Anorexia nervosa, a severe psychiatric illness characterised by an intense fear of gaining weight, disordered eating behaviour and extremely low weight, has the highest mortality rate among all psychiatric conditions.Reference Bulik, Slof-Op't Landt, van Furth and Sullivan1 Studies have shown that trait anxiety is associated with pathological eating behaviorsReference Lulé, Schulze, Bauer, Schöll, Müller and Fladung2 and predicts a lower likelihood of anorexia nervosa recovery,Reference Zerwas, Lund, Von Holle, Thornton, Berrettini and Brandt3 which suggests that emotion regulation for anxiety is a core target for treating anorexia nervosa. One promising approach for facilitating successful emotion regulation is mindfulness-based intervention (MBI). Mindfulness is thought to cultivate an attitude of acceptance together with detachment from the ‘self’.Reference Tang, Hölzel and Posner4 One study has suggested that anorexia nervosa patients have difficulty using acceptance.Reference Naumann, Tuschen-Caffier, Voderholzer and Svaldi5 Moreover, our previous work demonstrated that anorexia nervosa patients have higher brain activity in the precuneus, a key node of the default mode network (DMN), while performing the go/no-go task, which suggests that they are attached to self-related thoughts.Reference Noda, Isobe, Ueda, Aso, Murao and Kawabata6
Thus, a key question is whether MBI can successfully foster acceptance in patients with anorexia nervosa. To date, a few studies have reported effects of MBI on AN. However, empirical evidence of its efficacy remains scarce. In addition, previous studies are limited by their exclusive reliance on subjective measurements such as self-reported scales and lack of objective measures such as brain function.
We sought to determine the neural correlates of the effects of MBI on anxiety in anorexia nervosa patients. Specifically, we used a task designed to induce weight-related anxiety and asked participants to regulate their anxiety either using or not using an acceptance strategy. On the basis of a recent review,Reference Tang, Hölzel and Posner4 we hypothesised that the brain activity of regions supporting emotional and self-referential processing, which include the amygdala, anterior cingulate cortex (ACC), striatum, insula, prefrontal regions, posterior cingulate cortex (PCC) and precuneus, would decrease following the MBI and that the reduction in activity would be associated with a reduction in self-reported anxiety.
Method
The sample comprised 21 anorexia nervosa patients (Supplementary Table 1 available at https://doi.org/10.1192/bjo.2022.637). All participants completed self-reported psychological measurements (Eating Disorder Examination Questionnaire 6.0 [EDE-Q 6.0] and the State Trait Anxiety Inventory [STAI]) and underwent functional magnetic resonance imaging (fMRI) before and after the MBI (Supplementary Table 2). Patients performed an emotion regulation task during the fMRI scan. The task was designed to induce weight-related anxiety, and patients regulated their anxiety in one of three ways: try to not alter the emotion and let it be (ACCEPT), observe bodily sensations (BODY) or same as usual (SAU) (Supplementary Fig. 1). We conducted paired t-tests for the psychological measures. For the fMRI analyses, we used a block design for the first-level analysis, then conducted paired t-tests in predefined anatomical regions of interest (ROIs) for the ACCEPT > SAU contrast in the second-level. ROIs were chosen from a previous reviewReference Tang, Hölzel and Posner4 as follows: ACC, amygdala, striatum, insula, prefrontal cortex, PCC, and precuneus. Results for the BODY > SAU contrast are not presented here. We conducted correlation analyses between the change in self-reported anxiety and the change in brain activity for each ROI.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects were approved by the ethics committee of Kyoto University Graduate School and the Faculty of Medicine (C1295). Written informed consent was obtained from all participants. Detailed methods are provided in the Supplementary Materials (methods 1–7).
Results
None of the EDE-Q 6.0 subscale scores or body mass index differed significantly between the two timepoints. For the STAI, trait anxiety significantly decreased after the MBI (t = 2.44, P = 0.01; Bonferroni correction, P < 0.025). For the fMRI analyses, activity of the amygdala, ACC, striatum (putamen and caudate), prefrontal cortex (middle frontal gyrus [MFG]/inferior frontal sulcus [IFS] and orbital gyrus), PCC and precuneus decreased significantly after MBI (Fig. 1); however, no significant difference in the insula was found. In addition, the change in trait anxiety was significantly correlated with the change in ACCEPT > SAU contrast in the bilateral amygdala (right: r = 0.45, P = 0.02), ACC (r = 0.51, P = 0.01), bilateral MFG/IFS (left: r = 0.40, P = 0.04; right: r = 0.42, P = 0.03), bilateral orbital gyrus (left: r = 0.54, P = 0.01; right: r = 0.48, P = 0.01) and PCC (r = 0.53, P = 0.01), and a non-significant trend was observed in the left amygdala (r = 0.36, P = 0.06) and precuneus (r = 0.34, P = 0.07) (Supplementary Fig. 2).
