Major depressive disorder (MDD) is characterised by ‘depressed mood’ and/or anhedonia. Anhedonia is classically defined as a ‘diminished interest or pleasure in response stimuli that were previously perceived as rewarding during a pre-morbid state’,1 and is recognised as a core feature of MDD in both the DSM and the World Health Organization's ICD. Up to 70% of individuals with MDD present with anhedonia symptoms,Reference Shankman, Katz, DeLizza, Sarapas, Gorka, Campbell and Ritsner2 confirming anhedonia as a major feature of MDD. Individuals with anhedonia experience greater overall depressive severityReference Pelizza and Ferrari3 and decreased antidepressant treatment response.Reference Rizvi, Quilty, Sproule, Cyriac, Michael Bagby and Kennedy4,Reference Uher, Perlis, Henigsberg, Zobel, Rietschel and Mors5 In fact, anhedonia may be one of the most persistent symptoms of MDD, despite clinical remission from a major depressive episode (MDE): approximately 25% of individuals in remission continue to have residual symptoms of anhedonia.Reference Di Nicola, De Risio, Battaglia, Camardese, Tedeschi and Mazza6
Anhedonia treatment in MDD
Currently, serotonergic antidepressants are first-line treatments for MDD. However, there is mixed evidence regarding the efficacy of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) in improving anhedonia in individuals with MDD. Several studies of pharmacological interventions for anhedonia in MDD concluded that, although there was an improvement in anhedonia after treatment with an SSRI or SNRI, this improvement was significantly less than observed with other antidepressant agents, such as agomelatine.Reference Cao, Zhu, Zuckerman, Rosenblat, Brietzke and Pan7 A possible explanation for the limited anti-anhedonia effects of SSRIs is the fact that anhedonia is primarily associated with dopaminergic and opioidergic dysfunction, rather than serotonergic.Reference Treadway and Zald8 Further, SSRI-associated emotional blunting is a relatively common adverse event among patients receiving SSRIs;Reference Barnhart, Makela and Latocha9 therefore, SSRIs may sometimes exacerbate anhedonia.
The shortcomings of SSRIs in improving anhedonia present the need for other agents that specifically address the underlying pathophysiology of anhedonia. Dopamine is among the most frequently implicated neurotransmitters associated with reward processing and anhedonia,Reference Treadway and Zald8 and so it stands to reason that medications with dopaminergic properties may be effective in treating anhedonia. This is supported by reports on a number of medications with dopaminergic actions – including agomelatine, bupropion, pramipexole and psychostimulants – which have been associated with an improvement in anhedonia among individuals with MDDReference Cao, Zhu, Zuckerman, Rosenblat, Brietzke and Pan7 and other psychiatric/neurological disorders (e.g. Parkinson's disease).Reference Lemke, Brecht, Koester and Reichmann10 Aripiprazole, an atypical antipsychotic that has dopamine D2 receptor agonist properties and is an approved adjunct treatment for MDD, may also reduce symptoms of anhedonia.
The brain's reward circuitry
Advances in applied functional brain imaging to examine neural reward circuits have enhanced our understanding of reward deficits and various aspects of anhedonia, which involve communication between several key brain regions, including the ventral tegmental area (VTA), nucleus accumbens (NAc), ventral pallidum and anterior cingulate cortex (ACC). Theoretically, dysregulation in any of these regions may cause anhedonia. Among brain networks, the salience network is most implicated in reward processing: it selectively enhances salient reward stimuli required for higher cognitive processing from the flow of incoming stimuli with which humans are constantly presented.Reference Menon and Toga11,Reference Lesage, Stein, Pfaff and Volkow12 Several brain regions are implicated in the salience network: although the anterior insula and dorsal ACC act as the main hubs of the salience network, there is also significant contribution from the amygdala, ventral striatum, thalamus, hypothalamus, substantia nigra pars compacta and VTA.Reference Menon and Toga11,Reference Lesage, Stein, Pfaff and Volkow12
The current study
The purpose of the current study is to examine the relationship between anhedonia and functional neurocircuitry in key reward processing brain regions in an MDD population treated with the partial D2 agonist aripiprazole as an adjunct to the SSRI escitalopram. Data were collected as part of the first Canadian Biomarker Integration Network in Depression (CAN-BIND-1) study,Reference Kennedy, Lam, Rotzinger, Milev, Blier and Downar13 in which participants with MDD received escitalopram for 8 weeks. Non-responders to escitalopram were augmented with aripiprazole for a further 8 weeks, whereas responders continued 8 additional weeks of escitalopram only. Neuroimaging and clinical data were collected throughout the 16-week study. The group who received aripiprazole as an adjunct is the population of interest for this report. Our primary objective was to identify baseline and week 8 resting-state functional magnetic resonance imaging (fMRI) biomarkers associated with anhedonia improvement following adjunct aripiprazole treatment. Over the past several years, fMRI has been increasingly used to explore predictors of treatment response in populations with depression (e.g. for treatment with escitalopram, sertraline and pramipexole);Reference Godlewska, Browning, Norbury, Cowen and Harmer14–Reference Chin Fatt, Jha, Cooper, Fonzo, South and Grannemann17 however, to the best of our knowledge, this is the first study to explore resting-state fMRI predictors of anhedonia response to aripiprazole in a population with MDD.
