Autism spectrum disorder (ASD) is a heterogeneous group of neurodevelopmental disorder characterised by repetitive or stereotyped behaviours, and deficits in social relationships. In recent years, we have learnt a good deal about the genetics of ASD.Reference De Rubeis and Buxbaum 1 Similarly, our knowledge of the non-genetic risk factors associated with ASD has been refinedReference Lyall, Croen, Daniels, Fallin, Ladd-Acosta and Lee 2 – candidate prenatal and early life exposures include infection, obstetric complications, and nutritional and toxin-related exposures. Within the domain of nutritional exposures, there is growing interest in the possible links between gestational vitamin D deficiency and an increased risk of ASD.Reference Cannell 3 – Reference Fernell, Bejerot, Westerlund, Miniscalco, Simila and Eyles 6 This research has been inspired by birth cohort studies, which have provided evidence that prenatal vitamin D deficiency (as assessed in maternal sera) is associated with a range of later brain-related outcomes including impaired language developmentReference Whitehouse, Holt, Serralha, Holt, Hart and Kusel 7 , Reference Tylavsky, Kocak, Murphy, Graff, Palmer and Volgyi 8 and cognitive development in offspring.Reference Keim, Bodnar and Klebanoff 9 , Reference Morales, Guxens, Llop, Rodriguez-Bernal, Tardon and Riaño 10 Although several studies have reported that vitamin D deficiency or insufficiency is more common in children with ASD compared with controls,Reference Wang, Shan, Du, Feng, Xu and Staal 11 few studies addressed the specific hypothesis that vitamin D deficiency during gestation, a crucial time point in neurodevelopment, is related to the risk of subsequent ASD. One study compared neonatal vitamin D status in children with ASD v. their unaffected siblings (n=58 sibling pairs). This study reported lower 25-hydroxyvitamin D (25OHD) concentrations in neonatal samples from the ASD cases compared with their unaffected siblings.Reference Fernell, Bejerot, Westerlund, Miniscalco, Simila and Eyles 6 Recently, a Chinese ASD case–control study (n=68 cases) compared first trimester (11–13 weeks) maternal 25OHD concentrations and reported significantly lower concentrations of 25OHD in the ASD cases compared with their gender- and age-matched controls.Reference Chen, Xin, Wei, Zhang and Xiao 12
We recently reported an association between developmental vitamin D deficiency and a measure of social impairment within the autism spectrum, based on a large Dutch birth cohort.Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 13 In this study, vitamin D was measured at two occasions: at mid-gestation and at birth. Mid-gestation samples were collected from maternal sera and neonatal sera collected from cord blood. Autistic traits were based on parental ratings of the Social Responsiveness Scale (SRS), which quantifies behavioural features related to cognition, social communication and autistic mannerisms.Reference Constantino, Davis, Todd, Schindler, Gross and Brophy 14 Compared with individuals who were vitamin D sufficient (25OHD concentrations >50 nmol/L), those who were deficient (25OHD <25 nmol/L) had significantly higher (more impaired) SRS scores.
We had the opportunity to explore the association between developmental vitamin D status and risk of ASD, based on the same Dutch birth cohort in which we previously reported an association between low mid-gestational and neonatal vitamin D concentrations and autistic traits.Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 13 Within the cohort, we had access to an extensive set of potential confounding variables as well as the children's individual-level genome-wide genotype data. These data allowed us to precisely adjust our analyses for important confounding factors including ethnic background and genetic relatedness within the cohort. We predicted that, compared with individuals with sufficient concentrations of 25OHD, those who were 25OHD deficient (defined as <25 nmol/L) would be at increased risk of clinical ASD.
Method
The cohort
This study was embedded in the Generation R Study, a population-based prospective cohort from fetal life onward, based in Rotterdam, The Netherlands. The design and cohort of Generation R is extensively described elsewhere.Reference Jaddoe, van Duijn, Franco, van der Heijden, van Iizendoorn and de Jongste 15 , Reference Kruithof, Kooijman, van Duijn, Franco, de Jongste and Klaver 16 Briefly, the study is designed to identify environmental and genetic correlates of normal and abnormal health-related outcomes in mothers and their children.
