Cognitive impairment is more prevalent in individuals with schizophrenia than in the general population, and this is evident in verbal ability, memory, processing speed, attention and executive functions.Reference Fisekovic, Memic and Pasalic1,Reference Collins, Brown, Gold, Waltz and Frank2 Similar data has been reported for those diagnosed with bipolar disorder and substance misuse disorders.Reference Teng, Guo, Lei, Yang, Wu and Yu3 Data also highlight the role of cognitive impairment in individuals’ functional level, with higher degree of cognitive impairment limiting independent living, occupational status, social functioning and self-care.Reference Lepage, Bodnar and Bowie4
Evaluation of cognitive functioning in adults with severe mental health problems, such as schizophrenia, is proposed to be based on a comprehensive battery of neuropsychological tests, such as the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) or the MATRICS Consensus Cognitive Battery.Reference Rosca, Albarqouni and Simu5 These typically focus on assessment of multiple cognitive abilities, such as memory, executive functions, attention and visuospatial ability. Their main advantage is that they may identify patterns of strengths and weaknesses across multiple functional domains.Reference Fisekovic, Memic and Pasalic1,Reference Kern, Nuechterlein, Green, Baade, Fenton and Gold6 Disadvantages include the fact that they require approximately 1 h to be administered by trained clinicians, and their use is costly.Reference Kern, Nuechterlein, Green, Baade, Fenton and Gold6,Reference Nuechterlein, Green, Kern, Baade, Barch and Cohen7 Thus, these tests are not suitable for everyday clinical practice in acute psychiatric in-patient settings.
Montreal Cognitive Assessment (MoCA)
Brief psychometric tools evaluating cognitive functioning have been developed for use in clinical settings, such as the Brief Assessment of Cognition in Schizophrenia or the Brief Cognitive Assessment Tool for Schizophrenia. However, these tools have not been adequately established in clinical praxis, mainly because of lack of time and scoring difficulties, with no direct total score or cut-off scores available.Reference Wu, Dagg and Molgat8 As cognitive impairment is transdiagnostic, related to psychotic symptoms, it has prognostic value and has been reported in in-patient acute psychiatric settings;Reference de Jong, Oorschot, Kamperman, Brussaard, Knijff and van de Sande9 subsequently, validated screening tools for the assessment of cognitive function are needed. Additionally, as declined cognition is related to disability and non-adherence to medication, it can be used as a predictor of medication discontinuation and patient relapse.Reference Depp, Cain, Palmer, Moore, Eyler and Lebowitz10 Therefore, cognitive impairment is highly relevant in in-patient care, and especially compulsory psychiatric care, since it is related to poor medication adherence, deterioration of symptoms and lack of insight.Reference de Jong, Oorschot, Kamperman, Brussaard, Knijff and van de Sande9 The Montreal Cognitive Assessment (MoCA) tool is short and easy to apply; it was developed to detect mild cognitive impairment in the general population. However, it has been applied in many clinical populations, including adults with severe mental health problems (e.g. schizophrenia), mainly as a screening tool with adequate metric properties.Reference Rosca, Cornea and Simu11 Moreover, MoCA scores can be used to follow patients’ cognitive impairment over time because it can quantify one's cognitive capacity.Reference Rosca, Albarqouni and Simu5 MoCA is available in multiple languages and allows the assessment of executive functions, short-term memory, attention and working memory, which are commonly affected in individuals with severe mental health problems. Nevertheless, the use of MoCA tool for the assessment of cognitive impairment in adults with severe mental health problems is limited. A recent review identified only nine small studies in adults with schizophrenia that included the MoCA tool; among those, only two studies involved in-patients and none were in compulsory psychiatric settings. Additionally, there is scarce evidence on the association between the MoCA score and clinical factors in adults with severe mental health problems, such as the Positive and Negative Syndrome Scale (PANSS) score or medication variables.Reference Wu, Dagg and Molgat8,Reference Talreja, Shah and Kataria12
Aim
The aim of this study was to assess (a) the degree of cognitive impairment assessed by the MoCA tool in adults involuntarily admitted to hospital for compulsory psychiatric care, and (b) correlations with clinical variables, including polypharmacy, prescription of high-dose antipsychotics and psychotic symptoms. The metric properties of the MoCA tool were also assessed.