Method
Clinical and neuroimaging data were obtained from the first CAN-BIND trial, CAN-BIND-1 (Clinicaltrials.gov identifier: NCT01655706).Reference Kennedy, Lam, Rotzinger, Milev, Blier and Downar13,Reference Lam, Milev, Rotzinger, Andreazza, Blier and Brenner18 CAN-BIND is a Canada-wide research programme that aims to identify biomarkers of antidepressant treatment response.Reference Kennedy, Lam, Rotzinger, Milev, Blier and Downar13,Reference Lam, Milev, Rotzinger, Andreazza, Blier and Brenner18 From August 2013 to December 2016, 211 participants meeting the criteria for MDD, who were experiencing a current MDE, were recruited from six Canadian sites: University Health Network (Toronto), Centre for Addiction and Mental Health (Toronto), McMaster University (Hamilton), Queen's University (Kingston), University of Calgary and University of British Columbia (Vancouver). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by research ethics boards at each study site. This work was previously published by the first author (S.R.V.) as part of a Master's thesis through the Institute of Medical Science, University of Toronto.Reference Vaccarino19
Participants
Detailed inclusion/exclusion criteria, clinical outcomes and sample size calculations are described elsewhere.Reference Kennedy, Lam, Rotzinger, Milev, Blier and Downar13,Reference Lam, Milev, Rotzinger, Andreazza, Blier and Brenner18 In summary, eligible participants must have met criteria for an MDE for ≥3 months (based on DSM-IV-TR criteria, as confirmed by the Mini-International Neuropsychiatric Interview), were between the ages of 18 and 60 years, and had been free of psychotropic medications for at least five half-lives. Major exclusion criteria included diagnosis of another primary psychiatric disorder or neurological condition; current psychosis, substance use disorder or high suicide risk; failure of four or more adequate antidepressant trials; and previous failure or intolerance to escitalopram and/or aripiprazole. All participants provided written informed consent after receiving a complete description of the study.
Intervention
Study participants received open-label escitalopram (10–20 mg/day) for 8 weeks, after which escitalopram ‘non-responders’ (defined as <50% reduction in Montgomery–Åsberg Depression Rating Scale (MADRS) score from start of treatment to week 8) received adjunct aripiprazole (2–10 mg/day) for an additional 8 weeks. Dosing was at the discretion of the individual clinician.
Primary outcome measures
The primary outcome was anhedonia severity, as measured with the Dimensional Anhedonia Rating Scale (DARS).Reference Rizvi, Quilty, Sproule, Cyriac, Michael Bagby and Kennedy4 This 17-item self-report scale measures four dimensions of anhedonia – desire, motivation, effort and consummatory pleasure – with lower scores reflecting greater anhedonia.Reference Rizvi, Quilty, Sproule, Cyriac, Michael Bagby and Kennedy4 Participants are asked to choose two or three of their favourite activities/experiences to act as prompts for each of four categories: pastimes/hobbies, food/drinks, social activities and sensory experiencing. There were no limitations on which prompts a participant could choose, and they were not required to use the same prompts across sessions. The DARS displays good reliability and divergent validity.Reference Rizvi, Quilty, Sproule, Cyriac, Michael Bagby and Kennedy4 Improvement in anhedonia was measured as percentage change in DARS score, with a higher percentage equating to greater improvement. The DARS was completed by participants at baseline, week 8 and week 16.
Two analyses were completed with functional neuroimaging statistical analysis software (methods described below). First, resting-state fMRI predictors of DARS score change in the adjunct aripiprazole group were analysed from week 8 (aripiprazole baseline) to week 16. Second, resting-state fMRI predictors of DARS score change among the adjunct aripiprazole group were analysed from baseline to week 16.