A total of 9778 mothers were enrolled in the study, of whom 8878 (91%) were enrolled during pregnancy. Delivery dates of the pregnant women were between April 2002 and January 2006. Baseline response was 61% and follow-up rates in children until the age of 6 years exceeded 80%. Relevant to this study, Generation R is a multi-ethnic cohort, with the Dutch comprising roughly half of the sample. The ethnic background of the children was defined by the parents’ country of birth and further classified using definitions from Statistics Netherland. 17 If both parents were born abroad, the country of birth of the mother was used to define the child's ethnicity. The main non-Dutch ethnic groups were Surinamese, Turkish, Moroccan and Cape Verdean, which together accounted for almost 30% of the cohort. Written informed consent was obtained from the mothers, and the study was approved by the institutional review board of the Erasmus Medical Centre.
Vitamin D status: 25-hydroxyvitamin D
Vitamin D status was assessed by measuring 25OHD. 18 Samples were quantified using isotope dilution liquid chromatography–tandem mass spectrometry. The analytical system consisted of a Shimadzu Nexera UPLC coupled to an AbSciex 5500 QTRAP equipped with an atmospheric-pressure chemical ionisation source. Assay accuracy was assessed using certified reference materials purchased from the National Institute of Standards and Technology (NIST SRM 972a Levels 1–4). Samples were analysed at the Queensland Brain Institute in Brisbane, Australia, between July 2013 and August 2014. Further details of the assay methodology and a thorough analysis of the prevalence and sociodemographic correlates of 25OHD concentrations in the Generation R cohort have been described elsewhere.Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 19 25OHD concentration was defined as the sum of 25-hydroxyvitamin D2 (25OHD2) and 25-hydroxyvitamin D3 (25OHD3) species measured in serum.Reference Eyles, Anderson, Ko, Jones, Thomas and Burne 20 Within the total Generation R cohort, 25OHD was measured from prenatal and cord serum in a total of 7935 expecting mothers and their children. The first sample was taken at mid-gestation (mean (s.d.) gestational age 20.6 (1.2) weeks; range 18.1–24.9, n=7256). The second sample was collected at birth, from neonatal cord blood (mean (s.d.) gestational age 40.0 (1.5) weeks; range 27.6–43.6, n=5023).
In the current analyses, we examined three stratified levels of 25OHD concentrations: deficient (<25 nmol/L), insufficient (25–49.9 nmol/L) and sufficient (≥50 nmol/L). Although there is some debate about the definition of these strata,Reference Ross, Manson, Abrams, Aloia, Brannon and Clinton 21 there is no debate that children with 25OHD concentrations of <25 nmol/L have vitamin D deficiency (as defined by an increased risk of rickets and increased parathyroid hormone). Because the fetus is entirely dependent on the maternal supply of 25OHD, and because of the relatively long half-life of 25OHD (approximately 2 weeks), cord blood assays serve as a proxy measure of late gestational 25OHD status (with deficient cord levels suggesting a 25OHD-deficient environment during late gestation).Reference Hillman and Haddad 22 , Reference Salle, Delvin, Lapillonne, Bishop and Glorieux 23
ASD
The ascertainment of ASD in a general population cohort is challenging. The Netherlands has no general medical register but general practitioners hold all medical records, including information on treatment and diagnoses by medical specialists (e.g. paediatricians and child psychiatrists). A diagnosis of ASD is generally based on clinical consensus by a specialised multidisciplinary team. The diagnostic workup typically involves an extensive developmental case history obtained from parents as well as teachers, based on repeated observations of the child.