Method
Design and study setting
This was a nationwide cross-sectional study, conducted at the Athalassa Psychiatric Hospital (APH) (capacity of 132 beds), which is the only in-patient facility for compulsory psychiatric care in the Republic of Cyprus since the enactment of the Psychiatric Hospitalisation Act 77(I) in 1997.13 Compulsory psychiatric care in Cyprus is provided when violent behaviour toward oneself or others is present, mainly along with psychotic symptoms, or when the mental capacity of individuals has deteriorated to the extent of them being harmful to themselves.13 Although there are two psychiatric clinics – one at each state general hospital – only voluntarily admitted patients may receive psychiatric care at these facilities, thus they were not included in the present study.
Study participants
Study participants were adults under compulsory psychiatric care in the APH. Data collection was conducted between December 2016 and February 2018. Inclusion criteria for participation in the study were as follows: (a) age 18–65 years; (b) diagnosis of mood disorder, substance use disorder or schizophrenia spectrum/other psychotic disorder according to DSM-V14 criteria; (c) hospital stay of at least 3 days; and (d) capacity to provide signed informed consent for participation in the study. In-patients with a diagnosis of neurocognitive disorders (e.g. Alzheimer's disease or delirium), intellectual disabilities, developmental disorders or personality disorders (less than 3 days hospitalisation) were excluded.
A total of 761 adults were admitted to the APH during the study period. Twenty-two in-patients were excluded because of their age (nine were <18 years and 13 were >65 years), 152 were excluded because their hospital stay was <72 h, 21 were excluded because they were diagnosed with an intellectual disability, 79 were excluded because they refused to give informed consent, two were excluded because they died during their hospital stay and 184 were excluded because they did not complete the MoCA process, mainly because of the severity of their symptoms and/or pharmacological sedation. The sample included 303 participants. A detailed description of the study design is published elsewhere.Reference Kaikoushi, Middleton, Chatzittofis, Bella, Alevizopoulos and Karanikola15
Assessments and procedure
Two research members independently assessed all participants within the first 72 h after admission to the APH via a structured data collection sheet. Each data collection sheet included a structured questionnaire for sociodemographic and clinical data, which was constructed by the authors, and two structured tools for the assessment of cognitive function and psychotic symptoms. Each patient's data collection sheets from both assessors were kept in the patient's medical file. Any disagreements between the two assessors were resolved by discussion and consensus regarding the final assessment sheet. This was included in the present analysis only if signed informed consent for participation in the study was given by the participant on the final day of their hospital stay – an adequate level of illness insight was expected by this point, enabling participants to give their consent.
Sociodemographic (gender, age, marital status, nationality, educational level, occupational status, in receipt of financial reimbursement, body mass index) and clinical data (personal psychiatric history, family psychiatric history, substance use, main symptoms leading to their hospital admission (admission cause), psychiatric diagnosis and prescribed medication) were collected.
Admission cause was categorised as disorganised behaviour (agitation, self-care deficiency, disruption), suicidal/self-harming behaviour or aggressive behaviour toward others. Data on non-adherence to pharmacotherapy and substance use in the weeks before admission were also recorded, along with admission cause. Psychiatric diagnosis was classified according to the following grouping: (a) schizophrenia, (b) other psychotic disorder on the spectrum of schizophrenia (brief psychotic disorder, schizoaffective disorder, delusional disorder, schizophreniform disorder, substance/medication-induced psychotic disorder), (c) mood disorder (bipolar and related disorders, depressive disorders, substance/medication-induced bipolar or depressive disorder) and (d) other (anxiety disorders, psychotic disorder resulting from a medical condition).
The dose of each antipsychotic agent was recorded; the equivalent percentage of each antipsychotic was considered by converting its dose into the maximum percentage of the daily dose, as recommended by the European Medicines Agency (Maudsley Prescribing Guidelines).Reference Taylor, Barnes and Young16 Then, the sum of the percentages corresponding to the daily doses of all antipsychotics prescribed for each participant was calculated. A ‘high-dose prescription’ was deemed when this sum exceeded 100%. Antipsychotic polypharmacy was considered when co-prescription of more than one antipsychotic was prescribed for a participant.Reference Barnes and Paton17 Medication regimens that were prescribed as ‘as needed’/’when required’ were also recorded.