Neuroimaging acquisition and processing
Magnetic resonance imaging (MRI) data were collected at baseline and week 8, using 3.0 T MRI scanners. Each scan lasted 10 min and participants focused on a fixation cross projected in the MRI machine during the scan. A whole-brain T2*-sensitive blood oxygenation level dependent echo-planar imaging series was used with the following parameters: 2000 ms repetition time, 30 ms echo time and voxel dimensions of 4 × 4 × 4 mm. Further details of the MRI acquisition protocol for this and other CAN-BIND imaging projects are available elsewhere.Reference MacQueen, Hassel, Arnott, Addington, Bowie and Bray20 Neuroimaging data were preprocessed with the Optimization of Preprocessing Pipelines for Neuroimaging-fMRI (OPPNI-fMRI) pipelineReference Churchill, Spring, Afshin-Pour, Dong and Strother21,Reference Churchill, Raamana, Spring and Strother22 via Analysis of Functional Neuroimaging (AFNI) software (https://afni.nimh.nih.gov/pub/dist/doc/htmldoc).Reference Cox23,Reference Cox and Hyde24
Statistical analysis
Seed-based correlational analysis was employed to examine the relationship between resting-state functional connectivity (rsFC) of two regions of interest – NAc and ACC – and change in DARS score. These regions of interest, or ‘seeds’, were chosen because they are strongly and consistently implicated in reward processing and related to anhedonia severity in several previous studies.Reference Zisner and Beauchaine25–Reference Walter, Henning, Grimm, Schulte, Beck and Dydak27 For the standardised functional image of each patient, a region of interest ‘mask’ was applied to extract the BOLD time series. A whole-brain, seed-based connectivity map was then calculated for each participant, which shows the strength of rsFC between the region of interest and all other voxels in the brain. These maps were subsequently used in higher-level analyses, applying a mass univariate approach, to investigate patterns across participants.Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith28 Region of interest masks were created with the Harvard-Oxford Subcortical Structural Atlas for NAcReference Desikan, Ségonne, Fischl, Quinn, Dickerson and Blacker29 and the Talairach Atlas for ACC.Reference Talairach and Tournoux30–Reference Lancaster, Woldorff, Parsons, Liotti, Freitas and Rainey32
To investigate the relationship between rsFC and percentage change in DARS score, a general linear model (GLM) framework was applied: the main explanatory variable was change in DARS score, and age and gender were entered to control for confounding. To identify voxel clusters where this relationship was statistically significant, cluster thresholding was used (Z-statistic threshold of 3.1, cluster P-value threshold of 0.05). Several anatomical atlases were used to identify the Montreal Neurological Institute (MNI) 152 coordinates of the local Z-statistic maximum(s) at each cluster.Reference Desikan, Ségonne, Fischl, Quinn, Dickerson and Blacker29,Reference Mori, Wakana, van Zijl and Nagae-Poetscher33–Reference Rorden and Brett37 This analysis was repeated with MADRS score as an additional explanatory variable to establish whether significant findings were independent of depressive severity. The FMRIB Software Library (FSL) FEAT package was used for this analysis, which also corrects for multiple comparisons by using nonparametric permutation inference (FEAT version 6.00 for macOS, FMRIB, Oxford, UK; see www.fmrib.ox.ac.uk/fsl).Reference Woolrich, Behrens, Beckmann, Jenkinson and Smith28,Reference Woolrich, Ripley, Brady and Smith38
Independent component analysis (ICA) was applied to investigate the relationship between rsFC in the salience network and change in DARS score. ICA decomposition was performed with a temporal concatenation approach, via the FSL melodic command line.Reference Beckmann, DeLuca, Devlin and Smith39–Reference Beckmann and Smith41 Twenty components were extracted, employing the methodology of Iraji et al, who used resting-state fMRI data collected from 309 individuals to identify 12 resting-state networks.Reference Iraji, Deramus, Lewis, Yaesoubi, Stephen and Erhardt42 To investigate the relationship between rsFC in these resting-state networks and change in DARS score, a GLM framework was used, and age and gender were entered to control for confounding. Dual regression analysis was performed with this GLM matrix, to compare resting-state network maps across participants.Reference Nickerson, Smith, Öngür and Beckmann43,Reference Beckmann, Mackay, Filippini and Smith44 Using the FSL ‘randomize’ tool, nonparametric permutation inference with 5000 permutations was completed to correct for multiple comparisons across individuals.Reference Winkler, Ridgway, Webster, Smith and Nichols45 The salience network was identified from the independent components generated, using the map from Iraji et al for guidance.Reference Iraji, Deramus, Lewis, Yaesoubi, Stephen and Erhardt42 Both t-statistic and P-values for each voxel were generated, and P-values <0.05 represented salience network regions where rsFC and change in DARS score were significantly correlated. This analysis was repeated with MADRS score as an additional explanatory variable to establish whether findings were independent of depressive severity.
All analyses were also completed for participants who received escitalopram monotherapy for the duration of the study, for comparison purposes. These methods and results are presented in Supplementary Material available at https://doi.org/10.1192/bjo.2023.588.
Results
Overall, 211 participants were enrolled in the CAN-BIND-1 study (from August 2013 to December 2016). Non-responders to escitalopram (n = 95) were eligible to receive adjunct aripiprazole for an additional 8 weeks; of these participants, 69 had complete clinical and neuroimaging data to identify baseline neuroimaging predictors of week 16 response, and 71 participants had complete data to identify week 8 predictors of week 16 response. There were no significant differences in demographic or baseline clinical characteristics between those included and excluded from the subsequent analyses.
