In recent decades, there has been a philosophical shift in healthcare whereby patients have been recognised not just as passive recipients of care, but as experts in their own condition, who hold a right to be involved in treatment decisions.Reference Holman and Lorig1, 2 Shared decision-making (SDM) in healthcare is a practice where patient and provider expertise are brought together (i.e. through information exchange) to reach agreement on treatment decisions.Reference Patel, Bakken and Ruland3, Reference Charles, Gafni and Whelan4 Recognition of the importance of SDM has been driven by calls from the consumer rights movement to reject traditional paternalistic models of care and uphold patients' ethical and moral right to self-determination.Reference Charles, Gafni and Whelan4 Proponents of SDM argue that its practise enables better decision-making and ultimately, improved clinical outcomes.Reference Slade5
The practice of SDM is now widely enshrined in policy as a key facet of patient-centred care,6, Reference Frosch, Moulton, Wexler, Holmes-Rovner, Volk and Levin7 yet many patients in mental health settings report that it is yet to be routinely adopted.Reference Gray, Rofail, Allen and Newey8, Reference Harris, Brooks, Lythgoe, Bee, Lovell and Drake9 Although people with mental health conditions frequently express a preference to be involved in their care,Reference Adams, Drake and Wolford10 it has been argued that, in comparison with physical health conditions, the practice of SDM might be inhibited by the unique challenges associated with mental health conditions. In particular, the ability for practitioners to legally enforce care against the patient's wishes may act as a significant threat to SDM that inhibits the development of a collaborative partnership.Reference Morant, Kaminskiy and Ramon11 The concept of ‘capacity’ in mental health poses as a further obstacle, which may reduce the practitioners' willingness to implement SDM.Reference Hamann, Leucht and Kissling12
SDM may be particularly warranted in the prescribing of antipsychotic medication, the mainstay of treatment for people with serious mental illness (SMI).13 Most antipsychotics have comparable efficacyReference Leucht, Cipriani, Spineli, Mavridis, Örey and Richter14 and, aside from clozapine, similar effectiveness.Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins15–Reference Tiihonen, Walhbeck, Lönnqvist, Klaukka, Ioannidis and Volavka17 However, adverse effect profiles vary and there are appreciable differences in the risks of stigmatising effects such as tardive dyskinesia and those affecting physical health, for example, the risk of diabetes, atherosclerosis and sudden cardiac death.Reference Muench and Hamer18 Consequently, there is an argument for basing treatment decisions not only on effectiveness, but on side-effect burden. It seems important that those affected by side-effects should be involved in these decisions.
There is a clear need to understand why patient involvement in antipsychotic prescribing decisions does not take place. To our knowledge, no systematic review has sought to understand the barriers and facilitators to patient involvement in antipsychotic prescribing. To address this gap, the present study aims to systematically review published qualitative evidence on the barriers and facilitators to involving patients with SMI in antipsychotic prescribing decisions. Given the paucity of evidence examining SDM interventions for people with mental health conditions,Reference Duncan, Best and Hagen19 a synthesis of evidence of barriers and facilitators from key stakeholder perspectives (patient, carer and health professional) may yield insight into how future interventions could address the issue.
Method
Systematic search strategy
Electronic database searches of Ovid Medline, Embase, PsycINFO and CINAHL (Cumulative Index to Nursing and Allied Health Literature) were conducted from database inception to June 2016. Search terms were generated by team discussion and a review of the literature for four concepts; (a) patient, carer or health professional perspectives, (b) collaborative involvement, (c) antipsychotic medication, and (d) qualitative design. The concepts were combined and applied (the full search strategy is detailed in supplementary Table DS1 available online at http://doi.org/10.1192/bjo.2017.5) and a validity test was conducted using a set of four test papers known to be relevant to the review aim.
Search results were exported to EndNote, duplicates deleted and the remainder imported to Covidence (www.covidence.org), a web-based software product to assist the screening and organisation of systematic reviews. Titles and abstracts were independently screened against the eligibility criteria (Table 1) by two researchers, with conflicts resolved via third party agreement. Full texts for all potentially eligible papers were obtained. For texts that were published in abstract form only, targeted author searches were conducted to identify additional full text publications by the same author (one additional paper was identified using this method).Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 Full texts were independently screened by two researchers, with conflicts referred to a third author for resolution.
Table 1 Inclusion/exclusion criteria
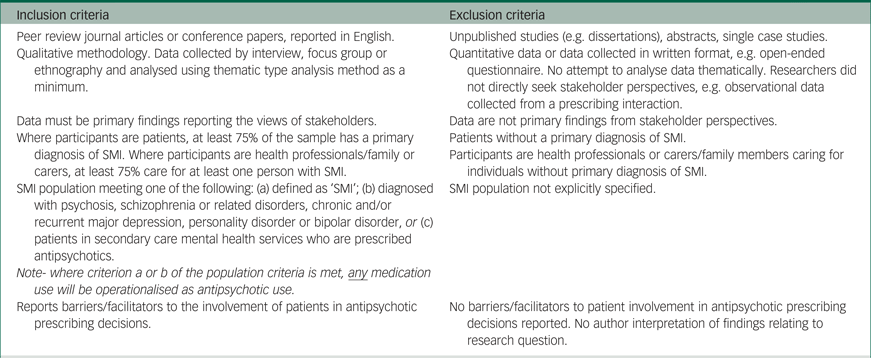
SMI, serious mental illness.
Critical appraisal and data extraction
Quality indicators were integrated from a variety of guidelines,21–Reference Mays and Pope23 and included whether the studies reported the methodology, selection of participants, data collection method and analysis method. No study was excluded on quality alone. Data extraction of quality indicators and descriptive study characteristics, such as country of origin and participant demographics, was undertaken by one of four authors and checked by a second reviewer. Two authors extracted study outcomes independently, and discrepancies were resolved by discussion.