The PANSS was used for the assessment of the severity of psychotic symptoms.Reference Kay, Fiszbein and Opler18 The PANSS includes 30 items; each one corresponds to a group of symptoms, and is rated between 1 to 7 (total range 30–210). The PANSS includes three subscales: the positive symptoms subscale (seven items), the negative symptoms subscale (seven items) and the general symptoms subscale (16 items).Reference Kay, Fiszbein and Opler18 The higher the value, the more intense the symptoms.
The MoCA was applied for the assessment of cognitive functioning. It includes 30 items and is deemed as a screening test to detect cognitive impairment. The MoCA is an unidimensional scale with an administration time of approximately 10 min. It evaluates language, visuospatial skills, attention, executive functions, memory, abstract thinking, calculation and orientation. Scale scores range from 0 to 30. The initial validation study resulted in a suggested cut-off score of 26 out of 30, with scores <26 suggesting cognitive impairment.Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin19,Reference Rossetti, Lacritz, Cullum and Weiner20 Different cut-off scores have been proposed based on population characteristics, age, race, ethnicity and level of education.Reference Yang, Abdul Rashid, Quek, Lam, See and Maniam21,Reference Gil-Berrozpe, Sánchez-Torres, García de Jalón, Moreno-Izco, Fañanás and Peralta22 Consequently, a range of cut-off scores to indicate cognitive impairment have been suggested, ranging from ≤23 to ≤27.Reference Carson, Leach and Murphy23,Reference Ng, Chew, Narasimhalu and Kandiah24 The cut-off score of 25 has been previously suggested to detect cognitive impairment in adults with long-term psychosis, but not in clinical populations in high-security psychiatric hospital settings.Reference Ng, Chew, Narasimhalu and Kandiah24
Ethics and consent
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the National Committee of Bioethics of Cyprus (approval number EEBK/EP/2014/08), the Research Committee of the Ministry of Health of the Republic of Cyprus (PN: 5.34:01.7.3E) and the Personal Data Protection Officer (5.43.01.7.6 Ε, PN: 0237/2014).
All participants provided consent to participate in the study following structured and detailed information about the aim and procedures of the study.
Data analysis
Internal consistency reliability analysis through measurement of Cronbach's alpha was performed for the MoCA and the PANSS scales to support the internal validity of the study tools, since both scales were used for the first time in adults admitted to hospital for compulsory psychiatric care in Cyprus. Frequencies were used to describe the categorical variables. Mean values and standard deviations (s.d.) were estimated for continuous variables. The MoCA total score (continuous variable) was calculated by adding the score of each item, and was further adjusted by adding 1 point for those individuals who had completed less than 12 years of education. Following normality test for continuous variables, comparisons between groups were assessed by parametric tests, and the chi-squared test was applied for comparisons between groups for categorical variables. The association of the mean total MoCA score with clinical and sociodemographic characteristics, polypharmacy (with and without ‘as needed’ medication regimens), and prescription of high-dose antipsychotics (with and without ‘as needed’ medication regimens) were estimated with the chi-squared test or t-test/analysis of variance, accordingly. Age and educational level were used as categorical variables in the linear regression model.
After assessing univariable associations (statistical significance set to 0.05 or lower), and aiming to test these associations after controlling for their confounding effect in the final model, the mean total MoCA score (continuous variable) for each of the sociodemographic (Table 1) and clinical characteristics, including prescribing patterns (high-dose antipsychotics, polypharmacy) (Table 2), was estimated in multiple forward linear (stepwise) regression models. The 95% confidence intervals were estimated. SPSS software version 20.00 for Windows 11 was used for data analysis.
Table 1 Differences in the Montreal Cognitive Assessment total score between groups, in terms of sociodemographic characteristics (t-test, one-way analysis of variance)

MoCA, Montreal Cognitive Assessment.
Table 2 Differences in the Montreal Cognitive Assessment total score between groups, in terms of clinical characteristics (t-test, one-way analysis of variance)

MoCA, Montreal Cognitive Assessment.
Results
Sociodemographic and clinical characteristics of study participants (n = 303)
The sample included 187 men and 116 women. Approximately 10.2% (n = 31) were aged 18–24 years, 32.0% (n = 97) were aged 25–34 years, 24.8% (n = 75) were aged 35–44 years and 33.0% (n = 100) were aged 45–65 years. A total of 39.5% (n = 120) had finished higher secondary education, 78.2% (n = 237) were unemployed and 54.8% (n = 166) were receiving economical reimbursement from the state. Additionally, 88.8% (n = 269) were single. The sociodemographic characteristics of study participants are presented in Table 1.