To identify ASD cases, we undertook the following steps:
-
(a) Children were formally screened with the SRS. The authors of the scale recommend cut-offs for screening for ASD in population-based settings, consistent with short-form SRS-weighted scores of 1.078 for boys and 1.000 for girls.Reference Constantino, Davis, Todd, Schindler, Gross and Brophy 14
-
(b) Children who scored in the top 15% on the Child Behavior Checklist-1.5–5 total scoreReference Achenbach and Edelbrock 24 underwent a more specific screening using the Social Communication Questionnaire (SCQ). Children with scores of 15 or above on the SCQ were considered screen-positive.Reference Berument, Rutter, Lord, Pickles and Bailey 25
-
(c) The presence of psychiatric diagnoses and treatments in the children were routinely assessed from parents at all contact points between ages 6 and 9 years (centre visits and questionnaires).
We obtained medical records of children who were screen-positive on one or more criteria. To minimise false positives, only screen-positive children for whom a diagnosis could be confirmed by medical specialist records were considered ASD cases in this analyses. To minimise false negatives, we relied on multiple sources of information to select the children for medical record retrieval to ascertain ASD.
Within the cohort of children for whom at least one 25OHD assay was available (i.e. at mid-gestation or time of birth), there were (a) 68 screen-positive children on the SRS, of which 23 children with ASD were confirmed with medical records; (b) an additional 20 children were identified screen-positive through the SCQ, of which 6 children with ASD were confirmed with medical records; and (c) an additional 74 children were considered screen-positive based on maternal report, of which 39 children with ASD were confirmed with medical records. In total, these procedures identified 68 children with ASD, within our total sample of 4334 children. The observed prevalence of ASD in the cohort was 1.6%, which is consistent with the estimated prevalence in high-income countries (1.4%). 26 To maximise statistical power, children in the cohort who did not meet the screening criteria or children for whom ASD diagnosis was not confirmed were all treated as controls in the downstream analyses.
Ancestry information and genetic relatedness derived from observed common genetic variants
Ethnicity is strongly associated with vitamin D status (darker skinned individuals are more prone to vitamin D deficiency and insufficiency).Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 19 Genome-wide genotype data for the children allow us to build models that are better able to account for variation in ethnic background (compared with register-based or self-ascribed ethnicity) and for genetic relatedness between cohort members that may inflate an association statistic when genes affect the exposure or outcome variable. DNA was extracted from blood collected from the umbilical cord, or if this was not available, from a blood sample that was obtained by venepuncture during the child's visit to the research centre at a mean age of 6 years. Genotyping was performed using Illumina HumanHap 610 or 660 Quad chips, depending on collection time, following manufacturer protocols. A detailed description of the genotyping and quality control of the Generation R cohort is described elsewhere.Reference Medina-Gomez, Felix, Estrada, Peters, Herrera and Kruithof 27 Analyses involving genome-wide genotype data in this study were based on 3234 individuals on 518 245 single-nucleotide polymorphisms (SNPs).
Imputation of missing data
The sample sizes vary between analyses depending on the availability of mid-gestational and cord 25OHD concentrations, offspring genotype information and inclusion criteria related to ethnic background of the children. Note that twin pregnancies and mothers with no follow-up after birth were removed before analyses (see Supplementary Figure 1).
To optimise the sample size (number of ASD cases in our population-based cohort study is small, as expected for a disorder with a low life-time prevalence), we imputed a range of parental and offspring variables that were included as covariates in the models. Imputation was performed using the ‘Mix’ imputation package in R.Reference Schafer 28 ‘Mix’ uses an iterative multiple regression algorithm which is expectation–maximisation (EM) based and imputes mixed continuous and categorical data under the general location model. Imputation expectancies were based on covariates only and were independent of predictor variables (25OHD concentrations) and outcome variable (ASD status). Four out of eight variables had missing data with missing proportions ranging from 3% (‘Educational level of the mother’) to 10% (‘Mother smoking during pregnancy’). We held a conservative approach to imputation of missing variables, as such, we did not impute ethnic background of the child and neither did we impute exposure and outcome variables in the study.
