Down syndrome is a neurodevelopmental disorder caused by triplication of chromosome 21 and is associated with an ultra-high risk of Alzheimer's disease with ageing.Reference Wiseman, Al-Janabi, Hardy, Karmiloff-Smith, Nizetic and Tybulewicz1 Common mental disorders (CMDs), defined in this study as a diagnosis of depression, anxiety or obsessive–compulsive disorder (OCD) including seasonal affective disorder (SAD), post-traumatic stress disorder (PTSD) and disruptive behaviour disorders, are relatively common in individuals with Down syndrome.2 In England, the prevalence of psychiatric diagnoses among the general population is estimated at 17%,Reference McManus, Bebbington, Jenkins and Brugha3 whereas results from studies investigating the prevalence of CMDs among individuals with Down syndrome present a mixed picture. Studies with smaller samples have reported a prevalence ranging from 10.8 to 23.7%, depending on the classification used.Reference Mantry, Cooper, Smiley, Morrison, Allan and Williamson4 Depression is one of the most frequently described mental health problems in individuals with Down syndrome, with prevalence rates ranging from 5.2 to 18.4%,Reference Startin, D'Souza, Ball, Hamburg, Hithersay and Hughes5 compared with 3.3% in the general population.Reference McManus, Bebbington, Jenkins and Brugha3 However, a recent population-based study indicated that mood and anxiety disorders are less common in individuals with Down syndrome than in the general population.Reference Baksh, Pape, Chan, Aslam, Gulliford and Strydom6 Lastly, although younger individuals with Down syndrome are more prone to anxiety and externalising disorders, older individuals with Down syndrome are more likely to receive a diagnosis of depression.Reference Tassé, Navas Macho, Havercamp, Benson, Allain and Manickam7
CMDs are associated with Alzheimer's disease-like cognitive decline in the general population.Reference Bendayan, Kelly, Hofer, Piccinin and Muniz-Terrera8 Anxiety is associated with poorer performance on memory tasks and has been linked to cognitive decline in older adults.Reference Gulpers, Ramakers, Hamel, Köhler, Voshaar and Verhey9 Depression severity is associated with decline in episodic memory, language, working memory, executive function, as well as processing speed domains,Reference McIntyre, Xiao, Syeda, Vinberg, Carvalho and Mansur10 which tends to persist even after remission of symptoms.Reference Godard, Baruch, Grondin and Lafleur11 Evidence has shown that cognitive impairment in older adults with depression is a strong predictor of dementiaReference Sáez-Fonseca, Lee and Walker12 and that depression is associated with a twofold increase in the risk of developing dementia.Reference Ownby, Crocco, Acevedo, John and Loewenstein13 Moreover, depressive and other psychiatric symptoms are also common among people with dementia and may be a response to early cognitive decline.Reference Bennett and Thomas14 The impact of CMDs on cognition has not been well-established in Down syndrome, despite depression and anxiety being common psychiatric conditions in Down syndrome.Reference Urv, Zigman and Silverman15 Some authors suggest that these psychiatric features might be regarded as prodromal symptoms of Alzheimer's disease.Reference Burt, Loveland, Primeaux-Hart, Chen, Phillips and Cleveland16 In addition, the presence of CMD symptoms may complicate diagnosis of Alzheimer's disease in people with Down syndrome, a population with lifelong cognitive impairments in whom it is difficult to establish the onset of cognitive decline. Clinicians are therefore often reluctant to diagnose dementia due to Alzheimer's disease in the presence of CMDs, and diagnosis of Alzheimer's disease may be significantly delayed in people with such comorbidity.Reference Eady, Courtenay and Strydom17 It is therefore critical to understand the potential impact of CMDs on longer-term cognitive outcomes in people with Down syndrome, as this could improve diagnostic certainty and allow for earlier access to treatment and better support.
Aims
We aimed to understand whether the presence of CMDs was associated with a decline in measures of cognition and adaptive behaviours in people with Down syndrome longitudinally, compared with those without a diagnosis of CMD.
