The economic burden of depression is substantial. The total cost of adult depression across England in 2007 was more than £7.5 billion, including £1.6 billion for health and social care and £5.8 billion for loss of earnings.Reference McCrone, Dhanasiri, Patel, Knapp and Lawton-Smith1 Many of these costs are due to refractory depression or treatment-resistant depression. For example, CrownReference Crown, Finkelstein, Berndt, Ling, Poret and Rush2 found that depression-related costs for in-patients with treatment-resistant depression were 19 times greater than those for other in-patients, and the costs for out-patients with treatment-resistant depression were 2.5 times greater than for other out-patients. This paper evaluates the cost-effectiveness of radically open dialectical behaviour therapy (RO DBT), a new treatment targeting overcontrolled personality, common in refractory depression.Reference Lynch, Whalley, Hempel, Byford, Clarke and Clarke3
Method
Design
The Refractory Depression – Mechanisms and Efficacy of Radically Open Dialectical Behaviour Therapy (RefraMED) study (trial registration: ISRCTN85784627) included a three-centre parallel-group randomised trial that compared RO DBT plus treatment as usual (TAU) with TAU alone and an integrated economic evaluation. We assessed participants at baseline and 7, 12 and 18 months after randomisation; the primary end-point of the trial was 12 months after randomisation.
Participants
Patients were eligible for the RefraMED trial if they: were 18 years or older; had an IQ >70; spoke English well enough to participate; had a current diagnosis of major depressive disorder in the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I);Reference First, Spitzer, Gibbon and Williams4 had refractory depression, defined below; and had a Hamilton Rating Scale for Depression (HRSD)Reference Williams, Kobak, Bech, Engelhardt, Evans and Lipsitz5 score of at least 15.
Definitions of refractory depression vary across studies. For example, Berlim & TureckiReference Berlim and Turecki6 found more than ten different descriptive terms for treatment-resistant/refractory major depression.
In the present study we define refractory depression as either chronic depression, that is depression lasting at least 2 years, or treatment-resistant depression, that is recurrent depression (which we operationalised as two or more previous episodes) which has not responded to an adequate dose of antidepressant medication for at least 6 weeks in the current episode.
Interventions
TAU
All participants received TAU, including prescribed antidepressant medication or psychotherapy.Reference Lynch, Hempel, Whalley, Byford, Chamba and Clarke7 In addition, control participants could access any treatment offered by the UK National Health Service (NHS) or privately, except standard DBT.
RO DBT
RO DBT is a transdiagnostic therapy designed to address a spectrum of disorders that are difficult to treat, in particular chronic depression.Reference Lynch8 It differs from other psychotherapies most notably by encouraging social bonding through emotional expression and ‘social signalling’, defined as any behaviour that an individual performs in the presence of another person, regardless of intention or awareness. At the time of the trial, RO DBT comprised 29 weekly individual therapy sessions each lasting 1 h and 27 skills training classes each lasting 2.5 h.Reference Lynch, Whalley, Hempel, Byford, Clarke and Clarke3,Reference Lynch8 Although participants continued to receive antidepressant medication as prescribed, we strongly discouraged them from seeking additional psychotherapy during RO DBT. Further information on RO DBT is contained in the clinical paper.Reference Lynch, Hempel, Whalley, Byford, Chamba and Clarke9
Economic perspective
The economic perspective was that of the UK NHS and personal social services, as preferred by the UK National Institute for Health and Care Excellence (NICE).10 We also explored the addition of productivity losses resulting from time off work due to illness, in sensitivity analysis.
Method of economic evaluation
The primary method of economic evaluation was cost–utility analysis, with effects measured in quality-adjusted life-years (QALYs), as preferred by NICE.10 We also undertook a cost-effectiveness analysis, with effects measured in terms of depressive symptoms, the primary outcome of the RefraMED trial.
Outcomes
For our primary economic evaluation, we estimated QALYs using the EQ-5D-3L measure of health-related quality of life.Reference Brooks and Group11 The EQ-5D-3L is a generic, preference-based measure for describing and valuing health-related quality of life.Reference Brooks and Group11 It rates health in five domains – mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. The health states described in the EQ-5D-3L were assigned a utility weight or score using responses from a representative sample of adults in the UK.Reference Dolan12 These weights were applied to the time between interviews and QALYs calculated using the area under the curve approach.Reference Richardson and Manca13 The EQ-5D has been validated in economic evaluations for common mental health disorders.Reference Mulhern, Mukuria, Barkham, Knapp, Byford and Soeteman14 For our secondary economic evaluation, effects were measured in terms of depression using the HRSD,Reference Williams, Kobak, Bech, Engelhardt, Evans and Lipsitz5 which was the primary clinical outcome of the RefraMED trial.