Statistical analyses
To estimate the effect of vitamin D deficiency on ASD, variations of linear models were fitted to the data. The choice of model depended on the distribution of close relatives in the sample and the method that was applied to control for population structure.
Main analyses
Logistic regression analyses were applied to test the effect of vitamin D deficiency status on ASD. To remove the effect of family relatedness in the sample, we removed one of each pair of siblings, prioritising siblings with valid vitamin D measurements from both mid-gestation and cord serum to maximise sample size. Because maximum likelihood estimates are often prone to small sample bias, we applied the Firth correction to our ASD analyses using the ‘logistf’ package in R.Reference Heinze and Schemper 29 The Firth correction uses a penalised likelihood estimation method to handle complete or quasi-complete separation in the data.
We compared full models in which we included the vitamin D predictor and parental and offspring demographic variables with nested models that did not include the vitamin D predictor. Parental variables included maternal and paternal age, maternal body mass index (BMI, measured mid-gestation), smoking status of the mother during pregnancy and educational level of the mother. Offspring variables included ethnicity of the child, gestational age at birth, birth weight and gender of the child. Significance of the vitamin D predictor was tested by comparing the fit of the full model with the fit of the reduced model, thereby considering that the penalty that is involved in the Firth correction is the same for the full and the nested model to make the two models directly comparable.
We fitted four full and reduced (nested) models to test the effect of deficient v. sufficient and insufficient v. sufficient at mid-gestation or cord. Subsequently, we estimated the population attributable fraction associated with the mid-gestation 25OHD status (assuming that the entire population could be moved into the 25OHD sufficient group), using Equation 10 of Bruzzi and colleagues.Reference Bruzzi, Green, Byar, Brinton and Schairer 30
![]() , with x being the number of cases and
being the relative risk for each of the cases in each stratum conditional on covariates.
, with x being the number of cases and
being the relative risk for each of the cases in each stratum conditional on covariates.
Sensitivity analyses
In addition to the main analyses, we undertook three series of sensitivity analyses.
First, we analysed only offspring with European ethnic background (based on the reported parental country of birth). The models fitted to these data were the same as the models in the main analyses, with the exception that the self-report ethnicity variable was not included as a covariate.
Second, because the exposure variable in this study (25OHD concentration) is highly associated with skin colour and thus with ethnic background,Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 19 we aimed to fit a model in which potential confounding effects related to ethnic differences between individuals were accounted for accurately. To this end, we fitted a mixed linear model in which we replaced the ethnicity covariate with a genetic component (i.e. genome-wide genetic relationship matrix) that very precisely captures both population stratification and genetic relatedness and consequently allows a test for association that is free from confounding because of sample structure. A detailed description including a simplified worked example of this method is described in our previous work.Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 13 Only children for whom individual-level genome-wide genotype data were available were included in these analyses.
Third, we fitted a model on the full sample replacing the categorical vitamin D predictor with a continuous measure of vitamin D and tested the association between 25OHD and ASD. These models included all the covariates that were also included in the main analyses.
Results
Supplementary Figure 1 depicts a flow chart of the inclusion of participants in this study. In total, 4334 children and their mothers were available with measures of vitamin D concentrations drawn from maternal blood at mid-gestation or drawn from cord blood at time of birth as well as data on the SRS and ASD status. Of these children, 3234 had individual-level genotype data available. Supplementary Table 1 provides an overview of the distributions of parental and offspring demographic variables in the study sample before and after imputation of missing covariates.
On average, 25OHD concentrations from cord blood were lower compared with concentrations at mid-gestation (mean 25OHD concentration in maternal serum at mid-gestation: 58.6 nmol/L; mean 25OHD concentration in cord blood: 35.9 nmol/L; t=35.8, P<001). The observed correlation between mid-gestation and cord samples was 0.50 (t=28.7, P<001).