Synthesis
An inductive thematic synthesis approachReference Thomas and Harden24 was conducted collaboratively by two authors (R.P. and C.M.), with regular consultation with wider team members. Figure 1 provides a detailed breakdown of the process. Primary findings were labelled as free codes according to (a) meaning and (b) participant perspective (i.e. patient, health professional or carer). We employed the constant comparative method to ensure translation of concepts from one study to another.Reference Thomas and Harden24, Reference Barnett-Page and Thomas25 This involved looking for the similarities and differences between papers and comparing new codes with our existing framework. Descriptive themes emerged during this stage to describe groups of codes which shared common meaning or experience. As the synthesis progressed, it became evident that some groups of codes would be better subsumed into larger themes, and other groups arose as distinct from others. The final stage of synthesis involved ‘going beyond’ the primary data, and has been described as the defining characteristic of synthesis.Reference Britten, Campbell, Pope, Donovan, Morgan and Pill26 For this stage, we drew parallels between various descriptive categories and began to generate a new interpretation, not explicitly presented in the primary findings. Particular barriers and facilitators were considered in terms of which contexts and which stakeholder perspective they emerged from.

Fig. 1 Diagram showing the development of analytical themes. Further analysis at the descriptive stage led to the understanding that the setting and nature of the interaction with health professionals (‘The consultation setting and processes affecting involvement’) formed the central theme, which was bi-directionally related to the remaining themes. HP, health professional; P, patient.
Results
The search resulted in 29 studies reported across 30 included papers for synthesis;Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Besenius, Bradley and Nolan27–Reference Tranulis, Goff, Henderson and Freudenreich55 the flow of studies is outlined in Fig. 2. All study characteristics are detailed in the supplementary material (Tables DS2 and DS3). Of the 30 studies, 13 were conducted in the UK,Reference Besenius, Bradley and Nolan27, Reference Carrick, Mitchell, Powell and Lloyd28, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Deslandes, John and Deslandes33, Reference Francis and Patel36, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Hon41, Reference Lester, Tait, England and Tritter44, Reference Malik and Das46, Reference Martean and Evans47, Reference Rogers, Day, Randall and Bentall49, Reference Seale, Chaplin, Lelliott and Quirk51–Reference Stewart, Anthony and Chesson53 ten in the USA,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Chafetz29, Reference Deegan, Rapp, Holter and Riefer31, Reference Delman, Clark, Eisen and Parker32, Reference El-Mallakh, Howard, Bond and Roque34, Reference Goscha and Rapp38, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50 four in Canada,Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Hagen, Nixen and Peters40, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Tranulis, Goff, Henderson and Freudenreich55 and one each in NorwayReference Lorem, Frafjord, Steffensen and Wang45 and SwedenReference Svedberg and Lutzen54. Sixteen studies examined data from only patients' perspectives,Reference Carrick, Mitchell, Powell and Lloyd28, Reference Chafetz29, Reference Delman, Clark, Eisen and Parker32, Reference Deslandes, John and Deslandes33, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39–Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Malik and Das46, Reference Rogers, Day, Randall and Bentall49, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50, Reference Stewart, Anthony and Chesson53, Reference Tranulis, Goff, Henderson and Freudenreich55 six from only health professionals' perspectives,Reference Chaplin, Lelliott, Quirk and Seale30, Reference El-Mallakh, Howard, Bond and Roque34, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Martean and Evans47, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Shepherd, Shorthouse and Gask52, Reference Svedberg and Lutzen54 and only one from carers' perspectives.Reference Francis and Patel36 The six remaining studies examined a combination of stakeholder perspectives,Reference Besenius, Bradley and Nolan27, Reference Deegan, Rapp, Holter and Riefer31, Reference Goscha and Rapp38, Reference Lester, Tait, England and Tritter44 two of these including carer viewpoints.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Mikesell, Bromley, Young, Vona and Zima48 This resulted in 886 participants; 647 patients, 215 health professionals and 24 carers.

Fig. 2 PRISMA flowchart. SMI, serious mental illness.