Resource use
We collected resource-use data using a version of the Adult Service Use Schedule (AD-SUS)Reference Kuyken, Byford, Taylor, Watkins, Holden and White15 designed for populations with depression. Research assessors completed this in interview with participants and recorded all use of hospital and community-based health and personal social care. For medications, we asked participants to report prescribed antidepressants, antipsychotics, sleeping tablets and painkillers. To avoid unmasking of research assessors, participants reported their use of all talking therapies on a separate self-reported questionnaire. We collected information about time off work due to illness alongside the AD-SUS using the productivity questions from the World Health Organization Health and Work Performance Questionnaire (HPQ).Reference Kessler, Barber, Beck, Berglund, Cleary and McKenas16 We asked participants to complete the AD-SUS and HPQ at baseline, to cover the previous 6 months, and at the 7-, 12- and 18-month interviews, to cover the time since the previous interview, thus covering the full period from baseline to final follow-up. We abstracted information on RO DBT resource use, including the number of individual and group sessions attended by each participant, from therapy records.
Unit costs
With the exception of RO DBT, we estimated mean costs of health and social services for each group by multiplying participants’ reported use by unit costs from national sources.Reference Curtis and Burns17–19 All unit costs, summarised in supplementary Table 1 (available at https://doi.org/10.1192/bjo.2019.57), were for the financial year 2014–2015, uprated where necessary using the Hospital and Community Health Services Index.Reference Curtis and Burns17 We based medication costs on the median dose of the most common category of drug (e.g. antidepressant, antipsychotic) reported by participants. We used participant-reported start and finish dates to estimate duration of their time using that drug, assuming full adherence. We estimated the costs of depression-related absenteeism and presenteeism for participants in paid employment using the human capital approach based on the national gross average wage.Reference Drummond, Sculpher, Claxton, Stoddart and Torrance20,Reference Woo, Kim, Hwang, Frick, Choi and Seo21
We estimated RO DBT costs using the micro-costing approach developed by the Personal Social Services Research Unit (PSSRU) at the University of Kent.Reference Netten, Knight, Dennett, Cooley and Slight22 We costed individual sessions from the average therapist Agenda for Change salary bands, including employer's costs (national insurance and pension contributions) and overhead costs (buildings, utilities, management and administration) from national sources.Reference Curtis and Burns17,Reference Curtis23 We weighted salary costs to cover therapists’ time away from their own patients using information from 16 RO DBT therapists on the time they spent running RO DBT therapy sessions and on other activities, which suggested a direct:non-direct ratio of 1:0.91. Individual sessions lasted 60 min on average. Supplementary Table 2 details our method of valuing therapist time.
We costed RO DBT group sessions on the basis that they were closed to other patients and went ahead irrespective of how many participants attended.Reference Barrett, Byford and Curtis24 We allocated the total cost of each group session across all participants invited to attend, regardless of whether or not they did attend. Group sessions lasted 2.5 h on average. The number of therapists running groups varied by group size. Groups larger than three patients were typically run by two therapists. The valuation of the cost of these group sessions is summarised in supplementary Table 3.
We did not include DBT-specific training costs because equivalent costs for the control group could not be easily identified and costed, making comparison difficult. In clinical practice, therapists undertake a wide range of training as part of professional development. It is therefore reasonable to assume that RO DBT training, if rolled out, would form part of this professional development in the same way as training received in other therapies, such as cognitive–behavioural therapy (CBT). Similarly, we excluded from analysis the cost of participants who failed to attend RO DBT individual sessions, given the absence of equivalent data for the control group. Only when comparing two specific therapies is it possible to record and cost non-attendance for both groups. In this study TAU varied; thus, participants reported attendances with therapists and other health professionals, but not non-attendances. Furthermore, we assumed that therapists would undertake other work activities during the time freed by non-attendances, thus reducing the cost of those non-attendances, potentially to zero.