There were no differences in demographics or psychiatric history between escitalopram responders and non-responders, although non-responders had received a greater number of previous antidepressant trials than responders (t = −2.67, P < 0.01). Also, there were no significant differences in baseline MADRS or DARS scores between the two groups.
Among those receiving adjunct aripiprazole, there was a significant improvement in DARS score after 8 weeks of aripiprazole treatment (t = −3.48, P < 0.001). This improvement was independent of change in MADRS score (Pillai's Trace: 0.315, P < 0.001).
Demographic and clinical data are presented in Table 1.
Table 1 Baseline demographics of study population
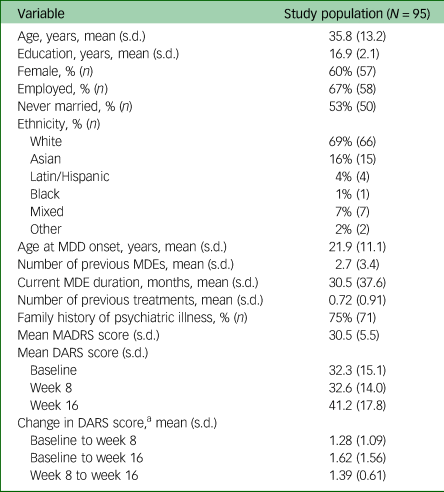
MDD, major depressive disorder; MDE, major depressive episode; MADRS, Montgomery–Åsberg Depression Rating Scale; DARS, Dimensional Anhedonia Rating Scale.
a. Expressed as a fraction, final value divided by initial.
Seed-based correlation analysis: rsFC association with anhedonia change (week 8 to week 16)
There was a positive correlation between change in DARS score from week 8 to week 16 and week 8 rsFC between the ACC (which was used as the seed) and the posterior cingulate cortex (PCC) (P = 0.03), as well as the bilateral ventral-posterior praecuneus (P = 0.01) at week 8. Change in DARS score was also negatively correlated with week 8 rsFC between the ACC and right dorsal-anterior praecuneus (P = 0.03), middle frontal gyrus (left: P = 0.004; right: P = 0.02) and left superior frontal gyrus (P = 0.004).
Change in DARS score from week 8 to week 16 was also positively correlated with week 8 rsFC between the NAc (which was used as the seed) and bilateral ventral-posterior praecuneus (P = 0.001), cerebellum lobule IX (P < 0.001), pons (P = 0.01) and splenium of the corpus collosum (P = 0.03). Change in DARS score was negatively correlated with week 8 rsFC between the NAc and left supramarginal gyrus (P = 0.02), parietal operculum (P = 0.02), middle occipital gyrus (left: P = 0.004; right: P = 0.01), superior parietal gyrus (P < 0.001) and bilateral dorsal-anterior praecuneus (P < 0.001). All results are presented in Table 2, and presented visually in Fig. 1.
Table 2 MNI152 coordinates of Z-maxima for association between DARS score change and rsFC, week 8 to week 16a
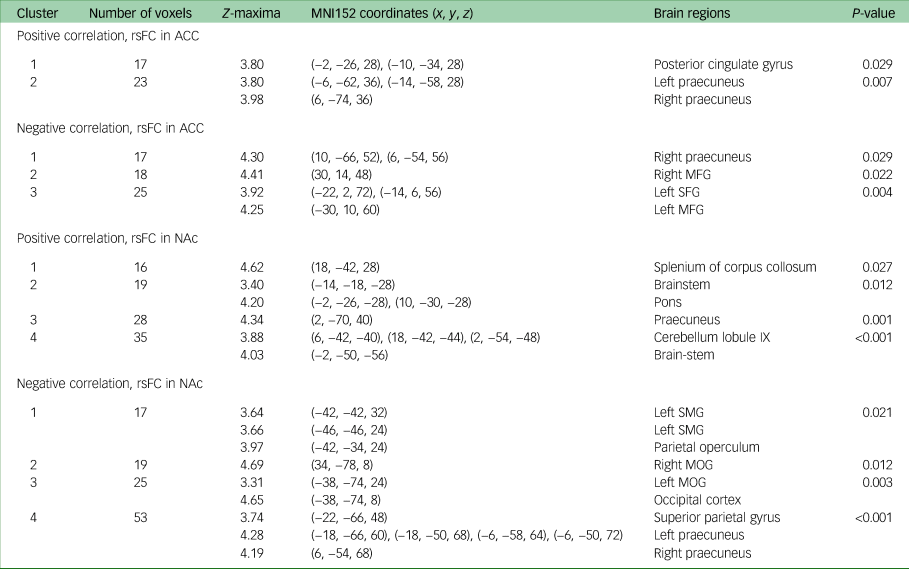
MNI, Montreal Neurological Institute; DARS, Dimensional Anhedonia Rating Scale; rsFC, resting-state functional connectivity; ACC, anterior cingulate cortex; MFG, middle frontal gyrus; SFG, superior frontal gyrus; NAc, nucleus accumbens; SMG, supramarginal gyrus; MOG, middle occipital gyrus.
a. Controlled for Montgomery–Åsberg Depression Rating Scale score, gender and age.