Of the 22 studies that included patients,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Besenius, Bradley and Nolan27–Reference Chafetz29, Reference Deegan, Rapp, Holter and Riefer31–Reference Deslandes, John and Deslandes33, Reference Gibson, Brand, Burt, Boden and Benson37–Reference Malik and Das46, Reference Mikesell, Bromley, Young, Vona and Zima48–Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50, Reference Stewart, Anthony and Chesson53, Reference Tranulis, Goff, Henderson and Freudenreich55 seven included participants with diagnosis of psychosis/schizophrenia,Reference Goscha and Rapp38, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Malik and Das46, Reference Rogers, Day, Randall and Bentall49, Reference Stewart, Anthony and Chesson53, Reference Tranulis, Goff, Henderson and Freudenreich55 one involved participants with a diagnosis of bipolarReference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50 and one included participants with dual diagnoses.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 Nine studies had a sample of mixed diagnoses,Reference Chafetz29, Reference Delman, Clark, Eisen and Parker32, Reference Deslandes, John and Deslandes33, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Hon41, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lester, Tait, England and Tritter44, Reference Mikesell, Bromley, Young, Vona and Zima48 and four described their sample as having ‘SMI’ but did not provide any further details on diagnosis.Reference Besenius, Bradley and Nolan27, Reference Carrick, Mitchell, Powell and Lloyd28, Reference Deegan, Rapp, Holter and Riefer31, Reference Hagen, Nixen and Peters40
Of the 12 studies that included health professionals,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Besenius, Bradley and Nolan27, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Deegan, Rapp, Holter and Riefer31, Reference El-Mallakh, Howard, Bond and Roque34, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Goscha and Rapp38, Reference Lester, Tait, England and Tritter44, Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Shepherd, Shorthouse and Gask52, Reference Svedberg and Lutzen54 seven recruited a mixed sample of professionals such as psychiatrists, nurses, case managers and peer support specialists,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Besenius, Bradley and Nolan27, Reference Deegan, Rapp, Holter and Riefer31, Reference El-Mallakh, Howard, Bond and Roque34, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Goscha and Rapp38, Reference Lester, Tait, England and Tritter44 three recruited only psychiatrists,Reference Chaplin, Lelliott, Quirk and Seale30, Reference Martean and Evans47, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Shepherd, Shorthouse and Gask52 one recruited only nursesReference Svedberg and Lutzen54 and one recruited only case managers.Reference Mikesell, Bromley, Young, Vona and Zima48
Sixteen studies recruited participants from a community care setting,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Carrick, Mitchell, Powell and Lloyd28, Reference Chaplin, Lelliott, Quirk and Seale30–Reference El-Mallakh, Howard, Bond and Roque34, Reference Francis and Patel36–Reference Goscha and Rapp38, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Malik and Das46, Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Svedberg and Lutzen54, Reference Tranulis, Goff, Henderson and Freudenreich55 five from mixed settings,Reference Besenius, Bradley and Nolan27, Reference Chafetz29, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Martean and Evans47 one study each from an in-patient forensics ward,Reference Stewart, Anthony and Chesson53 primary care settingReference Lester, Tait, England and Tritter44 and the readership of an alternative health magazine,Reference Hagen, Nixen and Peters40 and five studies did not specify service setting.Reference Hon41, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Rogers, Day, Randall and Bentall49, Reference Shepherd, Shorthouse and Gask52
Twenty-one studies used individual interviews,Reference Besenius, Bradley and Nolan27, Reference Chafetz29, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Delman, Clark, Eisen and Parker32–Reference El-Mallakh, Howard, Bond and Roque34, Reference Francis and Patel36–Reference Hon41, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lorem, Frafjord, Steffensen and Wang45–Reference Martean and Evans47, Reference Rogers, Day, Randall and Bentall49, Reference Seale, Chaplin, Lelliott and Quirk51–Reference Tranulis, Goff, Henderson and Freudenreich55 six used focus groupsReference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Deegan, Rapp, Holter and Riefer31, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Lester, Tait, England and Tritter44, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50 and two used a mixture of both.Reference Carrick, Mitchell, Powell and Lloyd28, Reference Mikesell, Bromley, Young, Vona and Zima48 Twelve studies used thematic analysisReference Besenius, Bradley and Nolan27, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Delman, Clark, Eisen and Parker32, Reference El-Mallakh, Howard, Bond and Roque34, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Martean and Evans47–Reference Rogers, Day, Randall and Bentall49, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Svedberg and Lutzen54 and five used grounded theory.Reference Carrick, Mitchell, Powell and Lloyd28, Reference Goscha and Rapp38, Reference Hon41, Reference Malik and Das46, Reference Stewart, Anthony and Chesson53 Seven used another type of analysis, e.g. content analysis (non-quantitative type),Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Chafetz29, Reference Deslandes, John and Deslandes33, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Hagen, Nixen and Peters40, Reference Shepherd, Shorthouse and Gask52, Reference Tranulis, Goff, Henderson and Freudenreich55 while the remaining five studies did not specify the analysis methodReference Deegan, Rapp, Holter and Riefer31, Reference Francis and Patel36, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Lester, Tait, England and Tritter44, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50 but were deemed to have used a thematic type analysis by the study team.
A table presenting study quality indicators is presented in the supplementary material (DS4). No study was judged to meet all quality indicators. All studies met at least one criterion, with 20 out of 29 studies meeting at least half the criteria (≥4 out of the eight quality criteria met). The most reliably met quality indicator was the method of data collection, reported by all 29 studies.
Data synthesis
Figure 1 shows how the analysis progressed from descriptive themes to a more developed interpretation that went ‘beyond’ the original study findings. As the descriptive themes were compared and contrasted, it was recognised that the nature and setting of events within the consultation lay at the centre of the influencing factors. This led to further refinement of themes; for example, upon further consideration, it was noted that the context theme consisted of two core components: firstly, the nature of the consultation setting and, secondly, those factors that originated from the organisational context. As such, the first component was transposed to the new ‘central’ theme, alongside the processes taking place within the consultation.
Emerging themes
Five themes emerged from the analysis, which can be subsumed under two broader themes, the underlying influences on patient involvement and the influences on involvement specific to the consultation. Each broad theme and its sub-themes will now be described.
The underlying influences on involvement
Three underlying influences on patient involvement were identified: perceptions of patient capability for involvement, the organisational context, and expectations and desire for collaborative prescribing.