Statistical analysis
Costs and outcomes
We initially present descriptive data on costs and outcomes adjusted for baseline differences in costs and relevant outcomes plus pre-specified clinical predictors as outlined in the accompanying clinical paper.Reference Lynch, Hempel, Whalley, Byford, Chamba and Clarke9 We adjusted cost-effectiveness analyses for the same pre-specified clinical predictors and baseline values of the variables of interest (costs, EQ-5D-3L or HRSD). We analysed cost differences by t-tests with confidence intervals around adjusted mean differences estimated using non-parametric bootstrapping to reflect non-normality of cost data.Reference Briggs and Gray25 We imputed missing cost, QALY and HRSD data using ‘multiple imputation using chained equations’ (MICE), under the assumption that these data were missing at random.Reference White, Royston and Wood26 We set the number of multiply imputed data-sets (m) to be equal to the fraction of incomplete service-use information (30%; m = 30) at the 12-month follow-up, as recommended by White and colleagues.Reference White, Royston and Wood26 Multiple imputation reports the sensitivity of results to missing data and the assumption that the data are missing at random. There is evidence that multiple imputation provides less biased estimates of costs and effects than complete case analysis, unless data are missing not at random.Reference White, Royston and Wood26 We set the number of bootstrap replications to 1000 for each of the 30 multiply imputed data-sets (30 000 bootstrap replications).Reference Schomaker and Heumann27
Cost-effectiveness analysis
We conducted the pre-specified primary cost-effectiveness analysis after 12 months, as with the clinical analyses. We assessed cost-effectiveness by estimating incremental cost-effectiveness ratios, which divide the difference in costs between two interventions by the difference in outcomes.Reference Van Hout, Al, Gilad, Gordon and Rutten28 We did not conduct a power calculation for the cost-effectiveness analysis because this is problematic owing to the large variability in resource use and cost measures, and the complexity of forecasting the joint distribution of the difference in costs and benefits between treatment arms. Instead we followed recommendations to take a decision-making approach and focus on estimating cost and QALY differences, and estimating the likelihood that RO DBT is cost-effective compared with TAU, given the data available.Reference Briggs29 We generated the joint distribution of incremental mean costs and effects for RO DBT relative to TAU using non-parametric bootstrapping to explore the probability that one of the groups is the better choice given NICE's ‘willingness-to-pay’ threshold of £20 000–£30 000 per QALY. We characterised uncertainty in the cost and effectiveness estimates by cost-effectiveness acceptability curves.Reference Fenwick and Byford30
Sensitivity analysis
We conducted five sensitivity analyses to explore the impact of variations in methods and assumptions on the relative cost-effectiveness of RO DBT and TAU:
(a) a complete case analysis for comparison with the results using multiple imputation for missing data;
(b) a broader economic perspective, additionally including the cost of absenteeism from work, given that depression is known to have a substantial effect on employment;Reference Kessler, Barber, Birnbaum, Frank, Greenberg and Rose31
(c) adjustment of the cost of RO DBT group sessions to address the fact that group session attendance was particularly low and thus costs particularly high relative to the cost of groups reported in similar studies; on the assumption that group attendance is unlikely to be as low in routine NHS services, we replaced the estimated cost of the RO DBT groups with the national cost applied to participants reporting group therapy attendances (£14 rather than £99);
(d) analysis of cost-effectiveness after 18 months to explore over a longer period the effect of RO DBT relative to TAU;
(e) analysis of cost-effectiveness using cost per point difference in the primary clinical outcome, the HRSD, as the measure of effect.
Ethical approval and conduct
Before starting the trial we gained approval from the Hampshire Research Ethics Committee (National Research Ethics Service (NRES) reference 11/SC/0146) and the Research Governance Department of the University of Southampton, the sponsor of this trial. We asked trial participants for consent on three occasions: oral before telephone screening; signed before baseline assessment; and signed before randomisation.
Patient and public inclusion
The National Institute for Health Research (NIHR) Mental Health Research Network and ‘Involve’, the national advisory group on public engagement, helped us to recruit two patients to the Trial Steering Group and two to the Trial Management Group. They contributed to developing patient information leaflets, managing the trial and disseminating its findings.
Data availability
All non-confidential data and syntax for analyses reported here are available online (https://zenodo.org/record/1442883).
Results
Participants
We randomised 250 eligible patients, 162 (65%) to RO DBT and 88 (35%) to control. Recruitment started in Dorset in March 2012 with an internal pilot. Recruitment in Hampshire and North Wales started in September 2012. Recruitment at all three centres continued until April 2015. Of the full sample of 250, 164 (66%) were female; 138 (55%) were aged between 35 and 55; 232 (97% of 238 responders) were White; 106 (42%) reported being in a stable relationship; and 82 (34% of 241 responders) had a university qualification. Ninety-two participants (37%) reported a first depressive episode before the age of 16; 179 (84% of 213 responders) were chronically rather than recurrently depressed; and 191 (82% of 234 responders) had previously received psychotherapy. In addition, 198 (79% of the sample) met criteria for a comorbid personality disorder. Full details of the flow of participants through the RefraMED trial and their baseline demographic and clinical characteristics are contained in the accompanying clinical paper.Reference Lynch, Hempel, Whalley, Byford, Chamba and Clarke9
Missing data
At 12-month follow-up, full service-use data for the entire follow-up period were available for 125 participants (77%) in the RO DBT group and 61 (69%) in the TAU group. This is compared to complete data in the RO DBT group for 118 (73%) at 7 months and for 101 (62%) at 18 months and in the TAU group for 61 (69%) at 7 months and for 51 (58%) at 18 months. There were no statistically significant differences between groups in the proportion of missing data (χ2 = 0.25, P = 0.61). Missing resource-use items in completed questionnaires were assumed to indicate no service use and were given a zero value.