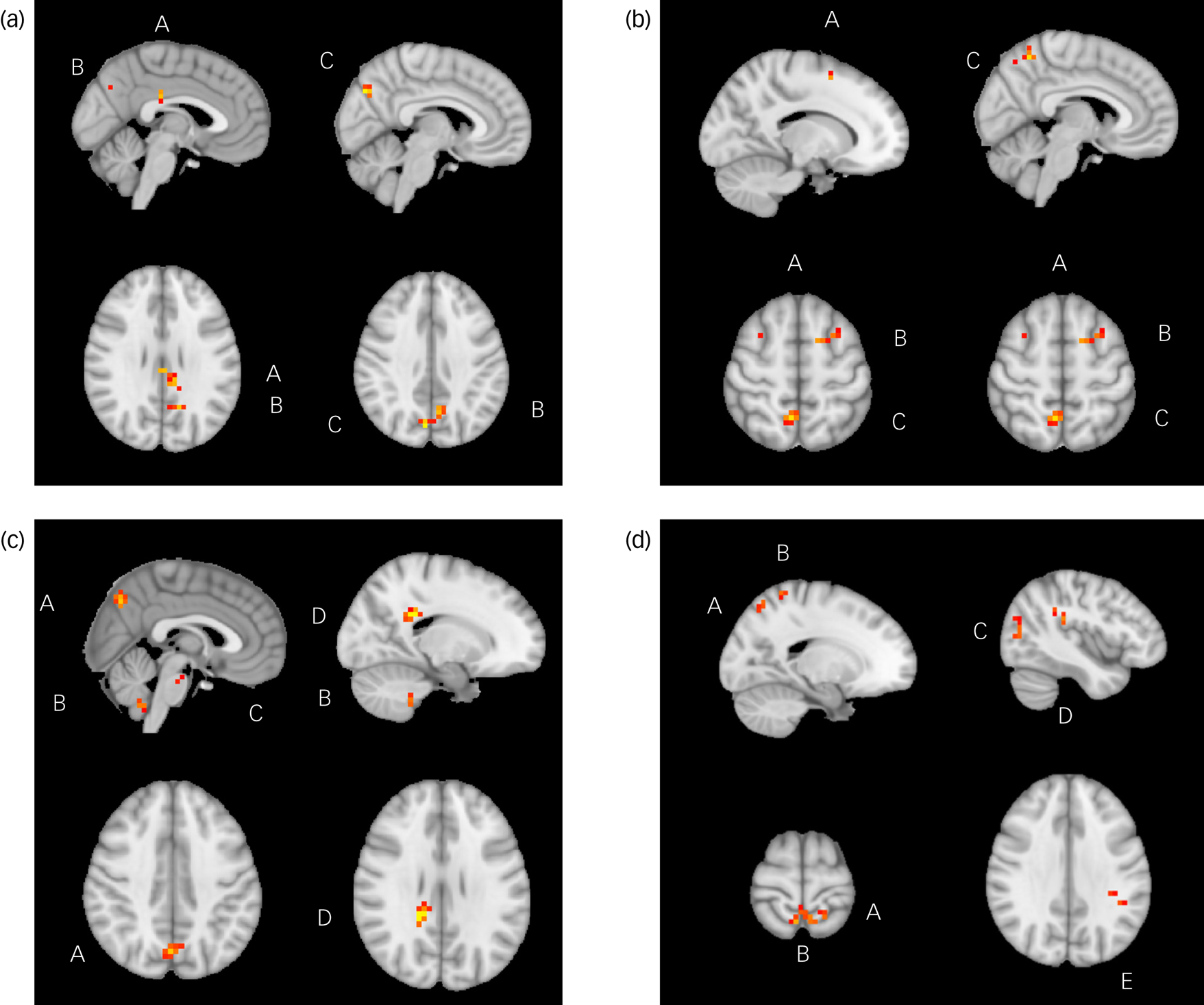
Fig. 1 Significant relationships between change in Dimensional Anhedonia Rating Scale (DARS) score and resting-state functional connectivity (rsFC), week 8 to week 16. (a) There were significant positive relationships between change in DARS score and rsFC between the anterior cingulate cortex and posterior cingulate cortex (A), left praecuneus (B) and right praecuneus (C). (b) There were significant positive relationships between change in DARS score and rsFC between the between the anterior cingulate cortex and superior frontal gyrus (A), middle frontal gyrus (B) and right praecuneus (C). (c) There were significant positive relationships between change in DARS score and rsFC between the nucleus accumbens and praecuneus (A), cerebellum lobule IX (B), pons (C) and splenium of corpus collosum (D). (d) There were significant positive relationships between change in DARS score and rsFC between the nucleus accumbens and superior parietal gyrus (A), praecuneus (B), middle occipital gyrus (C), parietal operculum (D) and supramarginal gyrus (E).