Perceptions of patient capability for involvement
Serious mental illness as a barrier
Concerns about the negative impact of patients' mental health problems on their capability for involvement emerged as a prominent theme. Health professionals, patients and carers agreed that there were times (particularly during ‘crisis’ periods) when the active symptoms of mental illness, cognitive impairment and lack of insight prevented patients from being meaningfully involved in medication decisions.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Besenius, Bradley and Nolan27, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Forchuk, Jewell, Tweedell and Steinnagel35, Reference Hon41, Reference Malik and Das46–Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50, Reference Shepherd, Shorthouse and Gask52, Reference Stewart, Anthony and Chesson53 Despite patients' doubts about their own capacity for involvement when severely unwell, they did not necessarily think it right for them to be excluded from decisions outside these times. Some patients felt that receiving a diagnosis of a mental illness could lead health professionals to ‘assume a certain level of incompetency’ (p. 32 in Mahone et al Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20), suggesting that judgements of patients' capability for involvement may be based on more generalised expectations of low capability, irrespective of capacity. Consistent with this, case managers taking part in one study perceived patients to lack key capabilities for involvement, e.g. in knowing how to research drugs.Reference Mikesell, Bromley, Young, Vona and Zima48 In one study, individual differences in psychiatrists' attitudes to capacity were apparent, with some attempting to maximise involvement despite lack of capacity (including attempts to understand the patient's wishes prior to being unwell), and others regarding its lack as an ‘absolute contraindication’ to patient involvement.Reference Shepherd, Shorthouse and Gask52 While there may be consensus between stakeholders that a degree of mental well-being and capacity are needed for involvement, precisely where that threshold lies appears to be more subjective and based on individual attitudes.
Patients' knowledge and confidence
Patients believed that the receipt of high-quality medication information was an important requisite for involvement. They widely reported having not been provided with the necessary knowledge to make medication decisions, including the range of drug options (e.g. depot rather than tablet medication) or the side-effects, the purpose, or even the name of their prescribed medication.Reference Carrick, Mitchell, Powell and Lloyd28, Reference Deslandes, John and Deslandes33, Reference Iyer, Banks, Roy, Tibbo, Williams and Manchanda42, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Malik and Das46, Reference Mikesell, Bromley, Young, Vona and Zima48 At times, poor understanding related to patients receiving low-quality information (e.g. medication leaflets), but this could also result from simply receiving no information, or only receiving information when it was too late, (e.g. being told about weight gain following increased weight).Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43 Patients felt that being given information of a higher quality and quantity could lead to increased involvement, not only through increasing their knowledge, but also by enhancing their confidence and levels of empowerment.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference El-Mallakh, Howard, Bond and Roque34, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Lester, Tait, England and Tritter44, Reference Mikesell, Bromley, Young, Vona and Zima48 The importance of patients' confidence was highlighted in one study of young adults with SMI, which indicated that involvement increased through gaining knowledge and by having a prescriber who encouraged involvement.Reference Delman, Clark, Eisen and Parker32 Confidence was also increased through successful prior experiences of involvement, achieving accomplishments, gaining control over life in general or simply through growing older.Reference Delman, Clark, Eisen and Parker32
Expressions of concern about patients having inadequate knowledge about medication were notably less prominent within health professional perspectives. In one study, although professionals acknowledged patients' entitlement to honest information, they also discussed the need to limit, or even deliberately withhold certain information about side-effects. This was to avoid patients becoming confused, as well as to ensure the best possible clinical outcome, e.g. telling a young person about the risk of weight gain may discourage them from taking the medication.Reference Mikesell, Bromley, Young, Vona and Zima48 This raises the prospect that patients sometimes receive inadequate information owing to health professionals' concern about their ability to use this information responsibly.
The organisational context
Pressure on prescribers from within the healthcare context was a clear, though less widely discussed, influence on the level of patient involvement. Prescribers working with patients in forensic settings spoke of the need to select a drug that would manage behaviour, ‘even if all the antipsychotic does is sedate them’ (p. 6 in Shepherd et al Reference Shepherd, Shorthouse and Gask52). Pressure from other staff, including nurses, who may push prescribers to select a drug they have ‘faith in’, could also restrict opportunities for involvement.Reference Shepherd, Shorthouse and Gask52
The wider organisational context was seen by health professionals as restricting their flexibility in prescribing. Resource limitations and restrictions imposed by funding bodies (statutory services or insurance companies) were regarded by health professionals as determining the choice of medication, in some cases restricting the opportunity to review decisions altogether.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Shepherd, Shorthouse and Gask52 For example, health professionals in one study were unwilling to review medication decisions once a patient was sufficiently well to be discharged from in-patient settings owing to the pressure to free beds.Reference Shepherd, Shorthouse and Gask52 This was also highlighted by carers, who perceived a reluctance to prescribe ‘newer’ drugs as a possible consequence of their cost.Reference Francis and Patel36 Professional and legal responsibilities enacted to ensure the safety and well-being of the patient and members of the public, including safeguarding against harm,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 were perceived to further hinder collaborative prescribing. Family members shared this concern, questioning ‘how far can he compromise his recommendation and still be a responsible physician?’ (p. 31 in Mahone et al Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20). Health professionals in one study felt that the growth of patient and consumer groups had encouraged their practice to move towards more patient-centred practice and, as such, was a facilitator to collaborative prescribing.Reference Seale, Chaplin, Lelliott and Quirk51
Expectations and desire for collaborative prescribing
Findings within this theme indicated that for collaborative prescribing to be a success, patients and prescribers need to value both their own and their partner's contribution to the process. Patients and prescribers agreed that both parties needed to possess the openness and willingness to allow patient involvement to take place.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Carrick, Mitchell, Powell and Lloyd28, Reference Mikesell, Bromley, Young, Vona and Zima48 However, stakeholder views showed that not all patients expect or want an active role in medication decisions, with some opting to take a passive approach, relying solely on the expertise of the prescriber.Reference Besenius, Bradley and Nolan27, Reference Malik and Das46, Reference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50, Reference Stewart, Anthony and Chesson53, Reference Tranulis, Goff, Henderson and Freudenreich55 Evidence highlighted a number of factors contributing to passivity, including patients' belief in the superior value of professionals' opinionsReference Sajatovic, Davies, Bauer, McBride, Hays and Safavi50 and being unaware of their own entitlement to contribute,Reference Rogers, Day, Randall and Bentall49 as well as past experiences of services where involvement had been denied.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference Svedberg and Lutzen54 The latter contributing factor included patients who had received services during adolescence (where professionals and parents made the choices) or who had been exposed to extended periods of care within a traditional medical model.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference Svedberg and Lutzen54 As one participant stated: ‘… you've been making this decision for me for so long, why start now …’ (p. 31 in Mahone et al Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20). Patients and health professionals noted the need to empower patients as vital.Reference Mikesell, Bromley, Young, Vona and Zima48
Not all patients were in doubt about the importance of their contribution to the prescribing process, with some identifying the need to value their own expertise.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 A contrasting minor theme within health professional perspectives indicated that although passivity posed a challenge to SDM, patients holding inflexible views about medication could be equally disruptive. Fixed views could take the form of reluctance to consider any medicationReference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 or, in the case of a study involving patients with personality disorder, an insistence on being prescribed medication despite a perceived lack of clinical justification.Reference Martean and Evans47 In such cases, rather than depending solely on the health professionals' opinion, patients failed to value the expertise of the prescriber.