Resource use
Resource use over the follow-up period is summarised in supplementary Table 4. Participants enrolled in the RO DBT group attended an average of 22.8 (median 26) individual RO DBT sessions and 19.3 (median 22.5) group RO DBT sessions. In addition, they attended an average of 3.4 (median 0) sessions of other types of talking therapy. Participants in the TAU group attended an average of 9.1 (median 6.5) sessions of various talking therapies. The use of all other health and social care services, including medications, was broadly similar between the two groups, suggesting that group allocation did not have a substantial effect on the intensity of other service use. Days reported off work and unproductive working hours were also similar between the two groups.
Costs
Table 1 summarises health and social care costs from the NHS and personal social services perspective over the 6 months prior to trial entry and over the 12- and 18-month follow-up periods. Disaggregated costs are based on complete case data, as data imputation was conducted at the aggregate level. The cost of all health and social care services used over the 6 months before entry into the trial were lower in the RO DBT group compared with the TAU group, but the difference was not significant (mean difference −£1029, bootstrap 95% CI −£2465 to £407; P = 0.160). Excluding the cost of RO DBT, the cost of all health and social care services used was lower over the 12-month follow-up period in the RO DBT group compared with the control group (adjusted mean difference −£909, bootstrap 95% CI −£1799 to −£19; P = 0.045) and also over the 18-month follow-up period (adjusted mean difference −£901, bootstrap 95% CI −£2755 to £952; P = 0.340). The RO DBT intervention cost approximately £5000 per person, including the cost of both individual and group sessions, resulting in total costs that were significantly higher in the RO DBT group compared with TAU over both the 12-month follow-up period (adjusted mean difference £4566, bootstrap 95% CI £3691–£5440; P < 0.001) and the 18-month follow-up period (adjusted mean difference £4463, bootstrap 95% CI £2915–£6011; P < 0.001).
Table 1 Disaggregated health and social care costs by group at baseline, 12- and 18-month follow-up
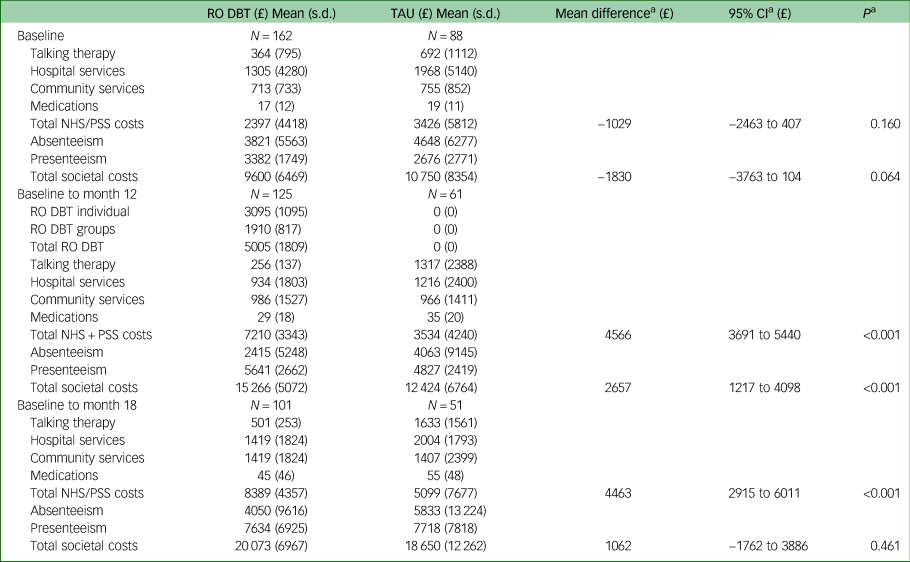
NHS, National Health Service; PSS, personal social services; RO DBT, radically open dialectical behaviour therapy; TAU, treatment as usual.
a. All analyses adjusted and bootstrapped.
Outcomes
Mean EQ-5D-3L scores and QALYs are presented in Table 2, together with unadjusted differences, differences after adjustment and imputation for missing data. EQ-5D-3L scores improved in both groups over the 18-month follow-up. Over the 7- and 12-month follow-up periods, EQ-5D-3L scores and QALYs were slightly higher in the RO DBT group compared with the TAU group, but over 18 months of follow-up, they were slightly higher in the TAU group. Differences were small and non-significant at all follow-up points.