Seed-based correlation analysis: rsFC association with anhedonia change (week 0 to week 16)
Change in DARS score from baseline to week 16 was positively correlated with baseline rsFC between the NAc and the supplementary motor area (P = 0.001), precentral gyrus (P = 0.001), and anterior and posterior cingulate cortices (P = 0.001) when controlling for both age and gender; and age, gender and MADRS score. Additionally, when controlling for age, gender and MADRS score (but neither age nor gender alone), change in DARS score was positively associated with baseline rsFC between the NAc and Heschl's gyrus (P = 0.001), planum temporale (P = 0.001), insula (P = 0.001) and central opercular cortex. All results are presented in Table 3, and presented visually in Fig. 1.
Table 3 MNI152 coordinates of Z-maxima for association between DARS score change and rsFC, baseline to week 16a
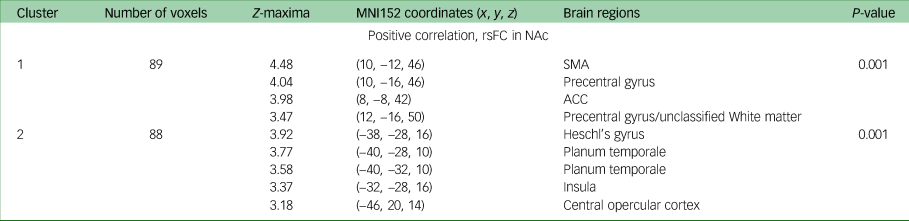
MNI, Montreal Neurological Institute; DARS, Dimensional Anhedonia Rating Scale; rsFC, resting-state functional connectivity; NAc, nucleus accumbens; SMA, supplementary motor area; ACC, anterior cingulate cortex.
a. Controlled for Montgomery–Åsberg Depression Rating Scale, gender and age.
Independent component analyses
The independent component map representing the salience network was identified: the greatest regions of activation were located in the insula, dorsal ACC, amygdala, thalamus and substantia nigra pars compacta/VTA. In both analyses, no significant associations were found between change in DARS score and rsFC in regions of the salience network, when controlling for age and gender and after correcting for multiple comparisons.
Discussion
Overall, our results demonstrate baseline and week 8 rsFC correlations between anhedonia improvement and adjunct aripiprazole treatment. Further, there was an improvement in anhedonia with adjunct aripiprazole, independent of change in depressive severity. Therefore, aripiprazole may be a promising treatment option for patients with MDD specifically experiencing anhedonia symptoms. As far as we are aware, this is the first study to report on the effectiveness of aripiprazole in treating anhedonia, and the brain regions associated with anhedonia improvement, in a cohort of participants with depression who had not responded to and 8-week trial of SSRI monotherapy.
Among those who received 8 weeks of escitalopram plus aripiprazole, stronger connectivity patterns between the ACC and PCC and ventral-posterior praecuneus were predictive of a decrease in anhedonia symptoms after 8 weeks of treatment. The PCC and praecuneus are two of the major regions implicated in the default mode network (DMN).Reference Fransson and Marrelec46 There is evidence to support the presence of distinct anterior and posterior subregions within the praecuneus, each with different functional activation patterns. The posterior praecuneus plays a role in memory retrieval.Reference Cavanna and Trimble47 The exact role of the PCC is not fully understood, but the majority of evidence suggests it is important for internally directed thought and cognitive control.Reference Leech and Sharp48 Considering the dorsal ACC is a key node of the salience network (i.e. the brain network most consistently implicated in reward processing), these findings suggest that a stronger connection between the DMN and salience network may predict anhedonia improvement after aripiprazole treatment. Conversely, a reduced connectivity between the ACC and the anterior praecuneus was associated with improvement in anhedonia. The anterior praecuneus, which is part of the DMN, is important for self-centred mental imagery.Reference Cavanna and Trimble47 It has been previously suggested in Menon's unifying triple network model that the relationship between the salience network and DMN is essential for attending to external stimuli, including rewarding stimuli, as the salience network suppresses DMN activity.Reference Lesage, Stein, Pfaff and Volkow12,Reference Menon49 The connectivity patterns between the salience network and DMN found in our current study, with both positive and negative correlations at specific regions of the DMN, support these previous findings and suggest a complex interplay between these two networks in reward processing.