The consultation setting and processes affecting involvement
The factors affecting involvement described so far illustrate the underlying contextual, attitudinal and belief-based underlying factors within the proposed model (Fig. 3) that underpin collaborative prescribing. A central theme within the data, where a large proportion of the study findings clustered, related to the consultation setting, as well as the processes and actions that take place within it, which directly help or hinder the collaborative process.

Fig. 3 Diagram of patient involvement. C, carer; HP, health professional; P, patient; SDM, shared decision-making. (P, HP, C) indicates which stakeholder's perspective this is from.
The consultation setting
The degree to which patients could be involved in prescribing was dependent on an environment that was conducive to collaborative discussion. Patients complained that their input in prescribing decisions was restricted by a lack of practical opportunity for discussion. Consultations were experienced as an ‘in and out’ process in which prescribers appeared visibly ‘bothered’ and rushed.Reference Delman, Clark, Eisen and Parker32, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Stewart, Anthony and Chesson53 Patients indicated that longer consultations allowed greater involvement, as these had allowed patients ‘time to go through it properly’.Reference Deslandes, John and Deslandes33, Reference Lester, Tait, England and Tritter44 Unavailability of time was rarely raised as a barrier to patient involvement by health professionals, the exception being a study conducted within a primary care setting.Reference Lester, Tait, England and Tritter44 However, health professionals in another study indicated the importance of not appearing rushed (i.e. remaining calm) and in giving patients adequate time to process and respond to information.Reference Chaplin, Lelliott, Quirk and Seale30, Reference Seale, Chaplin, Lelliott and Quirk51
Patients in one study reported that involvement opportunities were restricted by the infrequency of appointments, which health professionals were reluctant to increase, or which were precluded by external factors (relating back to the organisational context theme) such as insurance restrictions.Reference Delman, Clark, Eisen and Parker32 There was some evidence that individual psychiatrist attitudes and willingness to ensure their accessibility (e.g. provision of their direct phone number) could help to overcome such barriers.Reference Delman, Clark, Eisen and Parker32
Lack of continuity of care posed a further barrier to involvement, with patients' attempts to gain input further obstructed by interim health professionals: ‘you'll have to talk when you get a regular doctor’ (p. 74 in Lacasse et al Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43). In a study where a supplementary pharmacist prescriber had been trialled, greater continuity of care gave participants improved opportunity for discussion.Reference Deslandes, John and Deslandes33 Another factor that affected patients' ease when entering prescribing discussions was the number of people present; too many attendees could make patients feel overwhelmed.Reference Besenius, Bradley and Nolan27
Process factors
Relational factors
A significant factor influencing patient involvement in antipsychotic decisions was the behaviours of those involved in the prescribing discussion, with responsibility placed particularly on the health professional. The need for health professionals to convey their positive regard towards the patient was key, with health professionals highlighting the need to display empathy, warmth and respect in their interactions.Reference Chaplin, Lelliott, Quirk and Seale30, Reference Seale, Chaplin, Lelliott and Quirk51 These qualities were not always evident to patients, some of whom felt negatively perceived by health professionals (e.g. as ‘malingerers’ or ‘time wasters’),Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Lester, Tait, England and Tritter44 disrespected and treated with little compassion,Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43 e.g. ‘do you want to be fat, or do you want to be crazy?’ (p. 75 in Lacasse et al Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43). This patient perception was supported by one study where some staff held negative stereotypes of patients as unreliable, uneducated and potentially violent.Reference Lester, Tait, England and Tritter44 A widely reported barrier to involvement was when patients’ views were ‘brushed off’Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference Gibson, Brand, Burt, Boden and Benson37, Reference Goscha and Rapp38, Reference Hagen, Nixen and Peters40, Reference Lorem, Frafjord, Steffensen and Wang45, Reference Stewart, Anthony and Chesson53 or, more rarely, overtly rejected by the health professional, e.g. when under a court order.Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43 Conversely, patients described the behaviour of health professionals who encouraged involvement as those who listened and responded (e.g. by acting upon side-effect information) and who demonstrated openness and the willingness to take their views seriously (e.g. by not minimising concerns).Reference Chafetz29, Reference Delman, Clark, Eisen and Parker32, Reference Deslandes, John and Deslandes33, Reference Green, Polen, Janoff, Castleton, Wisdom and Vuckovic39, Reference Lorem, Frafjord, Steffensen and Wang45 One strategy that health professionals raised as demonstrating interest involved taking down notes about patients' lives (e.g. the names of their children) so that such details could be recounted during subsequent consultations to give the impression of a ‘personal service’.Reference Chaplin, Lelliott, Quirk and Seale30
There was also some evidence that patients held negative views of staff, with some speaking of being unable to trust health professionals, eroding their ability to be open and honest about their medication.Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43 Health professionals acknowledged that lack of patient trust could act as a barrier to involvement, which they attributed to their power to enforce mental health law.Reference Shepherd, Shorthouse and Gask52 Further evidence of this power imbalance was evident in the way some patients acted to appease the prescriber. Sometimes, this was due to a belief in the need to respect the medical practitioners' expertise and authority.Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Mikesell, Bromley, Young, Vona and Zima48 Patients felt a pressure to ‘please’ the provider, ‘keep the peace’ and allow the meeting to progress quickly.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference Mikesell, Bromley, Young, Vona and Zima48 At other times, open dialogue was inhibited by a fear of the prescriber becoming angry or showing disapproval, or by patients' fear of being perceived as difficult or even manipulative.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lester, Tait, England and Tritter44, Reference Mikesell, Bromley, Young, Vona and Zima48 Less frequently, fears were attributed to the powers that health professionals held to bring serious negative consequences to patients, such as being removed from the general practitioner surgery list.Reference Lester, Tait, England and Tritter44 In response to this, non-prescribing nurses interviewed in one study actively coached some patients to assert opinions when meeting with the prescriber, for example, by working with them to develop their courage.Reference Svedberg and Lutzen54