Table 2 EQ-5D-3L scores and QALYs at baseline, 12- and 18-month follow-up
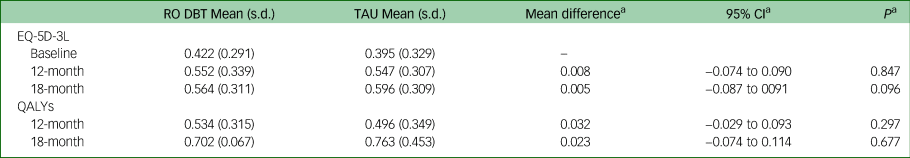
QALY, quality-adjusted life-year; RO DBT, radically open dialectical behaviour therapy; TAU, treatment as usual.
a. All analyses adjusted, imputed and bootstrapped.
Cost-effectiveness
The base-case 12-month additional cost of RO DBT plus TAU compared with TAU alone was £7048, which was associated with a difference of 0.032 QALYs, yielding an incremental cost-effectiveness ratio (ICER) of £220 250 per QALY. This ICER was well above the NICE upper threshold of £30 000 per QALY.
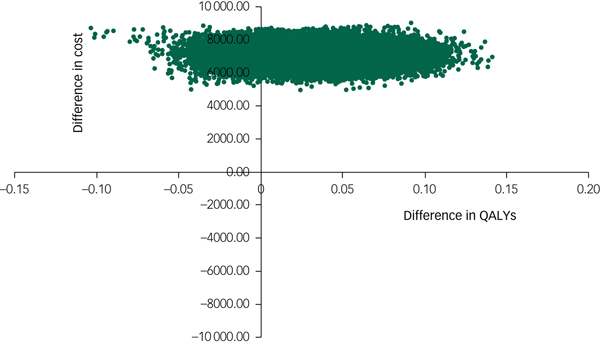
The cost-effectiveness plane shown in Fig. 1 illustrates the scatterplot of 30 000 bootstrapped cost and effectiveness pairs for RO DBT versus TAU from the perspective of NHS and personal social services at the primary 12-month end-point. All scatter points lie above the x-axis, illustrating that total health and social care costs are higher for the RO DBT group than the TAU group in all cases. The majority of scatter