Method
Study design
The London Down Syndrome (LonDownS) Consortium study is a longitudinal study of people with Down syndrome. All participants undergo extensive neuropsychological examination using the LonDownS Consortium battery, which has been described in previous studies.Reference Startin, Hamburg, Hithersay, Davies, Rodger and Aggarwal18 The battery involves a series of neuropsychological tests measuring memory, language, executive function and motor skills. Semi-structured interviews and questionnaires measuring adaptive behaviour and Alzheimer's disease-related decline are also administered to each participant's caregiver, alongside questions about demographic details and medical history. Participants and carers completed the LonDownS assessment at baseline (T 1) and subsequent follow-up (T 2) an average of 36 months after the baseline assessment.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by the North-West Wales Research Ethics Committee (13/WA/0194).
Inclusion criteria
Inclusion criterion for the initial LonDownS Consortium study was all adults with Down syndrome over the age of 16. Capacity was evaluated for participants at each assessment and written informed consent was obtained from all participants where possible. For those that lacked capacity, their carers acted as consultees. Carers were required to indicate their decision about the participant's inclusion based on their knowledge of the participant and their wishes, in accordance with the UK Mental Capacity Act 2005. Down syndrome diagnosis was genetically confirmed using saliva or blood samples.
Classification of CMD and exclusion criteria
At baseline, a comprehensive medical history was obtained in collaboration with caregivers. Participants with an ongoing diagnosis of depression, anxiety, OCD and related conditions such as SAD, PTSD and behavioural disorders at the time of their cognitive assessment were categorised as having a diagnosis of a CMD. In addition, participants with CMD-like symptoms reported by their doctor, and participants who were also prescribed medication typically used to treat CMDs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs) such as citalopram, fluoxetine, mirtazapine, sertraline and trazodone, were included. These participants were classed as having a CMD. Those without CMDs were therefore defined as individuals with no diagnosis, symptoms or prescription of relevant medications at the baseline visit. To focus on longer-term cognitive impact rather than acute effects of CMDs, participants who did not have a CMD at baseline but who had developed CMD by the time of their follow-up visit were removed from analyses. Finally, to be eligible for inclusion in this analysis, participants were required to have no diagnosis of dementia at baseline.
Cognitive and behavioural outcome measures
Cognitive task outcomes were measures of memory (Cambridge Neuropsychological Test Automated Battery Paired Associates Learning (CANTAB PAL) and Cambridge Cognitive Examination (CAMCOG) Orientation subscale), general verbal and non-verbal cognitive abilities (Kaufman Brief Intelligence Test – Second Edition (KBIT-2) and CAMCOG Orientation subscale), executive functioning (CAMCOG Verbal Fluency subscale and Tower of London test) and attention (CANTAB Simple Reaction Time (CANTAB SRT)). These tasks were chosen to assess skills associated with brain areas most affected in both CMDs and Alzheimer's disease; they are suited to a range of ages and abilities, including non-verbal individuals and have good test–retest reliabilityReference Startin, Hamburg, Hithersay, Davies, Rodger and Aggarwal18 (Table 1).
Table 1 Cognitive outcome measures
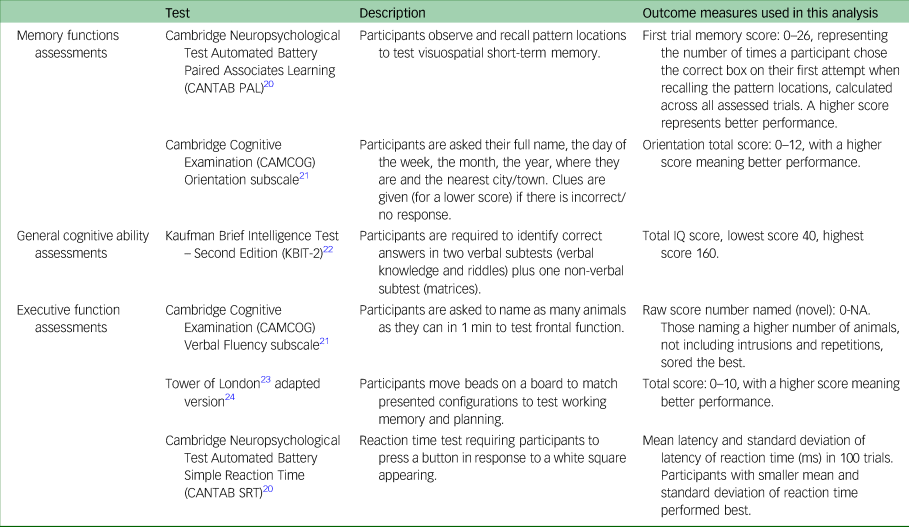
The Short Adaptive Behavior Scale (SABS)Reference Hatton, Emerson, Robertson, Gregory, Kessissoglou and Perry19 is an informant questionnaire that was used to assess changes in behaviours between T 1 and T 2. The SABS measures adaptive behaviours across three domains: personal self-sufficiency, community self-sufficiency and personal-social responsibility. The SABS results at baseline assessment were compared with the follow-up assessment results, with lower scores at follow-up suggesting functional decline.