MoCA and PANSS scores
The mean total MoCA score was 22.09 (s.d. 5.24) (reported scale range (RSR): 3–30) and the mean total PANSS score was 98.60 (s.d. 25.36) (RSR = 41–162). The mean PANSS general symptoms subscale score was 49.60 (s.d. 11.67) (RSR = 24–78), the mean positive symptoms subscale score was 28.80 (s.d. 9.06) (RSR = 7–45) and the mean negative symptoms subscale score was 20.18 (s.d. 9.38) (RSR = 7–45).
Internal consistency reliability analysis
Cronbach's alpha was 0.757 for the MoCA scale and 0.896 for the PANSS scale.
Association of MoCA scores with sociodemographic and clinical characteristics
Christian Orthodox participants (mean 21.88, s.d. 5.12) and those who received state financial support (mean 21.19, s.d. 5.36) reported significantly lower mean total MoCA scores compared with those of other religions (mean 23.84, s.d. 5.92) and those who did not receive financial support (mean 23.18, s.d. 4.88). Similarly, those with a positive psychiatric history (mean 21.71, s.d. 5.37), those who reported non-adherence to pharmacotherapy in the weeks before the admission (mean 21.32, s.d. 5.56) and those who were prescribed high doses of antipsychotics during their hospital stay (including ‘as needed’ medication regimens: mean 21.31, s.d. 5.70; excluding ‘as needed’ medication regimens: mean 20.71, s.d. 5.78) had significantly lower mean total MoCA scores compared with those with no psychiatric history (mean 23.42, s.d. 4.51), those who reported adherence to pharmacotherapy (mean 23.10, s.d. 6.61) and those were not prescribed high doses of antipsychotics (including ‘as needed’ medication regimens: mean 22.56, s.d. 4.90; excluding ‘as needed’ medication regimens: mean 22.60, s.d. 4.94). Those aged 25–35 years reported significantly higher MoCA scores (mean 23.67, s.d. 4.78) compared with those aged 35–65 years [35–44 years: mean 21.13, s.d. 5.49; 45–65 years: mean 21.19, s.d. 5.43). Moreover, those with primary education (6 years) (mean 18.63, s.d. 2.43) had significantly lower MoCA scores compared with those with higher education (lower secondary education: mean 21.34, s.d. 3.54; tertiary education: mean 22.36, s.d. 2.43) (Table 2).
Additionally, the mean total MoCA score was statistically significantly and inversely associated with total PANSS score (r = −0.15), PANSS general symptoms subscale score (r = −0.18) and PANSS negative symptoms subscale score (r = −0.16). There was no association between total MoCA score and PANSS positive symptoms subscale score (r = −0.066).
Regression analysis
Multiple linear forward stepwise regression analysis was conducted with sociodemographic and clinical factors, to assess the association with the mean total MoCA score. The set of variables associated with the mean total MoCA score in the final regression model were educational level, location of living, age, score on the PANSS general symptoms subscale and prescription of high-dose antipsychotics (without ‘as needed’ medication regimens). Table 3 presents the quantification of the relationship between mean total MoCA score and these variables. With every increase of one point in the score of the PANSS general symptoms subscale, the MoCA score (on the average) decreases by 0.09 (95% CI −0.13 to −0.04) units. Moreover, those who were prescribed high-dose antipsychotics (no ‘as needed’ medication regimens included) had a 1.28 (95% CI −2.55 to −0.01) lower mean total MoCA score compared with those who were not prescribed high-dose antipsychotics (without ‘as needed’ medication regimens).
