Clozapine and gastrointestinal hypomotility
Clozapine is the most effective antipsychotic for managing treatment-resistant schizophrenia (TRS). Reference Keepers, Fochtmann, Anzia, Benjamin, Lyness and Mojtabai1–Reference Galletly, Castle, Dark, Humberstone, Jablensky and Killackey3 However, use of clozapine is also associated with clozapine-induced gastrointestinal hypomotility (CIGH), constipation and in some cases severe and life-threatening gastrointestinal complications such as ileus, bowel obstruction and bowel infarction. Reference Every-Palmer, Nowitz, Stanley, Grant, Huthwaite and Dunn4–Reference Handley, Every-Palmer, Ismail and Flanagan6 The mortality rate of severe CIGH (15.0–33.0%) exceeds that of other potentially life-threatening adverse effects, such as agranulocytosis (2.2–4.2%) and myocarditis (12.7%), especially if progression to ileus occurs (43.7%). Reference Handley, Every-Palmer, Ismail and Flanagan6–Reference Siskind, Sidhu, Cross, Chua, Myles and Cohen9 Emerging evidence suggests that clozapine-induced dysbiosis of the gut microbiota may contribute to the development of adverse effects, such as pneumonia, constipation and other gastrointestinal complications. Reference Partanen, Häppölä, Kämpe, Ahola-Olli, Hellsten and Rask10,Reference Vasileva, Yang, Baker, Siskind, Gratten and Eyles11
Laxatives and clozapine
Experts assert that prophylactic prescribing of laxatives in patients prescribed clozapine should be carried out and is ethical for the following reasons:
-
(a) Clozapine has been shown to have unequivocal direct effects on colonic motility in animal models, inhibiting neurogenic contractions at lower concentrations and myogenic contractions at higher concentrations. Reference Every-Palmer, Lentle, Reynolds, Hulls, Chambers and Dunn12
-
(b) That 31.2% of patients on clozapine are estimated to report constipation, and that patients using clozapine are three times more likely to develop constipation than those on other antipsychotic drugs. Reference Shirazi, Stubbs, Gomez, Moore, Gaughran and Flanagan5
-
(c) More clozapine-treated patients have CIGH than report constipation. CIGH is often silent, making subjective constipation screening methods largely unreliable for timely diagnosis. Reference Every-Palmer, Inns and Ellis13 Bowel motility studies in clozapine-treated patients show that around 80% of clozapine-treated patients have gastrointestinal hypomotility when transit times are measured objectively. Reference Every-Palmer, Nowitz, Stanley, Grant, Huthwaite and Dunn4,Reference Every-Palmer, Inns, Grant and Ellis14
-
(d) That the potentially life-threatening consequences of poor or untimely managed CIGH and constipation outweigh the harms of prophylactically prescribing laxatives, even to patients that do not have constipation secondary to CIGH. Reference Every-Palmer, Nowitz, Stanley, Grant, Huthwaite and Dunn4,Reference Atkinson, Attard, Attard, Iles and Patel15 The harms of prescribing laxatives, especially stimulant laxatives long term, have historically been overstated without strong evidence. Reference Whorwell, Lange and Scarpignato16
-
(e) That universally screening patients on clozapine for CIGH with objective gastrointestinal motility tests is impractical. Reference Correll, Agid, Crespo-Facorro, de Bartolomeis, Fagiolini and Seppälä17
-
(f) That prophylactic laxatives are ethical, well accepted and guideline-recommended in other similar clinical scenarios, such as with opioids. Reference Kumar, Barker and Emmanuel18
Bulk-forming laxatives can constipate with inadequate water intake and thereby increase the risk of abdominal bloating or obstruction. Hence, prescribing guidelines have recommended they be avoided in CIGH. Reference Atkinson, Attard, Attard, Iles and Patel15,Reference Taylor, Barnes and Young19 Further, regularly scheduled rather than ‘as required’ laxative prescriptions are preferred because ‘as required’ prescribing can lead to under-utilisation of laxatives. Reference Boyd and de Zuniga20 The co-prescribing of laxatives with clozapine has been reported as highly variable (35–87%), differing between clinical settings. Reference Boyd and de Zuniga20–Reference Nakamura and Nagamine23 A large pharmacovigilance study of patients reported to have serious CIGH found that only 9% had been receiving laxatives before the evolution of symptoms. Reference Every-Palmer and Ellis24
Clinical decision support alerts to enhance prescribing
Digital interventions such as clinical decision support (CDS) alerts have been used successfully to promote laxative use with opioids. Reference Scheepers-Hoeks, Neef, Doppen and Korsten25 Point-of-care CDS alerts are common built-in functions of electronic medication management (eMM) systems in hospitals, intended to inform prescribing decisions and facilitate medication safety. Reference Kuperman, Bobb, Payne, Avery, Gandhi and Burns26 To our knowledge, it remains to be assessed whether a CDS alert could be used to improve co-prescribing of laxatives with clozapine.