Statistical analysis
Change scores were calculated for cognitive tasks and questionnaire scores over the follow-up period for each participant. The score at T 1 was subtracted from the score at T 2. Multiple linear regressions were performed on change in cognitive task and SABS scores over time to determine whether individuals diagnosed with a CMD at baseline showed negative change over time compared with individuals without a CMD diagnosis.
In the cognitive task analysis, the outcome measure was change in cognitive task scores on each of the cognitive tasks between visits. For the analysis of adaptive behavioural changes, the outcome measure was change in SABS score in three summary domains – personal self-sufficiency, community self-sufficiency and personal-social responsibility – as well as the total score. In all the statistical models the main predictor was CMD.
Relevant additional predictors were included in each model: these were age, gender and level of intellectual disability. Apolipoprotein E (APOE) status was also included, as people with Down syndrome who are APOE ɛ4 allele carriers show earlier neurodegenerative changes and clinical symptoms of Alzheimer's disease.Reference Bejanin, Iulita, Vilaplana, Carmona-Iragui, Benejam and Videla25 All statistical analyses were then performed using R (version 4.0.5 for MacOS)26 in RStudio 1.4.1106 with a significance level of 0.05.
Results
Participants
From the total number of individuals recruited for LonDownS (n = 474), 94 were excluded from the analyses as they presented with a dementia diagnosis at the initial assessment and a further 7 were excluded as they had developed a CMD by follow-up. These participants were excluded in order to focus specifically on the effects of mental health conditions over time to prevent the potential confounding factor of diminished task scores caused by dementia-related illness or subsequent development of CMDs. Of the remaining 373 participants, 115 had complete cognitive task and behavioural data at baseline and after at least 2 years, 27 (23.48%) of whom were classed as having a CMD (Fig. 1; see Table 2 for the demographic characteristics of the final sample).

Fig. 1 Flow chart showing participant exclusion and final cognitive data sample size.
LonDownS, London Down Syndrome Consortium study. CMD, common mental disorder; T 2, follow-up assessment.
Table 2 Participant demographics by common mental disorder (CMD) status at baseline (n = 115)

APOE, apolipoprotein E.
Changes in cognitive and behavioural scores over time
Both groups (i.e. with a CMD diagnosis and without a CMD diagnosis) showed differences in mean change scores over time (Table 3). Both groups showed declining scores for the Tower of London, CANTAB SRT mean latency, CAMCOG Verbal Fluency and CAMCOG Orientation between the baseline and follow-up visits. Participants with a CMD had greater decline in Verbal Fluency score than those without. However, both groups also showed improved performance in KBIT IQ, CANTAB PAL and CANTAB SRT standard deviation scores between visits. To examine associations between CMD and cognitive score, separate multiple regression analyses were used to model whether having a diagnosed CMD significantly predicted participants’ change scores on each cognitive task, controlling for age, gender, level of intellectual disability and APOE status (Table 4). Results showed that presence of a CMD as a predictor of change in cognitive task score was not statistically significant for any tasks (P > 0.05). Thus, no association was found between presence of a CMD and task change score.
Table 3 Mean task scores in affected versus unaffected individuals and change over time

T 1, baseline; T 2, follow-up; CAMCOG, Cambridge Cognitive Examination; KBIT, Kaufman Brief Intelligence Test – Second Edition; CANTAB, Cambridge Neuropsychological Test Automated Battery; PAL, Paired Associates Learning; SRT, Simple Reaction Time.
a. Bold denotes significance at P ≤ 0.05.