Table 3 Association of mean total Montreal Cognitive Assessment score with clinical and sociodemographic characteristics in a multiple linear stepwise regression model (N = 303)
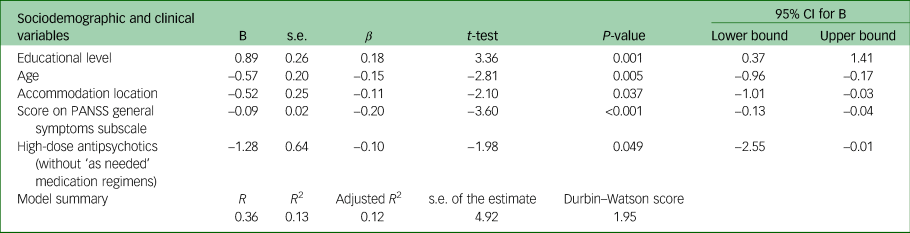
Variables excluded from the model: financial reimbursement, vocational status, gender, race, ethnicity, religion, family status, spoken language, mental health history, history of involuntary hospital admission, substance use history, family history of psychiatric disorders, dual diagnosis, high-dose antipsychotics (with ‘as needed’ medication regimens included), polypharmacy (with/without antipsychotics), psychiatric diagnosis, main symptoms leading to hospital admission, health-related behaviours before the admission, PANSS total score, negative symptoms PANSS subscale score, positive symptoms PANSS subscale score, relapse frequency. PANSS, Positive and Negative Syndrome Scale.
Discussion
To the best of our knowledge, this was the largest study assessing cognitive impairment by using the MoCA tool in adults under involuntary care. Previous studies using the MoCA tool were conducted in smaller samples, and no links to clinical symptoms and medication use were explored.Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead and Collin19,Reference Yang, Abdul Rashid, Quek, Lam, See and Maniam21 In contrast, our study assessed medication doses, cognitive impairment and psychotic symptoms simultaneously.
We found a positive association between cognitive impairment and general psychotic symptoms and prescription of high-dose antipsychotics, when additional sociodemographic and clinical confounders were considered. These data suggest that those who were prescribed with higher doses of antipsychotics experienced more severe psychotic symptoms and more intense cognitive disturbances. Indeed, previous data supported the association of intense psychotic symptoms with cognitive disturbances.Reference Gil-Berrozpe, Sánchez-Torres, García de Jalón, Moreno-Izco, Fañanás and Peralta22 Other studies showed a link between prolonged experience of mental health problems, including psychotic symptoms, and advanced therapeutic schemas in terms of high doses of antipsychotics.Reference Kaikoushi, Karanikola, Middleton, Bella and Chatzittofis25
One way to interpret the present association between cognitive impairment, general psychotic symptoms and prescription of high doses of antipsychotics is that high-dose antipsychotics and cognitive impairment are attributable to the severity of the underlining disease. Indeed, there is evidence that cognitive decline in psychosis starts at least a decade before the onset of psychotic symptoms, and this is more profound in individuals with schizophrenia, who experience a decrease in IQ at a rate of 0.35 points per year.Reference Jonas, Lian, Callahan, Ruggero, Clouston and Reichenberg26 Additionally, a second acceleration of cognitive decline approximately two decades after disease onset, with an IQ decrease of 0.59 points per year, has also been reported.Reference Jonas, Lian, Callahan, Ruggero, Clouston and Reichenberg26
Based on the cross-sectional design of the present study, the opposite may also be suggested, i.e. that exposure to high doses of antipsychotics may have resulted in the reported cognitive impairment. Indeed, there are studies that support the theory that exposure to antipsychotic medication may cause cognitive disturbances in adults with severe psychotic disorders. Specifically, an association between impaired neurocognitive function and high dopamine D2 receptor occupancy (above 77%) caused by antipsychotics has been reported.Reference Sakurai, Bies, Stroup, Keefe, Rajji and Suzuki27 Shin et al found that high D2/D3 receptor occupancy by antipsychotics was related to cognitive impairment in adults with schizophrenia, whereas dose reduction of antipsychotics improved cognitive function.Reference Shin, Kim, Seo, Lee, Howes and Kim28
Additionally, there is evidence that clinicians tend to more frequently prescribe high doses of antipsychotics in acute settings.Reference Patel, Bishara, Jayakumar, Zalewska, Shiers and Crawford29 Our study not only confirmed this in adults under compulsory care, but also highlighted the risk for cognitive impairment associated with the prescription of high-dose antipsychotics. Yet, there is no evidence that antipsychotics prescribed at higher than the maximum licensed dose is more effective, either for persistent aggression, acute psychosis, treatment-resistant schizophrenia or relapse prevention.Reference Choong, Coupe and Vijaya30
The present association between total MoCA score and PANSS general and negative psychotic symptoms subscales scores supported the known-groups validity of the MoCA tool, as did the finding of no association between MoCA score and PANSS positive symptoms subscale score. Moreover, since the strength of these associations was mild, it is suggested that these tools measure different constructs, supporting the MoCA's construct validity. Similarly, since there was no association between PANSS positive symptoms subscale score and total MoCA score, it seems that these tools assessed different constructs, supporting the construct validity of the MoCA scale. Furthermore, these associations were in line with existing literature.Reference Alkan, Davies and Evans31 Specifically, previous data suggested that cognitive decline was unrelated to the severity of positive psychotic symptoms, whereas PANSS negative symptoms subscale score was a significant predictor for cognitive performance in patients with schizophrenia, especially for processing speed, reasoning/problem-solving and overall composite scores using MATRICS.Reference Alkan, Davies and Evans31
The adverse association between positive mental health history and total MoCA score may be explained by the clinical course of severe mental illness. Specifically, a number of factors have been associated with functional and structural cognitive decline, such as duration of psychotic state after the onset of treatment, and the number and frequency of relapses early in the illness trajectory.Reference Andreasen, Liu, Ziebell, Vora and Ho32,Reference Rund, Melle, Friis, Johannessen, Larsen and Midbøe33 Overall, there is a need for early and effective interventions in severe mental illness to reduce the possibility of cognitive decline, and further longitudinal and intervention studies are proposed.