points fall in the north-east quadrant, where RO DBT is more effective than TAU but also more costly. Uncertainty in the ICER was explored in a cost-effectiveness acceptability curve (CEAC) shown in Fig. 2. This illustrates that RO DBT in its current format has zero probability of being cost-effective compared with TAU at the NICE willingness-to-pay threshold of £30 000.
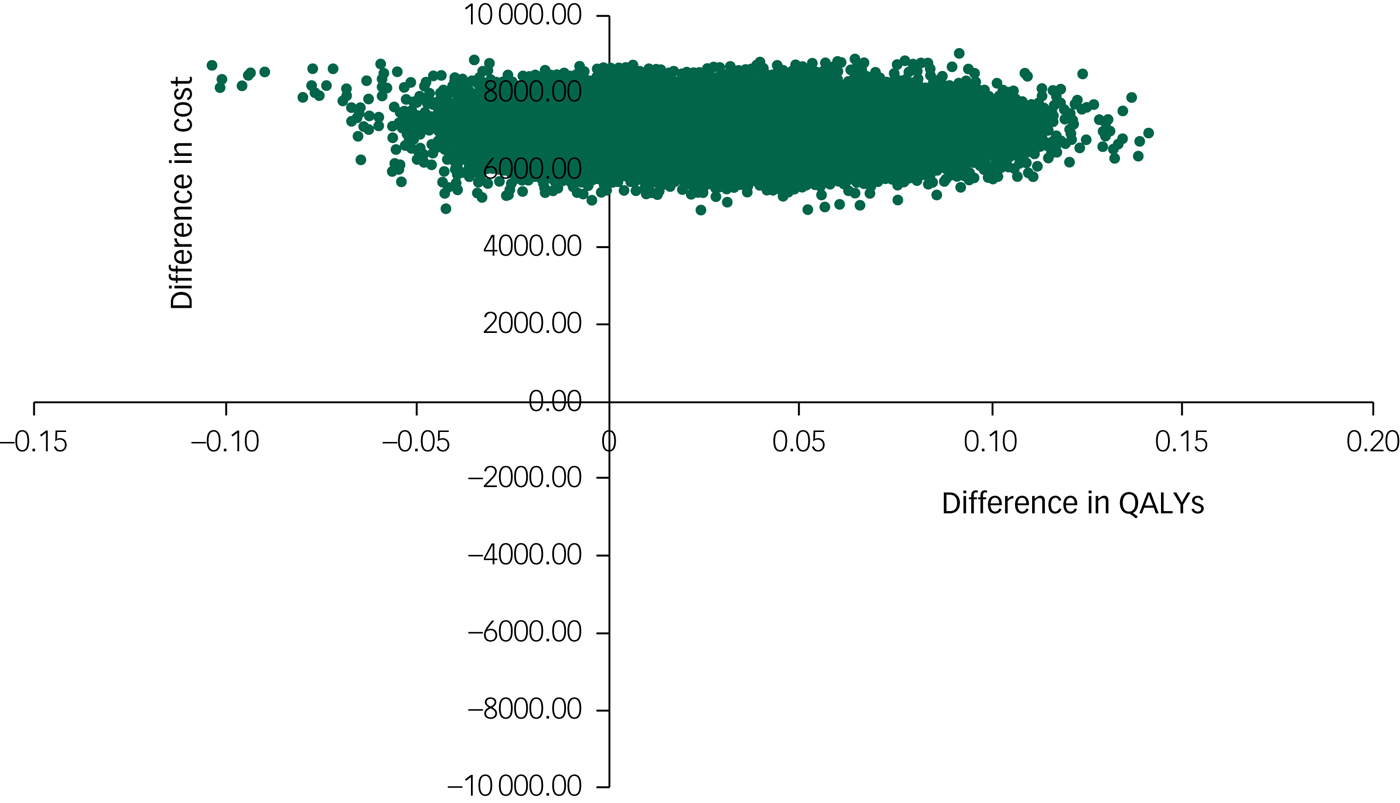
Fig. 1 Scatter plot of differences in costs versus differences in quality-adjusted life years (QALYs) for radically open dialectical behaviour therapy (RO DBT) versus treatment as usual after 12 months from the perspective of the National Health Service and personal social services.
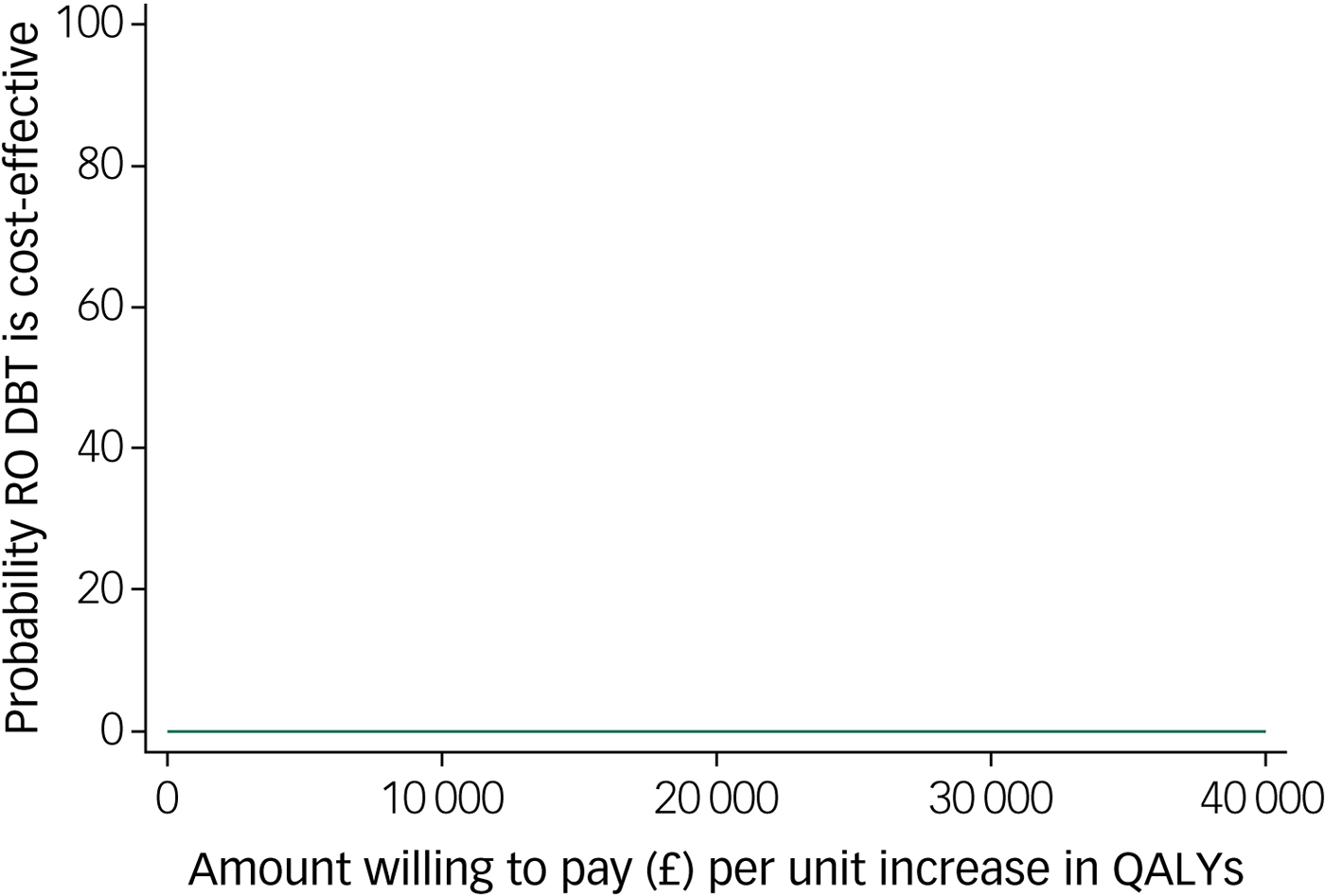
Fig. 2 Cost-effectiveness acceptability curve for quality-adjusted life years (QALYs) showing the probability that radically open dialectical behaviour therapy (RO DBT) is cost-effective compared with treatment as usual after 12 months from the perspective of the National Health Service and personal social services.