Table 4 Cognitive tasks score multiple regressiona
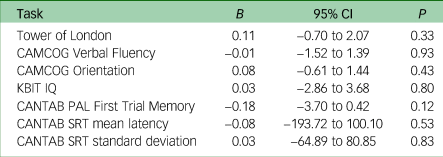
CAMCOG, Cambridge Cognitive Examination; KBIT, Kaufman Brief Intelligence Test – Second Edition; CANTAB, Cambridge Neuropsychological Test Automated Battery; PAL, Paired Associates Learning; SRT, Simple Reaction Time.
a. All models were adjusted for age, gender, level of intellectual disability and apolipoprotein E status.
Both groups showed differences in behavioural change scores over time (Table 5). Both groups showed improvement in mean behavioural scores between visits, and those with a CMD showed greater improvement (+16.73) than those without (+9.84) in the SABS total. Multiple linear regression was used to analyse associations between CMDs and scores representing behavioural changes reported on the SABS over time (Table 6). No significant effect of CMD state at baseline on SABS change score over time was demonstrated (P > 0.05).
Table 5 Mean Short Adaptive Behavior Scale (SABS) scores in affected and unaffected individuals and change over time

Table 6 Short Adaptive Behavior Scale (SABS) score multiple regressiona

a. All models were adjusted for age, gender, level of intellectual disability and apolipoprotein E status.
In summary, presence of a CMD was not significantly associated with cognitive and behavioural changes over time and there were no significant differences in change scores between participants with and without a CMD.
Seven participants were diagnosed with a CMD between their baseline and follow-up assessments. Among those who completed tasks on both visits, most participants with new onset of a CMD performed worse overall (Table 7). However, there are incomplete data for this subset of participants because those with unstable illness were unable to complete all tasks, and therefore these participants were not included in the above regressions.
Table 7 Change scores of participants who received a diagnosis of common mental disorder between visits
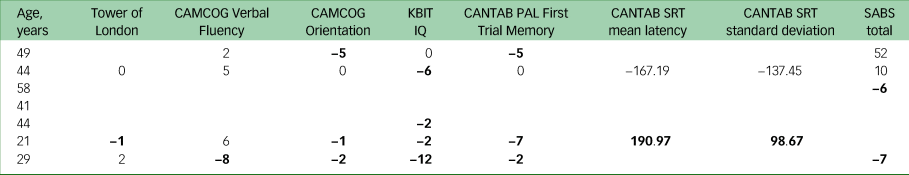
CAMCOG, Cambridge Cognitive Examination; KBIT, Kaufman Brief Intelligence Test – Second Edition; CANTAB, Cambridge Neuropsychological Test Automated Battery; PAL, Paired Associates Learning; SRT, Simple Reaction Time; SABS, Short Adaptive Behavior Scale.
a. Bold denotes declining scores.
Discussion
Main findings
This longitudinal study investigated associations between CMDs and cognitive decline in people with Down syndrome without dementia. We hypothesised that the presence of CMDs may be a significant risk factor for increased Alzheimer's disease-related cognitive decline over time in this population. We found no significant association between the presence of a stable or treated CMD at baseline and cognitive or functional decline over time; however, those with new onset of a CMD during the follow-up tended to have worse scores on cognitive and/or functional ability assessments.
Interpretation of our findings
CMDs, particularly depression, are known to be associated with cognitive impairments, which can significantly affect a person's independent functioning and quality of life in the short term. CMDs are also considered risk factors for Alzheimer's disease, with depression earlier in life being associated with risk of dementia in the general population.Reference Gulpers, Ramakers, Hamel, Köhler, Voshaar and Verhey9 Depression and anxiety symptoms are also frequently observed alongside mild cognitive impairment in the early stages of Alzheimer's disease and have been considered as prodromal features of Alzheimer's disease.Reference Bennett and Thomas14 Previous studies in people with Down syndrome have found associations between depression and impairment in adaptive behaviour,Reference Collacott and Cooper27–Reference Prasher and Hall29 with the severity of impairment being more pronounced in those with early-onset depression.Reference Collacott and Cooper27 Notably, a year of pharmacological treatment has demonstrated efficacy in mitigating these disturbances, even without significant changes in depressive symptomatology.Reference Prasher and Hall29 Furthermore, depression in individuals with Down syndrome is associated with impairments in daily living activities, social abilities, lower mental age and poorer memory performance.Reference Burt, Loveland and Lewis28,Reference Foley, Bourke, Einfeld, Tonge, Jacoby and Leonard30 Finally, speech impairment has been found to negatively correlate with depression and anxiety.Reference Määttä, Tervo-Määttä, Taanila, Kaski and Iivanainen31