Table 1 summarises the prevalence of 25OHD deficiency in mid-gestation and cord blood stratified by ASD diagnosis. The proportions of cases and controls with developmental 25OHD deficiency were substantial for both mid-gestation samples and cord samples with higher proportions for ASD cases of mid-gestation samples but not in cord samples.
Table 1 Prevalence of deficiency in mid-gestation and cord 25OHD samples stratified by ASD a
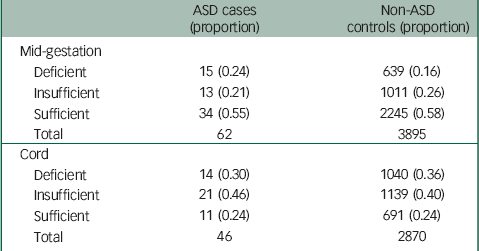
| ASD cases (proportion) | Non-ASD controls (proportion) | |
|---|---|---|
| Mid-gestation | ||
| Deficient | 15 (0.24) | 639 (0.16) |
| Insufficient | 13 (0.21) | 1011 (0.26) |
| Sufficient | 34 (0.55) | 2245 (0.58) |
| Total | 62 | 3895 |
| Cord | ||
| Deficient | 14 (0.30) | 1040 (0.36) |
| Insufficient | 21 (0.46) | 1139 (0.40) |
| Sufficient | 11 (0.24) | 691 (0.24) |
| Total | 46 | 2870 |
ASD, autism spectrum disorder; 25OHD, 25-hydroxyvitamin D.
a Deficient is 25OHD concentrations <25 nmol/L, insufficient is 25OHD concentrations 25 to <50 nmol/L; sufficient is 25OHD concentrations ≥50 nmol/L.
Vitamin D and ASD
For the main analysis, we observed that compared with individuals with sufficient concentrations of 25OHD at mid-gestation, those that were deficient had a more than twofold increased risk of ASD (odds ratio (OR)=2.42; 95% confidence interval (CI) 1.09–5.07; P=0.03) (Table 2). None of the other comparisons were significant. Based on the main analysis, the population attributable risk for the comparisons related to mid-gestation 25OHD was 10.8%.
Table 2 Association between mid-gestation and cord 25OHD deficiency and clinical autism case–control status

| n | OR (95% CI) | χ2(1) | P | |
|---|---|---|---|---|
| Maternal serum, mid-gestation: deficient v. sufficient | 2770 | 2.42 (1.09–5.07) | 4.64 | 0.03 * |
| Maternal serum, mid-gestation: insufficient v. sufficient | 3103 | 0.86 (0.43–1.62) | 0.21 | 0.64 |
| Cord blood, at birth: deficient v. sufficient | 1673 | 0.94 (0.36–2.42) | 0.02 | 0.90 |
| Cord blood, at birth: insufficient v. sufficient | 1761 | 1.25 (0.61–2.69) | 0.37 | 0.54 |
* Significant at alpha of 0.05.
25OHD, 25-hydroxyvitamin D.
a Estimates are based on general linear model; a Firth correction was applied to the model.
b Covariates included in the model are ethnicity of child, gender of child, birth weight of child, gestational age at time of birth, age of mother at intake, age of father at intake, smoking history of mother during pregnancy, educational level of mother and body mass index of mother at mid-gestation.
c Deficient is 25OHD concentrations <25 nmol/L, insufficient is 25OHD concentrations from 25 to <50 nmol/L, sufficient is 25OHD concentrations ≥50 nmol/L.