Sensitivity analyses
We based the primary economic analysis on a cost–utility analysis conducted at the 12-month follow-up, from the perspective of NHS and personal social services, with imputation of missing data. Supplementary Table 5 summarises how variation in methods and assumptions affected the ICERs. In sensitivity analyses 1 to 4 (complete case, inclusion of productivity losses, reduction in the cost of RO DBT group sessions and analysis at the 18-month follow-up), with all other factors being equal to the base case, RO DBT remained cost-ineffective compared with TAU, with costs per QALY all well above the NICE threshold of £30 000 per QALY. For illustration, replication of the analysis at the 18-month follow-up is reported in supplementary Figs 1 and 2. The results were very similar to those at the 12-month follow-up, with all replications in the scatter plot (supplementary Fig. 1) falling above the threshold willingness to pay of £30 000 per QALY and the CEAC (supplementary Fig. 2) indicating a zero probability that RO-DBT was cost-effective compared with TAU. In the final scenario, we undertook a cost-effectiveness analysis using the primary clinical outcome (the HRSD score) as the measure of effect, with all other factors being equal to the base-case analysis. This yielded an ICER of £7048 per unit improvement on the HRSD. Supplementary Fig. 3 illustrates the scatterplot of 30 000 bootstrapped cost and effectiveness pairs for RO DBT versus TAU based on differences in HRSD score using the £1000 per HRSD point threshold proposed by Romeo and colleagues.Reference Romeo, Patel, Knapp and Thomas32 The results were also very similar to the cost–utility analysis, with all replications falling above the threshold willingness to pay and the CEAC indicating a zero probability that RO DBT was cost-effective compared with TAU (supplementary Fig. 4).
Discussion
Main findings
Our clinical report indicated that RO DBT achieved moderate but not statistically significant improvement in depressive symptoms in a highly problematic treatment group.Reference Lynch, Hempel, Whalley, Byford, Chamba and Clarke9 This economic analysis adds important information about whether the moderate gains might still be a worthwhile use of scarce NHS resources. RO DBT with TAU was not cost-effective compared with TAU alone in the treatment of people with refractory depression, either from the perspective of the NHS and personal social services or when productivity losses were included. RO DBT is a resource-intensive intervention that does not achieve sufficient gains in outcomes or savings in the use of other health and social services to justify the additional cost of the intervention over the cost of TAU. Cost-ineffectiveness was driven by the high amount of contact time in RO DBT, some of which may have been an artefact of the rigour of a clinical trial. Whether such an intensive service would be made available in routine mental health services in the UK NHS is debatable.
Comparison with previous economic studies of DBT for disorders of overcontrol
This is the first economic evaluation of RO DBT for depression and one of very few studies to explore the economic implications of a DBT-informed approach for disorders of overcontrol. Previous economic evaluations have focused on standard DBT for borderline personality disorder (BPD). One economic study alongside a randomised trial compared standard DBT with TAU for self-harming patients with BPD.Reference Priebe, Bhatti, Barnicot, Bremner, Gaglia and Katsakou33 Although it focused on the cost of the intervention and other health and social care and did not include a formal cost-effectiveness analysis, it is a useful comparator. The mean costs of health and social care over 12 months for the BPD population (intervention group about £2500 per patient; control group about £3400) were similar to those of our depression population (intervention group about £2200; control group about £3500). The cost of standard DBT (£3200 per patient) was lower than the cost of RO DBT in the current study (£5000) but still substantially higher than that of therapies provided by the UK NHS, such as CBT. The authorsReference Priebe, Bhatti, Barnicot, Bremner, Gaglia and Katsakou33 concluded that standard DBT was effective in reducing self-harm in people with BPD but acknowledged that it would incur higher costs.