Our health region’s CDS working group decided to implement a CDS alert prompting clinicians to prescribe laxatives when prescribing clozapine for in-patients in 2019 (Fig. 1). In this study, our primary aim was to evaluate whether implementation of this alert improved timely laxative co-prescribing with clozapine. We also wanted to evaluate the effect of alert implementation on in-patient prevalence of constipation.

Fig. 1 Constipating medicine alert display in MedChartTM when clozapine is prescribed without a concurrent or already existing laxative prescription.
Method
Study design and setting
This observational, pre–post study of a local governance driven system change was performed using retrospective in-patient data from the Health New Zealand Te Whatu Ora – Waitaha Canterbury health region. This health region includes ∼1400 in-patient beds (>95% tertiary hospital beds) and serves about 600 000 people. In-patient prescribing across the majority of the health region (excluding emergency and intensive care departments) has been conducted using the commercial eMM system MedChartTM (version 8.1.1, Dedalus Group) since December 2016. The health region’s local data warehouse holds in-patient prescribing data, demographic data, Charlson comorbidity index scores and diagnoses coded using the International Classification of Diseases – 10th Revision, Australian Modification (ICD-10-AM). Reference Innes, Hooper, Bramley and DahDah27
On 9 September 2019, an interruptive CDS alert was implemented that prompted prescribing of a laxative with clozapine (Fig. 1). This alert was configured to display when a prescriber signed-off a new or edited clozapine prescription in the absence of a laxative being co-prescribed or already present on the patient’s drug chart. Within the alert display, a prescriber could either cancel or continue with the prescription(s) being created without being required to document a reason. The alert was configured to only display for patients 16 years of age or older and prescriptions of clozapine that were regularly scheduled. The principles and evidence-based methods used to guide CDS alert development and implementation in our health region have been published previously. Reference Chin, Chuah, Crawford, Clendon, Drennan and Dalrymple28 The laxatives that were configured to prevent the alert from triggering are listed in Supplementary Table 1.
Study population
In-patient admissions between 1 January 2017 and 31 December 2023 were divided into pre- (1 January 2017–8 September 2019) and post- (10 September 2019–31 December 2023) alert implementation groups. Admissions were included if they met the following criteria: (a) regularly scheduled clozapine was prescribed at least once; (b) the patient was 16 years or older at the time clozapine was prescribed; and (c) during the admission clozapine was prescribed for a duration of at least 24 h. Duration of clozapine prescribing for at least 24 h could include multiple prescriptions not separated by more than 24 h (e.g. first clozapine prescription was charted 12 h, and a second was made within 15 min of the first being stopped, which was charted for a further 48 h). First clozapine prescriptions, defined as the first commenced clozapine prescription within a given admission, were extracted and the prescription creation time used for assignment to the respective pre- and post-alert implementation groups.
Study outcomes
The primary outcome measure was the proportion of first clozapine prescriptions that had regularly scheduled non-bulking laxatives co-prescribed within 24 h. Secondary outcomes were the proportion of first clozapine prescriptions that had any laxatives of any frequency (regular, or ‘as required’) co-prescribed within 24 h, the co-prescribing patterns of laxatives with first clozapine prescriptions within 24 h (number of different laxatives, laxative types, dose frequencies of laxatives) and the proportion of in-patient admissions with constipation coded as a discharge diagnosis. Medicines were considered co-prescribed if they were prescribed already, concurrently or up to 24 h after clozapine was first prescribed.
