDF criteria.
The same trend is seen in the associations between SGA use and metabolic syndrome. All odds ratios are elevated in the SGA group, with a significant sign test indicating that SGA use is generally associated with a trend toward metabolic dysfunction. These results confirm findings from previous studies in sub-populations and expand their generalisability to the household population. In our study, hypertension and abdominal obesity as defined by the NCEP–ATP III are most strongly associated with SGA use in the general population, again indicating that extreme abdominal obesity is more prevalent in respondents taking SGAs than in the general population. As abdominal obesity has been the metabolic indicator most closely linked with increased risk of cardiovascular disease, these results are especially troubling. Significant results in other indicators were likely not found because of limited sample size and power. Large variance in the sample and correspondingly wide confidence intervals can be seen; we hypothesise that with a larger sample size, these associations would also be statistically significant.
These findings are also confirmed in the linear regression analysis. BMI was significantly different in respondents taking SGAs, although when stratified by gender, the difference became insignificant. Females who were taking SGAs were shown to have a mean waist circumference of 9.44 (95% CI 2.41–16.47) cm greater than their counterparts who did not take SGAs. Males who were taking SGAs also had a larger mean waist circumference, although it was not statistically significantly different at 7.26 (95% CI −0.73 to 15.25) cm. When examined as a continuous variable, both systolic and diastolic blood pressure were non-significantly higher in respondents taking SGAs than those that did not. This analysis could not be adjusted for use of an antihypertensive medication because of sample size restrictions; it is likely that such an adjustment would alter this result.
Limitations
Although the literature has established, and we have confirmed, that the use of SGAs has rapidly increased in recent years, the overall prevalence of SGA use remains low. In the latest cycle (2012–2013) of the CHMS, we estimated that the per cent of Canadians taking any SGA was 0.93–2.50. Even with a large population-based survey, this left a relatively small number of people taking SGAs in which to examine the associations we aimed to quantify in this study. The small sample size, combined with a complex sampling design and Statistics Canada sample size release requirements, led to some important considerations in our analysis. For example, those that took multiple SGAs could not be separately studied from those that took a single SGA. SGA use rates could also only be separately calculated for risperidone and quetiapine, and trends for use of other SGAs such as clozapine and olanzapine are not reported. The small sample size is also an important factor to consider when examining the reported 95% confidence intervals, which were often wide and therefore imprecise. Given these limitations, increasing the sample size in future studies would be helpful to obtain more precise estimates and allow for a successful and reliable use of the bootstrap variance estimation procedure.
Potential confounders that we were unable to consider could also affect our results. Although we adjusted some of our estimates for gender, we were not always able to do so because of sample size considerations. Other potential confounders we could not include in our analysis for the same reason, such as age, underlying diagnosis and socioeconomic variables, could also bias our results. Furthermore, a high percentage of people taking SGAs in our study reported having a mood disorder. Other treatments for mood disorders, such as selective serotonin reuptake inhibitors, have been shown to cause weight gain.Reference Aronne and Segal29 It was not possible to remove participants on one of these other medications from our analysis because of the sample size consideration; a re-analysis that takes this potential confounder into consideration would be suggested if future studies have sufficient sample size to do so. However, we have been able to show that that associations between SGA use and metabolic dysfunction seen in RCTs have carried over into the general household population.Reference Aronne and Segal29, Reference Printz, Clark, Stricks and Malaspina30 Because of the limitations discussed here, the results of this study should be interpreted in context and evaluated within the broader scope of the literature pertaining to SGAs and metabolic side-effects.
In conclusion, these findings, coupled with the continued increase in SGA use in Canada, indicate that SGAs are having a significant effect in the Canadian population. Knowing that the association between SGAs and metabolic dysfunction has been established by a variety of methodologies and has been studied both in clinical and household populations, it is imperative that clinicians are educated and seriously undertake metabolic monitoring of patients taking SGAs. Furthermore, as previous researchReference Haupt, Rosenblatt, Kim, Baker, Whitehead and Newcomer31, Reference Morrato, Druss, Hartung, Valuck, Allen and Campagna32 has shown that rates of metabolic monitoring in patients with psychiatric disorders who are taking SGAs remain low despite published guidelines, strategies to increase adherence to these recommendations should be developed and implemented.










eLetters
No eLetters have been published for this article.