Stakeholders perceived health professionals' communication as key to the success of collaborative prescribing. Professionals needed to take time in explaining relevant information about medication in non-technical, person-centred language, and actively elicit preferences around medication.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Mikesell, Bromley, Young, Vona and Zima48, Reference Seale, Chaplin, Lelliott and Quirk51, Reference Shepherd, Shorthouse and Gask52 Health professionals recognised that their ability to demonstrate such skills may require additional training, for example, in motivational interviewing.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 It was also considered important that discussions extended beyond medication to broader aspects of the patients' lives, including their illness, background, views and the underlying reasons for how they felt and behaved.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Chaplin, Lelliott, Quirk and Seale30, Reference Delman, Clark, Eisen and Parker32, Reference Lacasse, Hayes Piel, Lietz, Rider and Hess43, Reference Lester, Tait, England and Tritter44 While communication was important in ‘getting to know’ the patient, some health professionals emphasised the need to develop this relationship over time.Reference Chaplin, Lelliott, Quirk and Seale30
Although most findings focused on the health professionals' role in facilitating patient involvement, there was some recognition of the patient's part in instigating change, by speaking out against decisions made without their involvement and by communicating side-effects to their prescriber.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Carrick, Mitchell, Powell and Lloyd28, Reference Lorem, Frafjord, Steffensen and Wang45 Health professionals validated these facilitators, emphasising the need for patients to ‘speak up’ and be ‘reliable communicators’.Reference Mikesell, Bromley, Young, Vona and Zima48
Another means of facilitating patient communication was through the involvement of non-prescribing staff members and family members who could advocate for patients, helping to overcome barriers such as low confidence or dismissive prescriber attitudes.Reference Delman, Clark, Eisen and Parker32, Reference Goscha and Rapp38, Reference Lester, Tait, England and Tritter44 It was recognised, however, that some patients were unaware that they could involve an advocate, indicating that some individuals may be denied such support.Reference Besenius, Bradley and Nolan27 The need to strengthen patients' capability for involvement was recognised by non-prescribing nurses who, when working with patients they perceived to be cooperative, actively sought to enhance their ability in medication decisions, by explaining the pros and cons of medications.Reference Svedberg and Lutzen54
Tools and support aids
Evidence from a number of studies suggested that collaborative discussions could be aided by the use of tools and support aids, which helped to improve the quality of discussions and ensured patients’ perspectives were heard. One such strategy was the identification of patient priorities and goal setting. Studies evaluating experiences of using an SDM computer program found that this software enabled the identification of patient priorities, leading to increased involvement.Reference Deegan, Rapp, Holter and Riefer31, Reference Goscha and Rapp38 It was notable that where meaningful goals had not been identified or where a shared understanding of patient priorities had not been developed, involvement was less likely.Reference Carrick, Mitchell, Powell and Lloyd28, Reference Delman, Clark, Eisen and Parker32, Reference Goscha and Rapp38 This is exemplified when health professionals focus solely on symptom levels, rather than taking a broader, holistic view of overall well-being.Reference Carrick, Mitchell, Powell and Lloyd28, Reference Delman, Clark, Eisen and Parker32
Patients and health professionals found that SDM packages encouraged conversationReference Goscha and Rapp38 and ensured that patients' concerns were quickly recognised and addressed.Reference Deegan, Rapp, Holter and Riefer31 Health professionals believed that this was of particular benefit to patients whose mental health problems interfered with their communication skills.Reference Deegan, Rapp, Holter and Riefer31 Such electronic packages were also found to increase patients' disclosure, with health professionals reporting that patients found it ‘easier to tell the computer’.Reference Deegan, Rapp, Holter and Riefer31, Reference Goscha and Rapp38 For example, use of the package led one mother to reveal fears that taking her sedating medication would cause her to sleep through alerts from her child's sleep apnoea monitor.Reference Deegan, Rapp, Holter and Riefer31 This understanding enabled health professionals to direct support to this concern.Reference Deegan, Rapp, Holter and Riefer31
A small number of highly specific barriers to the implementation of shared decision aids were raised. These included the difficulties for patients with literacy problems in using decision aids,Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20 difficulties in ensuring that patients arrived promptly enough to make use of the software package,Reference Deegan, Rapp, Holter and Riefer31 and the challenge for staff in working with the large amounts of information generated by the software tool.Reference Deegan, Rapp, Holter and Riefer31
Discussion
Our synthesis has led to the identification of a number of key factors that affect patients' involvement. Within the hypothesised model (Fig. 3), three factors outside the consultation – (a) patients' capability, (b) the organisational context, and (c) the expectation and desire for collaborative prescribing – determine the consultation setting and processes affecting involvement. Stakeholders' experiences of the consultation setting and processes also feed back into the three factors outside the consultation, forming a cyclical process. For example, there was evidence that patients' past experiences of being uninvolved in consultations had contributed to a low expectation and desire for involvement.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20, Reference Delman, Clark, Eisen and Parker32, Reference Svedberg and Lutzen54 Similarly, the model postulates that the three factors identified outside the consultation influence each other. For example, patients who do not feel they possess the knowledge to contribute to decisions then lack desire or expectation for involvement.