Sensitivity analyses
In the analyses restricted to offspring with European ethnicity, we observed that the pattern of findings persisted. Comparing individuals with deficient levels at mid-gestation with those with sufficient levels, we estimated an OR of 2.42 (95% CI 1.21–6.43; P=0.02) (Supplementary Table 2). Similarly, when we replaced ethnicity with the genetic component that captures both population stratification and family relatedness, the pattern of findings identified in the main analyses remained significant, assuring that the observed association is free from confounding with ethnicity. OR for the mid-gestation deficient v. sufficient comparison was 2.19 (95% CI 1.42–3.38; P=0.01) (Supplementary Table 3). When we treated 25OHD concentrations as a continuous variable, the association between 25OHD and ASD status was no longer statistically significant (OR=0.99, 95% CI 0.98–1.00, P=0.11) (Supplementary Table 4).
Discussion
Based on a sample of 4334 children and their mothers, of whom 68 children were diagnosed with ASD, we show that gestational 25OHD deficiency is associated with a higher risk of being diagnosed with ASD. This finding is restricted to mid-gestation 25OHD status – in our sample we do not find evidence that cord blood 25OHD status is associated with ASD. In light of the robust findings linking developmental 25OHD concentrations (i.e. both mid-gestation and neonatal 25OHD concentrations) with autistic traits as measured with the SRS in this same population,Reference Vinkhuyzen, Eyles, Burne, Blanken, Kruithof and Verhulst 13 this may reflect a lack of power for the current ASD comparisons based on cord blood samples. The number of cases with ASD in the analysis of cord blood was 46 of whom 30% were 25OHD deficient at time of birth, compared with 36% of the non-ASD children. Confidence intervals around the estimates are fairly large implying that substantial effect sizes are required to reach statistical significance.
The association between 25OHD deficiency at mid-gestation and ASD was significant and the pattern of findings persisted when we restricted the analyses to the offspring of European ethnicity, and when we thoroughly accounted for ethnic variation and family relatedness using genetic data. These robust sensitivity analyses strongly reduce the chance that our findings were confounded by ethnic diversity and genetic differences in the sample.
Our results add to earlier findings from two previous studies that reported an association between 25OHD deficiency (obtained from neonatal dried blood spots) and 25OHD deficiency in first trimester maternal sera, with increased risk of ASD.Reference Fernell, Bejerot, Westerlund, Miniscalco, Simila and Eyles 6 , Reference Chen, Xin, Wei, Zhang and Xiao 12 Of interest, a Swedish study examined the risk of ASD in the offspring of women with a lifetime diagnosis of 25OHD deficiency (v. women without this diagnosis).Reference Idring, Rai, Dal, Dalman, Sturm and Zander 31 Although this study was not able to address the specific timing of this exposure (e.g. during the gestation of the index offspring), a robust association was reported between lifetime 25OHD deficiency and autism associated with intellectual disability.
Our study, based on a large, population-based, multi-ethnic cohort, was well suited to test our hypotheses. The prevalence of vitamin D deficiency was high in this cohort (17% of the mothers had mid-gestational 25OHD concentrations <25 nmol/L). Based on the current sample and with the standard caveats associated with the interpretation of population attributable fraction (i.e. assuming that the variables of interest are causally related and that other variables remain unchanged), optimising vitamin D status of this sample could be associated with a reduction in the prevalence of ASD of approximately 10%.