Data collection
In-patient prescribing, demographic, age-adjusted Charlson comorbidity index (ACCI) and ICD-10-AM data were extracted from the local data warehouse using structured query language (SQL) within Azure Data Studio (version 1.40.2 for Windows, Microsoft Corporation, Redmond, Washington, USA; https://azure.microsoft.com/en-us/products/data-studio). Demographic data included age, gender and ethnicity. The ACCI was used to indicate disease burden, calculated using ICD-10-AM data and age at the time of clozapine prescribing. Reference Charlson, Szatrowski, Peterson and Gold29,Reference Sundararajan, Henderson, Perry, Muggivan, Quan and Ghali30 Age-adjustment involved assigning an additional score of 1 for each decade over the age of 40 years. Reference Charlson, Szatrowski, Peterson and Gold29 Incidence of in-patient constipation was identified using ICD-10-AM diagnoses codes K59.0, K56.0, K56.4, K56.6 and K56.7. ICD-10-AM codes included constipation and more severe gastrointestinal events such as ileus and intestinal obstruction associated with severe CIGH.
Admissions where opioids were co-prescribed were identified. Opioid medicines included are shown in Supplementary Table 1. At our institution there is also an implemented alert that prompts prescribing of a laxative with opioids. An opioid was considered co-prescribed if it was present on the patient’s drug chart at the time clozapine was first prescribed. Anticholinergic burden was calculated for admissions from medicines present at the time clozapine was first prescribed, using a modified version of the Anticholinergic Cognitive Burden (ACB) scale. Reference Joshi, Thomas, Braff, Green, Gur and Gur31 Total ACB scores were generated by summing the individual ACB values from all medications (Supplementary Table 2) and categorised them into five groups: no anticholinergic burden (ACB score 0), low anticholinergic burden (ACB score 1 or 2), moderate anticholinergic burden (ACB score 3 or 4), high anticholinergic burden (ACB score 5 or 6) or very high anticholinergic burden (ACB score > 6). Reference Joshi, Thomas, Braff, Green, Gur and Gur31 When clozapine was first prescribed during the admission (relative to the first day of the admission) and for which admission in the data-set for each patient was also identified (e.g. first or fourth admission). When clozapine was first prescribed was calculated in days by subtracting the admission date from the date when the prescription was created. The length of admission was calculated in days by subtracting the date of admission from the date of discharge. The time elapsed between when clozapine was first prescribed and a laxative was first co-prescribed was calculated to the nearest second. This was done by subtracting the creation time of the first co-prescribed laxative prescription from the creation time of the first clozapine prescription.
Data analysis
First clozapine prescriptions per admission were linked to laxative prescriptions (see Supplementary Table 1), ACCI scores, ICD-10-AM constipation codes, opioid prescriptions and ACB scores in R (version 4.2.3 for Windows, R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/) using unique patient and admission identifiers and subsequently deidentified. Descriptive statistics were used to summarise characteristics related to admissions, prescriptions and outcomes. Proportions of primary and secondary outcomes were compared pre- and post-alert implementation using chi-squared (χ2) tests. Cumulative laxative co-prescribing within 24 h of first clozapine prescription was plotted over time. Time from when clozapine was first prescribed to when a laxative was first co-prescribed was plotted. These plots were visually assessed for inflection points (i.e. where the plot appeared to start flattening). Rates of laxative co-prescribing for first clozapine prescriptions each month across the study period were plotted to visualise temporal trends.
Multivariable logistic regression analysis was conducted to assess whether alert implementation was a predictor of regularly scheduled non-bulking laxative co-prescribing while adjusting for the effects of other covariates. As a sensitivity analysis, the same analysis was done to assess predictors of co-prescribing any laxative. Covariates included in the models were gender, ACCI, co-prescription of opioids, ACB score, the chronological admission number for a patient and day of admission clozapine was first prescribed. Any admissions that were ongoing at the time of analysis were excluded from the multivariable logistic regression component of the analysis because ICD-10 data was unavailable, and ACCI scores could not be calculated. All statistical analyses were performed in R software and graphs were constructed using the R package ggplot2 (https://ggplot2.tidyverse.org/). Reference Wickham32 For statistical tests significance was set at two-sided P < 0.05.
Ethics
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. This study was approved by the University of Otago Human Ethics Committee as a minimal-risk study (HD24/003) and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Reference Vandenbroucke, von Elm, Altman, Gøtzsche, Mulrow and Pocock33 Under the above ethics approval, no patient consent was required because the data used in this study was secondary re-use of data collected as part of routine care and all data were de-identified before analysis. All data were securely handled in accordance with local standards.