In our study, the presence of stable and/or treated CMDs in individuals with Down syndrome was not significantly associated with cognitive decline or behavioural changes over a 3-year period. A key factor in our study is the use of clinically confirmed diagnoses of CMDs by the participants’ doctor, meaning that these mental health conditions have been diagnosed and that these individuals have likely been offered treatment, either with medication or with psychological or psychosocial interventions. It has been previously shown in Down syndrome that 1 year of antidepressant pharmacotherapy produced a significant recovery in adaptive functioning even though depressive symptoms were still present.Reference Prasher and Hall29 Half of our participants were receiving pharmacological treatment, and this may have mitigated the cognitive decline that can be associated with CMDs in these individuals. In the general population, antidepressants have been shown to help reduce cognitive deficits associated with CMDs. For instance, a systematic review and meta-analysis showed that SSRIs have beneficial effects on attention, executive function, memory and processing speed in people with depression.Reference Prado, Watt and Crowe32 A recent clinical trial showed that vortioxetine, a multimodal antidepressant, was associated with significant improvement of executive function, attention, learning, memory and processing speed in adults with depression from the general population.Reference McIntyre, Lophaven and Olsen33 SNRIs can also be effective in improving cognitive functions such as declarative and working memory,Reference Herrera-Guzmán, Herrera-Abarca, Gudayol-Ferré, Herrera-Guzmán, Gómez-Carbajal and Peña-Olvira34 verbal learningReference Raskin, Wiltse, Siegal, Sheikh, Xu and Dinkel35 and psychomotor speed.Reference Greer, Sunderajan, Grannemann, Kurian and Trivedi36 Pharmacological interventions may therefore have mitigated the detrimental cognitive effect potentially associated with CMDs in our sample.
Another factor that may have influenced the results of the study is related to the developmental trajectory of cognition in Down syndrome. The cognitive profile in Down syndrome is typically characterised by growth persisting throughout adolescence and early adulthood, followed by a gradual loss of ability,Reference Carr37 which is frequently associated with neurodegenerative processes.Reference Määttä, Tervo-Määttä, Taanila, Kaski and Iivanainen31 Therefore, the potential negative influence of CMDs on cognition, if present, may be less pronounced in individuals with Down syndrome.
Our study confirms that CMDs are common in people with Down syndrome. In our cohort, CMDs that occurred in participants without dementia were not related to cognitive and functional decline over time. However, the seven participants that developed a CMD between baseline and follow-up appeared to show some cognitive decline. Whether this was due to acute cognitive effects of CMDs or whether these individuals presented instead with neuropsychiatric prodromal symptoms of dementia is not clear. Of these seven participants, five were aged 41 and older, and therefore may have been experiencing some prodromal dementia symptoms. Nonetheless, our data suggest that CMDs may not be a significant additional risk factor for cognitive decline in adults with Down syndrome in the long term, particularly if well managed, in the context of an overwhelming genetic risk for Alzheimer's disease due to amyloid precursor protein (APP) gene triplication, although those with acute presentations may benefit from optimisation of treatment and cognitive surveillance.
Strengths and limitations
Our study had several strengths. By using each participant's own baseline, we were able to control for the effects of baseline cognitive and functional skills and track changes for that individual, thus improving the sensitivity of the assessment. We used neuropsychological test scores and informant ratings to consider changes across a wide range of domains. In addition, we included participants with severe intellectual disability, who are often excluded from research. The follow-up was longer than in most other studies of cognitive decline in Down syndrome, and although it is possible that decline in those with CMDs might have been demonstrated over a longer time period, this is unlikely, as in naturalistic studies of decline in people with Down syndrome aged 35 and older, decline has been observed within 2 years.Reference Hithersay, Baksh, Startin, Wijeratne, Hamburg and Carter38 This is also in keeping with previous studies testing the hypothesis of depression and anxiety as prodromal features of Alzheimer's disease in the general population that considered a similar time window between the diagnosis of CMDs and the onset of Alzheimer's disease (e.g.Reference Geerlings, Bouter, Schoevers, Beekman, Jonker and Deeg39,Reference Chen, Hu, Wei, Qin, McCracken and Copeland40 ).