The finding from this study lends weight to the growing body of epidemiological and animal model-based research linking gestational vitamin D deficiency and altered brain development. ASD is considered a neurodevelopmental disorder; abnormalities at different developmental stages may therefore be part of the pathophysiology. Rodent models based on transient prenatal exposure to vitamin D deficiency have found a range of persistent molecular, neurochemical and behavioural changes of interest to neuropsychiatry.Reference Eyles, Burne and McGrath 32 A number of studies have linked ASD with neuronal migration.Reference Reiner, Karzbrun, Kshirsagar and Kaibuchi 33 Migration-associated phenotypes such as changes in neuronal density and volume, aberrant minicolumns and heterotopias have been detected in patients with ASD.Reference DiCicco-Bloom, Lord, Zwaigenbaum, Courchesne, Dager and Schmitz 34 , Reference Chen, Liao, Lai and Chen 35 As neuronal migration starts at 6 weeks gestation and usually ends around 24 weeks gestation, the observed association between our mid-gestation samples, which were drawn at ~20 weeks gestation, and risk of ASD corroborates our previous findings linking vitamin D deficiency with abnormal brain development. Furthermore, neonatal vitamin D deficiency has been previously associated with an increased risk of schizophrenia.Reference McGrath, Eyles, Pedersen, Anderson, Ko and Burne 36 ASD and schizophrenia are known to share genetic risk variants;Reference Lee, Ripke, Neale, Faraone, Purcell and Perlis 37 our findings provide additional evidence of the shared risk architecture between these two neurodevelopmental disorders. For example, the active form of vitamin D (1,25OHD) is known to affect the function of voltage-gated calcium channels.Reference Latimer, Brewer, Searcy, Chen, Popovic and Kraner 38 Variants in genes coding for subunits of these same calcium channels (e.g. CACNA1C) have been linked to risk of both schizophrenia and ASD.Reference Casamassima, Hay, Benedetti, Lattanzi, Cassano and Perlis 39
With respect to study limitations, the sample of offspring with ASD was small, and the diagnoses were not confirmed with gold-standard research criteria. Additionally, we lacked resources to systematically retrieve records of all 4334 cohort members for ASD. However, such misclassifications would bias our study towards the null hypothesis (i.e. no association between the variables of interest). Although we had measures of 25OHD concentrations at two developmental time points, we lacked information at other stages of gestation and early life. In future studies, it will be of interest to explore the critical window during which low vitamin D may contribute to ASD risk – it is feasible that exposure in the first few years of life may also contribute to adverse brain outcomes. Defining the precise critical window during which developmental vitamin D deficiency adversely affects brain development at this stage would be premature.
This study has several important strengths. The study was based on a large representative multi-ethnic cohort. We used a gold standard assessment of 25OHD concentrations and we had access to genome-wide genotype data of the children that allowed us to adjust for population stratification (e.g. ethnic differences) and genetic relatedness between cohort members.
Clearly, our findings are based on a relatively small sample of ASD cases and require replication in a larger sample. Because of the low prevalence of ASD, case–control samples may be better suited to explore this particular hypothesis. Findings based on observational epidemiology remain susceptible to residual confounding and thus randomised controlled trials would be required to infer causality.
Developmental vitamin D deficiency at mid-gestation was associated with a twofold increased risk of ASD. The association between developmental vitamin D deficiency and ASD may have important implications from a public health perspective. It is feasible that a safe, inexpensive and publically acceptable vitamin D supplementation in at-risk groups may reduce the prevalence of this risk factor. Just as prenatal folate supplementation has reduced the incidence of spina bifida, we speculate that prenatal vitamin D supplementation may reduce the incidence of ASD.
Acknowledgements
We thank the participants and staff of the Generation R study. We also thank the members of the Program of Complex Trait Genomics at the Institute for Molecular Bioscience for their support (a list of members of this centre can be found at http://cnsgenomics.com/index.html).
Funding
The Generation R Study is made possible by financial support from the Erasmus Medical Centre, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development. V.J. received an additional grant from the Netherlands Organization for Health Research and Development (ZonMw-VIDI 016.136.361) and a Consolidator Grant from the European Research Council (ERC-2014-CoG-648916). The vitamin D assay was supported by the National Health and Medical Research Council (NHMRC APP1062846). Shipping of the samples was supported by FP7 Nutrimenthe (grant agreement 212652). The collection of ASD diagnostic information was supported by a Simons Foundation Autism Research Initiative (SFARI – 307280) and a ZonMw TOP grant number 91211021, both to T.W. J.M. received an NHMRC John Cade Fellowship (APP1056929). The researchers are independent from the funders. The study sponsors had no role in the study design, data analysis, interpretation of data or writing of this report.






eLetters
No eLetters have been published for this article.