Although it is well-documented that non-adherence to medication is associated with relapse, poorer clinical outcome, greater risk for hospital admission and longer duration of hospital stay,Reference Higashi, Medic, Littlewood, Diez, Granström and De Hert34,Reference Lecomte, Potvin, Samson, Francoeur, Hache-Labelle and Gagné35 the present relationship between cognitive dysfunction and non-adherence to medication is still unclear. In their review, Spiekermann et al reported that a number of studies comparing adherence to medication and cognitive function in patients with schizophrenia revealed a positive correlation between cognitive performance and medication adherence, although other studies did not.Reference Spiekermann, Schulz, Behrens, Driessen, Rahn and Beblo36 Moreover, although some researchers found that mental healthcare patients who reported adherence to pharmacotherapy had higher scores on executive function,Reference Maeda, Kasai, Watanabe, Henomatsu, Rogers and Kato37 memory impairmentReference Jeste, Patterson, Palmer, Dolder, Goldman and Jeste38 and higher total IQ measures,Reference El-Missiry, Elbatrawy, El Missiry, Moneim, Ali and Essawy39 others suggested that those who reported non-adherence to pharmacotherapy scored better on tests of executive functions, verbal learning and memory, and had a higher IQ than those who reported adherence to medication.Reference Yang, Ko, Paik, Lee, Han and Joe40,Reference Jónsdóttir, Opjordsmoen, Birkenaes, Simonsen, Engh and Ringen41 Nevertheless, there is a need for further investigation into this link.Reference Maeda, Kasai, Watanabe, Henomatsu, Rogers and Kato37
The present study also confirmed the previously reported association between low educational level and cognitive impairment in those with severe mental health symptoms who are under compulsory treatment.Reference Wu, Dagg and Molgat8,Reference Rossetti, Lacritz, Cullum and Weiner20,Reference Bernstein, Lacritz, Barlow, Weiner and DeFina42 Furthermore, the present inverse association between total MoCA score and age >25 years was in line with previous studies in clinical and non-clinical populations.Reference Rossetti, Lacritz, Cullum and Weiner20,Reference Carson, Leach and Murphy23,Reference Sakurai, Bies, Stroup, Keefe, Rajji and Suzuki27,Reference Classon, Van den Hurk, Lyth and Johansson43 However, there are studies in clinical populations that found that age has no effect on MoCA total score.Reference Wu, Dagg and Molgat8,Reference Yang, Abdul Rashid, Quek, Lam, See and Maniam21,Reference Bernstein, Lacritz, Barlow, Weiner and DeFina42 Overall, the trajectory of cognitive impairment in adults with severe mental illness continues to be poorly understood, and further studies on this subject are needed to safely interpret relevant associations.
The most important strength of this study is that it provides data on the association between cognitive impairment, prescription patterns and psychotic symptom severity in a large census sample of adults under compulsory treatment in a referral in-patient facility. Additionally, this is the first national study to describe the relationship between cognitive impairment and clinical and sociodemographic variables in this clinical population, providing a much-needed baseline time point for future assessment of progress.