When considering factors outside the consultation, there was evidence that involvement could be blocked by health professionals' attitudes, for example, a blanket view of patients' incapability. In discussing organisational challenges, professionals (with some agreement from carers) identified constraints in their day-to-day work, such as pressures from colleagues to prescribe certain drugs, which prevented patients' involvement. Although the development of consumer groups was raised as a contextual influence that has increased involvement, no other facilitators were raised. Further research into the nature of organisational barriers would be valuable in understanding how professionals could prioritise patient involvement above these constraints. For example, implementation theory suggests that ‘transformational’ leadership is important when implementing evidence-based practice.Reference Rycroft-Malone56 Patient perspectives revealed little awareness of the organisational barriers constraining health professionals. Although the barriers identified by health professionals seemed to be regarded as insurmountable, patient perspectives suggested that this was not always the case. Patients highlighted health professionals' failings to provide them with adequate information, which consequently had a detrimental effect on their perceived capability and confidence for involvement. Although there is a clear need to improve patients' knowledge, precisely how knowledge is best imparted is unclear, and the process is likely to be further hindered by the relational difficulties identified in the consultation.
There was evidence that some patients neither desired nor expected involvement, instead opting to entrust decisions to the professional. While it is important to respect that patients can make an informed choice not to be involved, it is likely that several factors encouraged passivity, such as past experiences of relatively paternalistic practice. One qualitative study involving people with chronic physical health conditions found that, compared with patients from high socioeconomic groups, those from lower socioeconomic groups were less likely to ask questions within consultations, believing it not to be within their role.Reference Protheroe, Brooks, Chew-Graham, Gardner and Rogers57 This suggests that there are individual differences between patients, a subgroup of whom do not feel entitled to be involved. This underlines the need for health professionals to not take passivity at face value, but instead to help patients to establish, as Protheroe et al Reference Protheroe, Brooks, Chew-Graham, Gardner and Rogers57 have discussed, ‘permission to participate’.
The most prominent theme within the synthesis, central to the model, was the consultation setting and process factors within it. In the consultation setting, patients felt that their involvement was constrained by lack of time within the consultation, yet only one study involving health professionals identified time as a barrier. This suggests that prescribers fail to recognise that patients regard time as a barrier to their involvement. Although patients and prescribers understood the importance of their relationship and, in particular, the prescribers' responsibility in fostering a collaborative one, the majority of barriers came from the patient perspective. They identified the following relational factors as hindering their interactions: professionals' disregard of patients' opinions, feeling negatively perceived by the health professional, lack of trust and a pressure to appease the prescriber. Although all these factors were conceptualised as relational processes in the synthesis, it is important to acknowledge that interactions are based on relationships built over time. In addition, it should be acknowledged that interactions are likely to be influenced by broader influences, such as cultural perceptions of medicine and views of medical practitioners as powerful.Reference Nimmon and Stenfors-Hayes58
It is notable that the majority of facilitators identified within the evidence emerged within the consultation theme. There was evidence that involvement may be facilitated through the use of decision aids such as SDM computer packages, which increased disclosure and developed a more comprehensive understanding of patients' concerns. This stakeholder perception is supported by findings from a recent Cochrane review, which found evidence that decision aids led to a range of benefits for people with a range of health conditions, such as improved clinician–patient communication, greater alignment between patient values and their care choices, improved knowledge, and reduced passivity.Reference Stacey, Légaré, Lewis, Barry, Bennett and Eden59 While decision aids may increase involvement, the fact that such tools are needed to generate open and rich discussion also points to difficulties in the face-to-face interactions with the prescriber. Indeed, the behaviour of non-prescribing staff in trying to coach patients in asserting opinion and the need to bring advocates to prescribing meetings are indicative of failings in the patient–prescriber relationship. While prescribers may not be wholly responsible for these difficulties, further work is needed to improve the quality of this critical relationship and to overcome barriers such as the power imbalance between patients and prescribers. This highlights the importance of health professionals employing good communication skills, by showing a holistic interest in patients and through listening and responding. Stakeholders agreed that these were fundamental to involvement and, while some prescribers may already possess these skills, additional training may be beneficial.Reference Mahone, Farrell, Hinton, Johnson, Moody and Rifkin20
This synthesis demonstrates that patient involvement in antipsychotic prescribing decisions depends on a complex multifactorial process in which all elements respond to and influence one another. As such, patient involvement cannot be expected to spontaneously emerge within prescribing discussions without first ensuring that they are underpinned by the necessary foundations for a collaborative relationship. This includes there being an organisational context where patient involvement can be prioritised above working pressures, but also relates to the beliefs and attitudes of both patients and prescribers, e.g. patient passivity and perceptions of patients' capability.