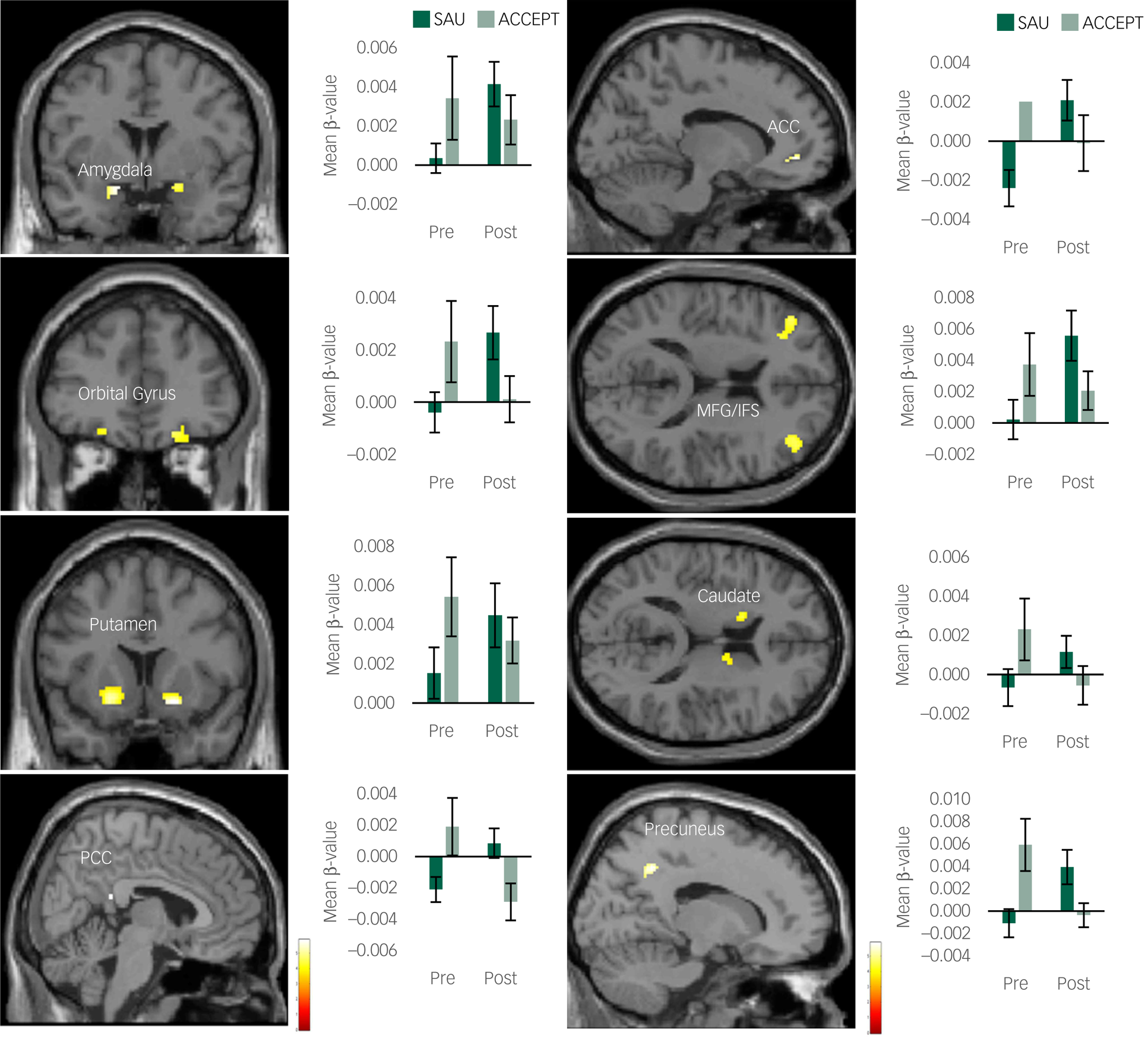
Fig. 1 Brain regions with a significant decrease in the ACCEPT > SAU contrast following the mindfulness-based intervention. ACC, anterior cingulate cortex; OFC, orbital frontal cortex; MFG, middle frontal gyrus, IFS, inferior frontal sulcus; PCC, posterior cingulate cortex; SAU, same as usual. Bar graphs show mean β-values extracted from the ACCEPT > SAU contrast in each condition. Error bars show standard errors. Graphs show only the left side for the amygdala, orbital gyrus, MFG/IFS, putamen and caudate.