The remaining four economic evaluations were all part of a systematic review and preliminary economic evaluation of evidence for psychological therapies for BPD.Reference Brazier, Tumur, Holmes, Ferriter, Parry and Dent-Brown34 They used clinical data from four randomised trials of standard DBT for BPD, and cost data from the trials where available and other published sources where necessary. Two evaluations suggested that standard DBT dominated the comparison arm (TAU in one study, person-centred therapy in the other), achieving better outcomes at a lower cost per patient. The third evaluation found slightly higher costs but better outcomes in terms of parasuicides avoided for standard DBT compared with TAU. The fourth evaluation found that standard DBT was more costly than TAU but delivered only moderate gains in outcomes (parasuicide events avoided and QALYs) at an ICER considerably above the NICE threshold. All these analyses showed considerable uncertainty because they analysed data from only 20–44 patients. Furthermore, they relied heavily on assumptions and data outside the original trials, since none of them included economic data. The authorsReference Brazier, Tumur, Holmes, Ferriter, Parry and Dent-Brown34 concluded that the findings did not support the cost-effectiveness of standard DBT for BPD, although it has the potential to be cost-effective.
In comparison, the RefraMED economic evaluation provides rigorous economic evidence about RO DBT in the NHS using NICE criteria established for clinical disorders. Interestingly, when it comes to treatment recommendations for personality disorders, NICE is equivocal about cost estimates. Despite costs for out-patient DBT far exceeding recommended thresholds, NICEReference Brazier, Tumur, Holmes, Ferriter, Parry and Dent-Brown34 still recommends DBT for treatment of BPD in the NHS. As noted by NICE,Reference Brazier, Tumur, Holmes, Ferriter, Parry and Dent-Brown34 costing thresholds for the treatment of personality disorders have yet to be identified and broad consensus has been repeatedly noted for a change in how we measure and pay for mental healthcare in the NHS – especially when it comes to treating chronic mental health problems.Reference Stanton35 The high rates of comorbid personality disorder (79%) in the RefraMED trial suggest that cost considerations for our trial – and other similar trials in chronic depression – may be best understood when evaluated similarly.
Study limitations
The interpretation of our economic results is subject to some important limitations. First, in terms of generalisability, there is a range of definitions of refractory depression and treatment-resistant depression, which should be taken into account when considering the generalisability of our findings. Second, our economic findings may also have limited generalisability outside NHS mental health services in England and Wales. Third, the study fell short of the target recruitment (250/276, 91%) and full follow-up data for the economic evaluation were available for only 74% (186/250) of the sample; consequently, the study was underpowered with respect to the pre-planned target effect size.Reference Lynch, Whalley, Hempel, Byford, Clarke and Clarke3
Implications for further research
Since our results suggest that RO DBT in its current form is not cost-effective relative to TAU according to NICE criteria, research should address how to refine RO DBT to maintain the present gains but at lower cost for longer. There are many ways of adjusting the delivery of RO DBT to reduce costs, and future work must address the effectiveness and cost-effectiveness of these amended manuals. For example, we could taper treatment by reducing the frequency of sessions after an initial intensive period or adopt a stepped approach offering group sessions initially and individual sessions only to those who fail to respond. Indeed, small studies of RO DBT skills training classes have reported promising improvements in effectiveness.Reference Chen, Segal, Weissman, Zeffiro, Gallop and Linehan36,Reference Keogh, Booth, Baird, Gibson and Davenport37 Another important avenue of future research will be the development and evaluation of a RO DBT support programme on mobile phones or the internet. Several participants suggested that this would be helpful during and after active treatment. Further exploratory analyses of the RefraMED data-set will be critical in developing a shorter version of the skills programme that could be used in NHS settings with limited funding.
Funding
This trial was funded by the Efficacy and Mechanism Evaluation (EME) Programme (grant 09/150/12). The EME is funded by the UK Medical Research Council (MRC) and administered by the National Institute for Health Research (NIHR). The NIHR was responsible only for monitoring the progress of the trial, notably by appointing the independent members of the Trial Steering Committees and Data Monitoring and Ethics Committee. Thus, the grant holders were responsible for: study design; collecting, analysing and interpreting data; writing this paper; and deciding to submit it for publication. The views expressed in this paper are those of the authors and not necessarily those of the NIHR, MRC, National Health Service or Department of Health.
Acknowledgements
We are grateful to: the trial participants; the independent members of the Trial Steering Committee and Data Monitoring and Ethics Committee; the trial therapists; the trial research assistants, paid and voluntary; the adherence raters; the trial administrative staff; the clinical studies officers; and administrative and R&D staff at Dorset HealthCare University NHS Foundation Trust, Southern Health NHS Foundation Trust and Betsi Cadwaladr University Health Board for their support of the trial.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2019.57.






eLetters
No eLetters have been published for this article.