Results
Population characteristics
Between 1 January 2017 and 31 December 2023, we identified 1342 in-patient admissions where clozapine was prescribed. After considering inclusion criteria (Supplementary Fig. 1), 1194 admission (449 pre-alert implementation and 745 post-alert implementation) for 490 unique patients were included for analysis (Table 1). The majority of admissions were for males (62.0%, 740/1194) and those of European ethnicity (71.7%, 856/1194). The median (interquartile range (IQR)) age of patients when they were first prescribed clozapine during an admission was 54 (35–70) years. Patients had a median (IQR) of 2 (1–4) admissions included in the study and were first prescribed clozapine on day 1 (1–2) of their admission.
Table 1 Characteristics of the 1194 admissions included in the study and their respective first prescription of clozapine
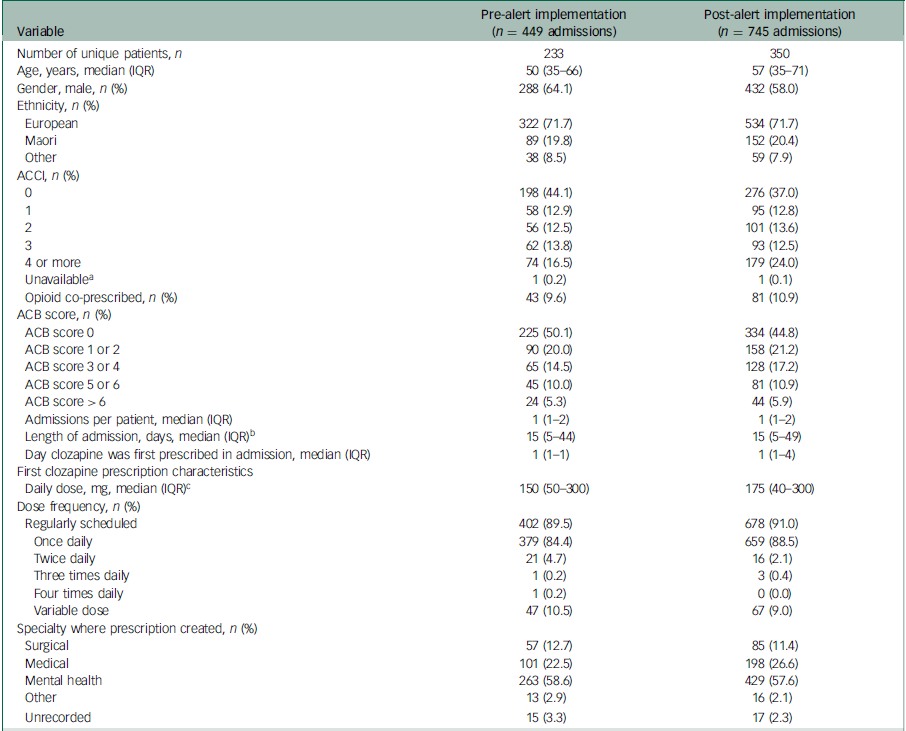
IQR, interquartile range; ACCI, age-adjusted Charlson comorbidity index; ACB, anticholinergic burden.
a. ACCI scores were not calculated for two admissions (one before and one after alert implementation) because the patients were still in-patients at the time of analysis and ICD-10 data were unavailable.
b. Length of admission could not be calculated for two admissions (one before and one after alert implementation) because the patients were still in-patients at the time of analysis.
c. Calculations exclude variable dose prescriptions.
Two admissions (one in each of the pre- and post-alert implementation groups) were ongoing at the time of analysis and excluded from the multivariable logistic regression component of the analysis. Logistic regression was not performed for the secondary outcome of constipation because of an insufficient number of admissions with coded constipation.
Primary outcome
Co-prescribing of regularly scheduled non-bulking laxatives within 24 h of the first clozapine prescriptions increased from 67.0% (301/449) pre-alert implementation to 76.1% (567/745) post-alert implementation (χ2 (1) = 11.61, P < 0.001, Table 2). In the multivariable logistic regression model, alert implementation was associated with an increased likelihood of regularly scheduled non-bulking laxative co-prescribing for first clozapine prescriptions within 24 h (odds ratio, 1.341; 95% CI, 1.021–1.756; z = 2.114, P = 0.035, Table 3). The most frequently co-prescribed regularly scheduled non-bulking laxative with clozapine was docusate and sennoside B as a combination tablet formulation, followed by macrogol-3350 and lactulose across both pre- and post-alert implementation groups (Table 2). Cumulative frequency plots that illustrate regular non-bulking laxative co-prescribing with first clozapine prescriptions over time revealed inflection points around 15 min after clozapine was first prescribed for both study groups upon visual inspection (Fig. 2). Within 15 min, regularly scheduled non-bulking laxatives were co-prescribed for 55.9% (251/449) and 70.7% (527/745) of first clozapine prescriptions pre- and post-alert implementation, respectively.