Discussion
We examined the neural correlates of the effects of the MBI on the acceptance of anxiety in anorexia nervosa patients. As hypothesised, the activity of all the regions involved in emotional and self-referential processing except for the insula decreased following the MBI.
According to recent reviews,Reference Tang, Hölzel and Posner4,Reference Sezer, Pizzagalli and Sacchet7 the key features of the effects of mindfulness mediation can be viewed as emotional and self-referential processing. In addition, the neural underpinnings of these effects are considered to be changes in the salience network and the DMN, respectively. The ACC is the core node of the salience network and has a strong connection with limbic structures, including the amygdala.Reference Sezer, Pizzagalli and Sacchet7 Decreased activity in the amygdala, which is the core structure involved in emotional processing,Reference Drevets and Raichle8 has consistently been shown in mindfulness studies.Reference Tang, Hölzel and Posner4 The PCC and precuneus are considered to be key nodes of the DMN, which represents self-referential processing and autobiographical memory.Reference Fransson and Marrelec9 Through its effect on the DMN, MBI may be effective for reducing mind-wandering in patients with anorexia nervosa, whose attention is typically easily distracted by internal thoughts.Reference Noda, Isobe, Ueda, Aso, Murao and Kawabata6 Overall, our results are consistent with those of numerous previous studiesReference Tang, Hölzel and Posner4 and support our hypothesis that MBI lowers emotional arousal and fosters the detachment of the ‘self’ in anorexia nervosa patients. Our results are also in accord with the proposal that contemplative practice may have a protective effect on mental health by modulating DMN activity.Reference Svob, Wang, Weissman, Wickramaratne and Posner10
Regarding the other brain structures, decreased activation in the putamen, caudate, orbital gyrus and MFG/IFS were demonstrated after MBI. A previous study showed altered activity in the limbic cortico-striatal circuits in anorexia nervosa.Reference Uniacke, Wang, Biezonski, Sussman, Lee and Posner11 Given that anorexia nervosa patients are likely to engage in obsessive and compulsive behaviours, the reduced activity in these regions in our study may suggest a potential benefit of MBI in ameliorating obsessive weight concern.
Furthermore, trait anxiety decreased following the MBI, and the change in trait anxiety was associated with changes in brain activity in most of the regions that showed a significant decrease following MBI. These results support the notion that MBI alleviates anxiety not only at a subjective evaluation level but also at a neural level in anorexia nervosa.
A major limitation of this study was that we could not conclude that the reduction in brain activity was due to MBI because we had no active control group. Randomised controlled trials will be needed to address this issue. Another limitation was that we did not screen for comorbidities. Thus, it is still unclear for which specific trait of anorexia nervosa MBI might potentially be beneficial. Detailed characterisation of symptom profiles and documentation of comorbidities will be essential in future studies. Despite these limitations, the present study provides new insight for elucidating the neural mechanisms underlying the effect of MBI in ameliorating anorexia nervosa.
Relevance to practicing psychiatrists
Psychotherapy for anorexia nervosa has not yet been established. However, the exacerbation of anorexia symptoms has become a serious issue during the coronavirus pandemic. In response to the ongoing coronavirus outbreak, there is an urgent need to develop effective psychological interventions that target the regulation of anxiety. Our results offer a basis for developing effective and practical interventions using mindfulness. Mindfulness has aspects of cognitive training and is an approach that could dramatically improve psychological interventions alongside existing psychotherapy for anorexia nervosa.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2022.637.
Data availability
The data that support the findings of this study are available from the corresponding author, T.N., upon reasonable request.
Acknowledgments
We thank everyone who participated in the study and the members of our laboratory who supported us in conducting this study. We thank Sarina Iwabuchi, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Author contributions
T.N. formulated the research question, performed the research, analysed the data and wrote the manuscript. M.I. formulated the research question, performed the research and wrote the manuscript. R.M. formulated the research question, performed the research and wrote the manuscript. K.T. formulated the research question, performed the research and wrote the manuscript. M.K. formulated the research question, performed the research and wrote the manuscript. T.A. conducted fMRI data processing and analysed the data. S.T. wrote and reviewed the manuscript. S.N. formulated the research question, supervised the research and reviewed the manuscript. T.M. supervised the research, wrote the manuscript and reviewed the manuscript.
Funding
This study was supported by JSPS KAKENHI (grant numbers JP19J40242, JP19K17088, 21H05173 and 21K07544).
Declaration of interest
None.





eLetters
No eLetters have been published for this article.