Increased connectivity between the NAc and ventral-posterior praecuneus predicted anhedonia improvement. As previously stated, the posterior region of the praecuneus is involved in memory retrieval.Reference Cavanna and Trimble47 Previous studies have found that effective processing and retrieval of reward-related memories are required for reward processing.Reference LeGates, Kvarta, Tooley, Francis, Lobo and Creed50 Additionally, in the current analysis, increased connectivity between the NAc and the pons, cerebellum lobule IX and the splenium of the corpus collosum correlated with anhedonia response (although the corpus collosum is not generally explored by fMRI, as it is white matter, there is emerging evidence that fMRI findings in white matter are detectable and reliableReference Gore, Li, Gao, Wu, Schilling and Huang51). These three brain regions are not classically implicated in anhedonia or reward processing, but they are necessary for the integration, processing and relay of neural signals in the brain. The raphe nuclei, where most serotonergic neurons originate, is located in the pons. Serotonin is involved in modulating dopamine activity in the NAc, and therefore plays a role in regulating reward response in the NAc.Reference Parsons and Justice52,Reference Dölen, Darvishzadeh, Huang and Malenka53 Lobule IX of the cerebellum is a ‘non-motor’ region of the cerebellum, implicated in emotional processing.Reference Depping, Wolf, Vasic, Sambataro, Hirjak and Thomann54 Increasing evidence suggests the cerebellum may be part of the DMN,Reference Habas, Kamdar, Nguyen, Prater, Beckmann and Menon55 further supporting the theory that connectivity patterns between reward regions and the DMN is predictive of anhedonia improvement after aripiprazole. However, the cerebellum is not typically studied for its role in reward processing, so data supporting this finding are limited.
This study provides support for the role of dopamine in both reward-related processing and anhedonia treatment. To our knowledge, there have been no human studies assessing the effect of aripiprazole on anhedonia in a population with MDD. However, in preclinical studies, aripiprazole had favourable effects on animal models of anhedonia.Reference Kim, Kim, Hong, Shin and Choi56,Reference Scheggi, Pelliccia, Gambarana and De Montis57 Further, favourable effects of aripiprazole on anhedonia symptoms have also been reported in bipolar depression and schizophrenia.Reference Mazza, Squillacioti, Pecora, Janiri and Bria58,Reference Liemburg, Aleman, Bous, Hollander and Knegtering59 Aripiprazole likely has anti-anhedonia effects directly via partial D2 receptor agonism, and indirectly via 5-HT2A serotonin receptor antagonism,Reference Liemburg, Aleman, Bous, Hollander and Knegtering59 which increases dopaminergic activity by reducing blockade of dopamine receptors.Reference DeLeon, Patel and Lynn Crismon60 In addition, aripiprazole is shown to increased dopaminergic activity in brain regions specifically implicated in reward processing, most notably the mesolimbic pathway.Reference Han, Huang and Deng61 Aripiprazole's effect on dopamine is dose-dependent, with higher doses resulting in greater D2 receptor occupancy; aripiprazole occupies 74–85% of D2 receptors at doses of 2–10 mg (the dose range used in the current study).Reference Yokoi, Grunder, Biziere, Stephane, Dogan and Dannals62
Interestingly, there was an inverse relationship between rsFC among key reward regions and decrease in anhedonia; that is, reduced connectivity at baseline between specific reward regions was associated with anhedonia improvement post-aripiprazole treatment. This may suggest that aripiprazole is more likely to act favourably on the subgroup of individuals with anhedonic brain-connectivity patterns, demonstrating that aripiprazole, and potentially other dopamine modulator agents, may be particularly effective in addressing reward-related deficits.
There were no significant findings in the ICA of the salience network, demonstrating no role of functional connectivity within the salience network in predicting anhedonia improvement. However, our current findings suggest that connectivity between regions of the salience network and other brain regions may predict anhedonia improvement after antidepressant treatment, considering our finding that that functional connectivity between the ACC (a major hub of the salience network) and regions of DMN was significantly associated with anhedonia improvement. This is supported by multiple other antidepressant biomarker studies reporting that connectivity between nodes of different brain networks is predictive of antidepressant response, but not connectivity within a network itself (reviewed by Dunlop et alReference Dunlop, Talishinsky and Liston63). However, the current study may be underpowered to detect relationships within the salience network, as ICA is data-driven, rather than model-driven, and therefore may not be suitable to detect more subtle associations in studies with smaller sample sizes.