However, since this was a descriptive, cross-sectional study, assumptions on causality are not relevant. On this basis, it was not possible to assess the degree of cognitive decline caused by the substance use itself, or psychosis. Moreover, since only patients receiving compulsory care were included in the study, one could argue that these patients potentially have more severe psychopathology and poor insight compared with voluntarily admitted in-patients. Further, although the study sample was more homogenous because it only included patients receiving compulsory psychiatric care, the results were less generalisable to the general psychiatric population as a result. We also need to clarify that almost half of the participants reported that the current episode leading to their involuntary hospital stay was their first mental health episode. Thus, the majority of the diagnoses provided in the present sample were preliminary diagnoses instead of established diagnoses. Based on this, only a brief grouping of diagnoses was applied. Yet, assessment of psychotic symptoms and cognitive function were performed transdiagnostically for all study participants, and this is the largest study to date assessing the association between severity of psychotic symptoms, cognitive function, high-dose antipsychotics and polypharmacy in adults receiving compulsory psychiatric care. Additional limitations included lack of data about length of hospital stay, cross-titration during switching and pharmacological management of side-effects, as well as failure to account for the detrimental effect that anticholinergic medications – which are frequently prescribed in patients with psychosis – have on cognition. Most importantly, no additional cognitive assessments or test batteries were used for additional cognitive assessment. Nevertheless, the MoCA test has limitations in detecting cognitive impairment in adults with schizophrenia, as it does not assess processing speed and verbal learning.Reference Gil-Berrozpe, Sánchez-Torres, García de Jalón, Moreno-Izco, Fañanás and Peralta22,Reference Musso, Cohen, Auster and McGovern44 Assessment of cognitive functioning with an additional tool would provide data on the sensitivity and specificity of the MoCA tool in this clinical population, and on the scale's cut-off value for this population. However, the present study supported the internal consistency reliability, the construct and the known-groups validity of this tool for the first time, to the best of our knowledge, in a clinical population under compulsory treatment. Furthermore, we did not follow a test–retest reliability design at admission and discharge. However, it has been shown that cognitive impairment is relatively stable over different test–retest intervals and across psychotic state changes,Reference Wu, Dagg and Molgat45 supporting our decision to only administer the MoCA test at admission. Our study did not also include a healthy control comparison group.
In conclusion, the present findings demonstrated the need for structured evaluation of cognitive functioning in adults under compulsory treatment. Integration of this assessment as a standard process in adults with low educational level, higher age and severe psychiatric symptoms who are prescribed high-dose antipsychotics is expected to enable prompt, state-of-the art interventions. Moreover, assessment and illness management in out-patient clinics and compulsory psychiatric settings, especially during the first psychotic episode, can be useful in identifying cognitive impairment at a relatively early stage of the disease. The application of an easily applied tool such as the MoCA would be very beneficial for healthcare practitioners to detect severe cognitive impairment. Additionally, prescribers should pay attention to the risks of cognitive decline associated with the prescription of high doses of antipsychotic medication. Educational programmes on prescribing skills in clinicians have been shown to successfully alter prescribing behaviours.Reference Kunstler, Lennox and Bragge46 Implementation of such programmes is proposed. Overall, optimisation of antipsychotic medication, avoidance of medication with detrimental effects on cognition (such as anticholinergics) and regular evaluation of prescribed medications are proposed. Similarly, further research on the association between high doses of antipsychotic drugs and cognitive function in patients under involuntary treatment in psychiatric settings is needed. Moreover, the results of this study also supported the need to develop and implement interventions that can improve adherence to pharmacotherapy, such as the use of long-acting injectable antipsychotics.Reference Haddad, Brain and Scott47 Considering the clinical role of mental health nurses in community settings, it is of crucial importance to implement educational programmes in cognitive assessment tools and batteries, promoting adoption of cognitive assessment procedures in everyday clinical practice.
Data availability
The data that support the findings of this study are available from the corresponding author, M.K., upon reasonable request.
Acknowledgements
We would like to thank all individuals who participated in the study, as well as the healthcare professionals of the setting in which this study took place.
Author contributions
M.K.: study design, draft preparation, analysis and interpretation of the data, project supervision; M.N.: draft preparation, data interpretation; K.K.: study design, preliminary data analysis, critical review of the final manuscript; N.M.: data analysis, critical review of the final manuscript; A.C.: study design, draft preparation, interpretation of the data, critical review of the final manuscript..
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.







eLetters
No eLetters have been published for this article.