Table 2 Laxative co-prescribing within 24 h of first clozapine prescriptions, co-prescribed laxative prescription characteristics and prevalence of in-patient constipation pre- and post-alert implementation

a. Combination tablet formulation
b. Regular: if one of the co-prescribed laxative prescription(s) was regular; as needed: if co-prescribed laxative prescription(s) only as required or as required + one-time dose; one-time dose: if co-prescribed laxative prescription(s) only one-time dose.
c. Chi-squared test P < 0.05.
Table 3 Multivariable logistic regression analysis for the effect of alert implementation on co-prescription of regularly scheduled non-bulking laxatives and any laxatives within 24 h of the first clozapine prescription
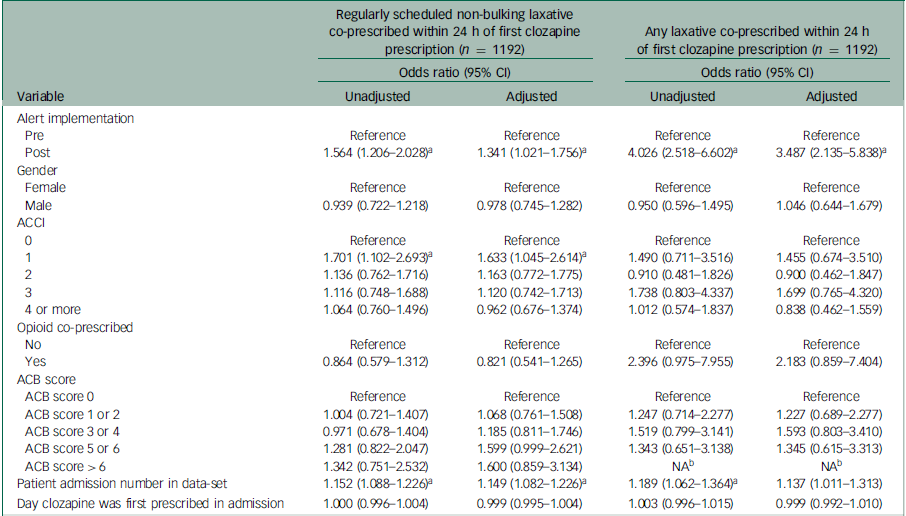
ACCI, age-adjusted Charlson comorbidity index; ACB, anticholinergic burden; NA, not available.
a. Wald test P < 0.05.
b. First clozapine prescriptions for admissions associated with an ACB score > 6 were not present in people who were not co-prescribed any laxative within 24 h.
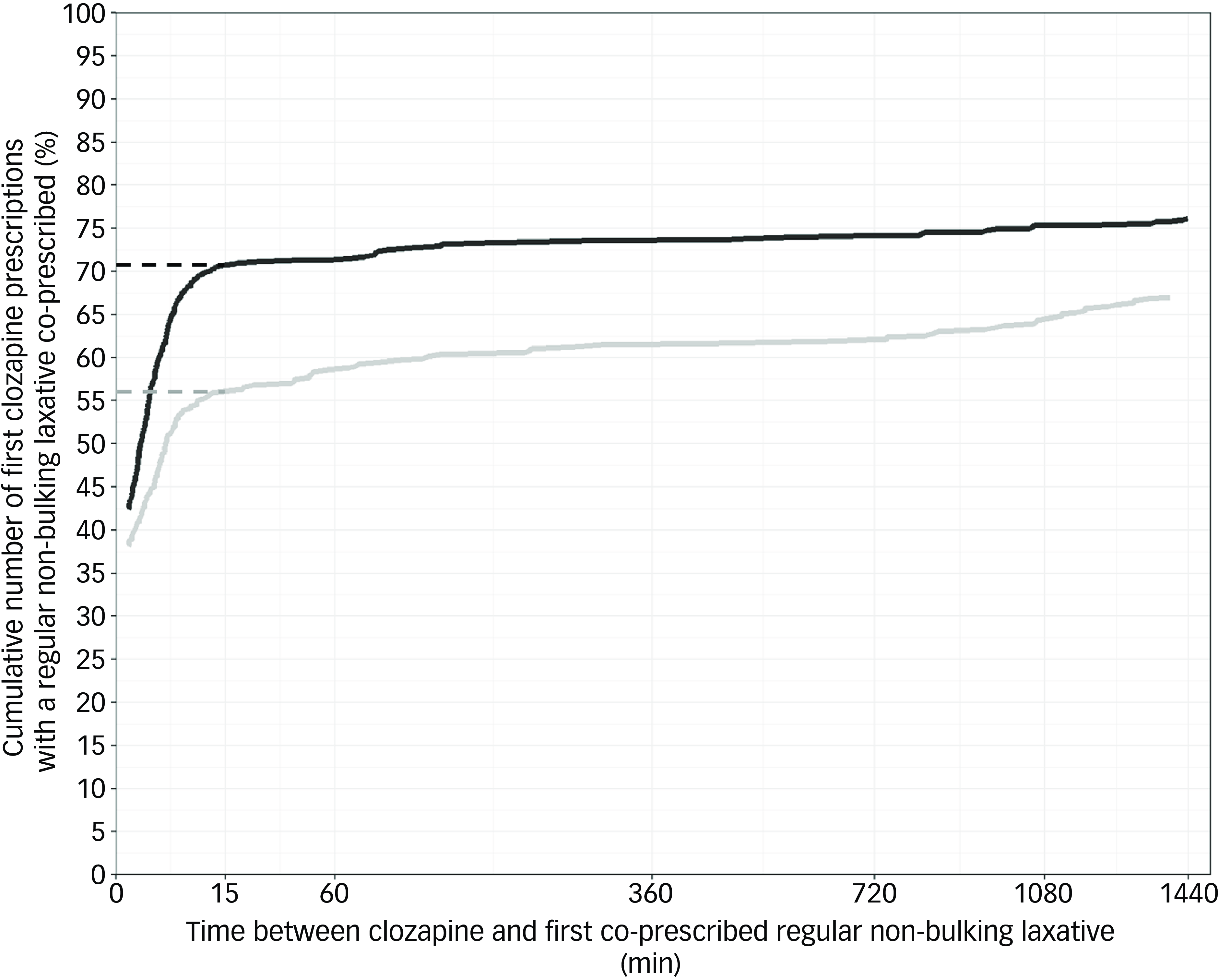
Fig. 2 Cumulative number of first clozapine prescriptions with a regular non-bulking laxative co-prescribed within 24 h. Dashed lines indicate the cumulative number of first clozapine prescriptions that had a regular non-bulking laxative co-prescribed within 15 min. Grey: pre-alert, black: post-alert. Plots do not start at x = 0 because earliest co-prescribing of a laxative after clozapine was several seconds later. Time between clozapine and first co-prescribed regular non-bulking laxative has been square root transformed.