However, this study also has some limitations. Although this is a longitudinal study with a relatively large sample, those included at baseline may have been under-representative of those with more severe episodes of mental illness. Another limitation of this study is that the use of SSRI and SNRI prescription as an indicator of CMD in individuals with Down syndrome may not always accurately reflect the presence of a CMD, as these medications are occasionally prescribed for other types of mental health condition, such as chronic pain disorder, eating disorders and severe mental illnesses. Also, we did not complete our own clinical assessments of the presence of CMD, but rather relied on diagnoses obtained from medical histories. Furthermore, we could not examine potential age differences in the impact of CMDs on cognition, as our sample was not large enough to allow for age-related grouping. It should be noted that our study did not specifically account for the presence of autism spectrum disorder (ASD), which can coexist and show overlapping symptoms with OCD in Down syndrome. ASD traits can be a confounding factor when examining the potential influence of CMDs on cognition, and future studies should take this into account. Finally, there were modest inter-participant differences in follow-up period that we did not statistically control for; however, we have controlled for participants’ age, as this has been shown to be one of the most important predictors of decline in analyses of Down syndrome studies of Alzheimer's disease.
Considerations for clinical practice
Despite widespread awareness of the high risk of Alzheimer's disease in people with Down syndrome, there remain many factors affecting diagnostic certainty when assessing for Alzheimer's disease in clinical settings. Some of these challenges are related to difficulties with self-report of symptoms, diminished communication skills and differences in psychopathology presentation.Reference Pape, Al Janabi, Ashton, Hye, Sheehan and Gallagher41 Others are driven by the reliance on data from informants who may have limited historical knowledge of an individual.Reference Prasher, Sachdeva and Tarrant42 A significant consideration for clinicians is whether symptoms of cognitive decline in those with a history of mental health issues are related to a CMD rather than early symptoms of Alzheimer's disease. Our findings suggest that although symptom overlap between CMDs and Alzheimer's disease is acknowledged,Reference Dekker, Sacco, Carfi, Benejam, Vermeiren and Beugelsdijk43 CMDs are not associated with significant objective cognitive decline over the longer term. A diagnosis of Alzheimer's disease should therefore be considered in those presenting with typical symptoms even in the presence of a comorbid CMD. This may reduce delays in both assessment and provision of support. However, evidence of cognitive decline from an individual's baseline would be important for an accurate diagnosis of Alzheimer's disease, and acute presentations of CMDs need to be treated before Alzheimer's disease can be diagnosed.
In conclusion, we found that stable and treated CMDs were not associated with longitudinal Alzheimer's disease-like cognitive decline and functional changes in adults with Down syndrome. This highlights the importance of baseline cognitive assessments being routinely offered for all people with Down syndrome prior to Alzheimer's disease onset. Further research is needed to investigate CMDs in earlier life and their later impact on cognition and behaviour, behavioural and psychiatric presentations as prodromal symptoms of dementia and the impact of treatment of CMDs in the Down syndrome population on the onset of Alzheimer's disease symptoms. Finally, the association between diagnosis of dementia and presence of CMDs is an area that warrants further study.
Data availability
The data that support the findings of this study are available from the corresponding author M.I. on request. The data are not publicly available as they contain information that could compromise the privacy of research participants.
Acknowledgements
We thank all the participants in this study and their families and carers for their time. We also thank Deborah Ness for helping to collect the data and Dr Kin Mok from John Hardy's laboratory at the University College London Queen Square Institute of Neurology for the genetic analysis. This research was supported by the National Institute for Health Research networks (Mental Health, Dementia and Neurology) and participating NHS trusts and we thank our NHS network of sites that helped to identify research participants.
Authors contributions
M.I., F.S., A.S., R.A.B. and S.E.P. contributed to the conception and design of the study; M.I., F.S., S.E.P. and M.-S.C. collected the data; M.I. analysed the data, with advice from A.B.; M.I. and F.S. wrote the manuscript with input from all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by: a Wellcome Trust Strategic Award (098330/Z/12/Z) conferred on the London Down Syndrome (LonDownS) Consortium; the Baily Thomas Charitable Fund (TRUST/RNA/AC/KW/3111/5776); and the Medical Research Council (MR/S011277/1, MR/S005145/1 and MR/R024901/1).
Declaration of interest
None.












eLetters
No eLetters have been published for this article.