We would argue that many of the belief and attitudinal barriers identified through this synthesis reflect differences between stakeholders in the understanding of the rights and responsibilities of each individual in the prescribing partnership. Without such a shared model, it is unreasonable to expect that patients will challenge and speak up in the determination of their own treatment, and that prescribers will challenge those obstacles that inhibit patient involvement. From the earliest points of engagement with services, patients need to understand that they have a right to involvement, which can only be removed in very specific circumstances. Additionally, both patients and prescribers need to understand that collaboration cannot be achieved without each partner being willing to value their own, as well as the other's, expertise. It may be that specific interventions could be adopted to support this understanding, for example, by educating patients on how their involvement may benefit their care and their satisfaction in interactions. Prescribers need to understand their responsibilities to foster opportunities for involvement wherever possible, by challenging barriers rather than conceding to them. This could involve explicit conversations between patient and prescriber about involvement to understand individuals' specific barriers. For example, patients who express a lack of interest in involvement should be asked why, to understand any barriers inhibiting their contribution and to determine steps to overcome these, such as information provision or simply giving them sufficient time for involvement.
Prescribing decisions involving people with SMI are influenced by a complex system of values, processes and constraints. The multifaceted and interconnected nature of these factors emphasises the need to take a global and holistic approach to enhancing SDM for antipsychotic treatments. Some parallels can be drawn with recent non-systematic work that has suggested that the involvement of people with mental health problems may depend upon factors beyond the nature of their individual interactions with prescribers.Reference Morant, Kaminskiy and Ramon11 Our systematic approach to reviewing the literature has provided a comprehensive assessment of the evidence base and, importantly, enabled the integration of multiple stakeholder views. As such, it provides a broader view than any single reference. Our analysis is the first to identify differences in perspectives between patient and prescriber, which provides meaningful and contextually relevant opportunities for intervention.
Strengths and limitations
Our inclusion criteria allowed for the incorporation of studies without a specific aim to examine barriers and facilitators to patient involvement in antipsychotic prescribing, for example, those focusing on topics such as adherence and SDM more broadly. While this captured the breadth of findings in this field, it also led to some subjectivity in identifying those findings specific to involvement in prescribing decisions. To guard against this, two reviewers independently extracted all findings; where disagreements in interpretation arose, they were discussed to arrive at a final version to be used for the synthesis.
As part of our inclusion criteria, we operationalised any medication use in people with SMI as relating to antipsychotic medication, which may have had an impact on the studies included in our review. For example, Lester's studyReference Lester, Tait, England and Tritter44 of views about the involvement of people with SMI in a primary care setting may refer to medications prescribed for other conditions in this population, such as physical health problems. However, this study's findings were deemed salient to the broader process of patient involvement in prescribing and were therefore retained.
The generalisability of our findings may also be limited by the characteristics of the primary studies included. All included studies originated from Western Europe or the USA/Canada. As such, the applicability of the findings and conclusions drawn may not translate to all international care settings. In addition, the greater proportion of studies conducted in community settings suggests that our findings may not readily apply to other settings, e.g. in-patient. Further primary research in a range of settings therefore seems warranted, as the particular barriers and facilitators to involving patients in prescribing are likely to differ.
We did not use any indicator of study quality to guide our synthesis. Although this is a limitation of this study, we would argue that our inclusion/exclusion criteria, which specified a minimum of a thematic type analysis, ensured that all studies provided a degree of ‘richness’. It is also possible that relevant studies may have been excluded from our review owing to the authors' failure to report key characteristics of their sample. For example, some papers which included patients in in-patient settings were excluded owing to the sample being unclear; it is likely that a substantial number of participants within these settings would in fact meet the criteria. Future studies should ensure that their methods and findings are clearly described.
Future directions
Few studies incorporated family members' views on patient involvement in prescribing, although it is noteworthy that several studies not eligible for review included carers' views on their own involvement in medication decisions. Future studies may consider seeking the perspectives that family members hold about the patient's involvement, and the benefits or challenges this may present to carers, particularly as these individuals play a key part in the management of SMI.
Some of the themes contained very few facilitators; therefore, further research should aim to elicit such facilitators, particularly those that overcome the organisational barriers of patient capacity and passivity perceived by professionals. Further research is needed to empirically test whether the factors implicated within this model truly influence patients' involvement in medication decisions, and whether these factors interact in the way hypothesised. Finally, the findings suggest that future research needs to develop and test new interventions that break down patient barriers to involvement, not only at the consultation (e.g. through SDM tools), but also at a more fundamental level that challenges situational, attitudinal and belief-based barriers, e.g. helping to educate patients on the value of their knowledge and rights to involvement.
Author contributions
R.P., P.B., K.L., H.B., K.R. and R.D. contributed to the design and conception of the study. All authors were involved in the screening of texts for inclusion in the review. R.P., C.M., V.B. and B.R. extracted the data. The analysis and interpretation of data was led by R.P. and C.M., with supervision from P.B., H.B. and K.L. All authors contributed to the analysis and interpretation of the synthesis. R.P. and C.M. led the write-up of the manuscript. All other authors provided critical review of the manuscript and assisted with revising the manuscript. All authors approved the final manuscript for submission.
Funding
This manuscript presents independent research (EQUIP) funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme (RP-PG-1210-12007). The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Acknowledgements
Many thanks to Peter Coventry, who assisted with the design of the study and initial abstract screening. Many thanks to Owen Price, Amy Blakemore and Christine Molloy for their feedback on the manuscript.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjo.2017.5







eLetters
No eLetters have been published for this article.