In conclusion, eight weeks of aripiprazole, adjunct to escitalopram, was associated with improved anhedonia symptoms. Several distinct rsFC patterns were predictive of anhedonia improvement after treatment with adjunct aripiprazole. Decreased functional connectivity between key reward regions was associated with anhedonia improvement, suggesting that aripiprazole may be an effective treatment for individuals experiencing reward-related deficits. Future studies are required to replicate the present findings in larger placebo-controlled studies, and explore their generalisability with other agents with partial D2 agonism and/or 5HT2A antagonism.
Limitations
Because only escitalopram non-responders received aripiprazole treatment, all participants in the aripiprazole treatment group were ‘treatment-resistant’ to at least one antidepressant. However, as aripiprazole is only approved as adjunct treatment for depression, when used in combination with a first-line antidepressant, this study cohort reflects a clinical group of individuals with depression who may be prescribed aripiprazole in clinical practice. Overall, the study population was moderately depressed and moderately anhedonic, and therefore results may not be applicable to individuals with more severe depression or anhedonia. Further, as presence of anhedonia was not an inclusion criterion, individuals without clinically significant anhedonia at baseline were part of the study cohort. This study was limited by the sample size, which may have been too small to detect associations, particularly in the ICA. Further, there was no placebo group to compare our findings against, and therefore we cannot make definitive conclusions about efficacy of aripiprazole in anhedonia or the specificity of resting-state biomarkers.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2023.588
Data availability
The data that support the findings of this study are available from the corresponding author, S.H.K., on reasonable request.
Author contributions
S.R.V. wrote the initial draft of the manuscript. S.R.V., S.J.R., W.L. and S.H.K. formulated the research questions and methodology. S.R.V. and S.W. completed the data analysis. S.R.V., S.J.R., W.L. and S.H.K. interpreted the data. G.M.M., B.N.F., R.W.L., R.V.M., A.V.R., S.C.S. and S.H.K. designed the CAN-BIND-1 trial. G.M.M., K.H., B.N.F., R.W.L., R.V.M., S.R., A.V.R., S.C.S. and S.H.K. completed data collection. All authors contributed to the editing and revising of the final manuscript and provided final approval for submission.
Funding
This project was completed as part of a Master's thesis at the Institute of Medical Science, University of Toronto, with financial support from the University of Toronto Entrance Award. The completed thesis can be found at https://hdl.handle.net/1807/109088. Data used for this project were collected during a CAN-BIND project. CAN-BIND is an integrated discovery programme carried out in partnership with, and with financial support from, the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario Government. The opinions, results and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Additional funding is provided by the Canadian Institutes of Health Research, Lundbeck, Bristol-Myers Squibb and Servier. Funding and/or in-kind support is also provided by the investigators’ universities and academic institutions. All study medications are independently purchased at wholesale market values.
Declaration of interest
S.J.R. has received consulting fees or research funding from Allergan Canada, Janssen, Neurocrine and Pfizer Canada; and also holds the copyright for the DARS. G.M.M. has been on the advisory board or has been a speaker for Allergen, Lundbeck, Lilly, Pfizer, Janssen and Johnson & Johnson. R.W.L. has received honoraria for ad hoc speaking or advising/consulting, or received research funds, from Abbvie, Asia-Pacific Economic Cooperation, Bausch, British Columbia Leading Edge Foundation, Brain Canada, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, CAN-BIND Solutions, Carnot, Grand Challenges Canada, Healthy Minds Canada, Janssen, Lundbeck, Medscape, Michael Smith Foundation for Health Research, Mitacs, Neurotorium, Ontario Brain Institute, Otsuka, Pfizer/Viatris, Shanghai Mental Health Center, Sunnybrook Health Sciences Center, Unity Health, Vancouver Coastal Health Research Institute and Vancouver General Hospital-University of British Columbia Hospital Foundation. R.V.M. has received research grants from CAN-BIND, Canadian Institutes of Health Research, Janssen, Lallemand, Lundbeck, Nubiyota, Ontario Brain Institute and Ontario Mental Health Foundation; and has received speaking and consulting honoraria from AbbVie, Allergan, Janssen, Kye, Lundbeck, Otsuka and Sunovion. A.V.R. has received speaker and consultant honoraria or research funds from Bristol Myers Squibb, Canadian Depression Research and Intervention Network, Canadian Foundation for Innovation and the Ministry of Economic Development and Innovation, Canadian Institutes of Health Research, Grand Challenges Canada, Janssen, Lundbeck, Ontario Mental Health Foundation, Pfizer and Sunovion. S.H.K. has received research funding or honoraria from the following sources: Abbott, Alkermes, Allergan, Bristol-Myers Squibb, Brain Canada, Canadian Institutes of Health Research, Janssen, Lundbeck, Neurocrine, Neurotorium, Ontario Brain Institute, Ontario Research Fund, Otsuka, Pfizer, Servier, Sunovion and Xian-Janssen. All other authors report no conflicting financial relationships with commercial interests.








eLetters
No eLetters have been published for this article.