Secondary outcomes
Co-prescribing of any laxative within 24 h of the first clozapine prescriptions increased from 87.3% (392/449) pre-alert implementation to 96.5% (719/745) post-alert implementation (χ2 (1) = 36.70, P < 0.001, Table 2). The multivariable logistic regression model for any laxative co-prescribing within 24 h showed that implementation of the alert was a significant predictor (odds ratio, 3.487; 95% CI, 2.135–5.838; z = 4.887, P < 0.001, Table 3) after adjusting for the other covariates. Docusate and sennoside B (as a combination tablet formulation), macrogol-3350 and lactulose were the most frequently co-prescribed laxative medicines across pre- and post-alert implementation groups (Table 2). There were no admissions where colonic secretagogues or 5HT4 receptor agonists were prescribed. Similar numbers of first clozapine prescriptions were co-prescribed only ‘as required’ laxatives pre- (20.0%, 90/449) and post-alert implementation (19.7%, 147/745). Cumulative frequency plots for any laxative co-prescribing over time relative to the first clozapine prescription for both study groups also showed inflection points at around 15 min after clozapine was first prescribed upon visual inspection (Fig. 3). Within 15 min, a laxative was co-prescribed for 73.3% (329/449) and 92.8% (692/745) of first clozapine prescriptions pre- and post-alert implementation, respectively. Constipation was recorded in 3.8% (17/449) of admissions pre-alert implementation and 4.4% (36/745) post-alert implementation (χ2 (1) = 0.72, P = 0.400). Rates of laxative co-prescribing across the study period are shown in Supplementary Fig. 2.
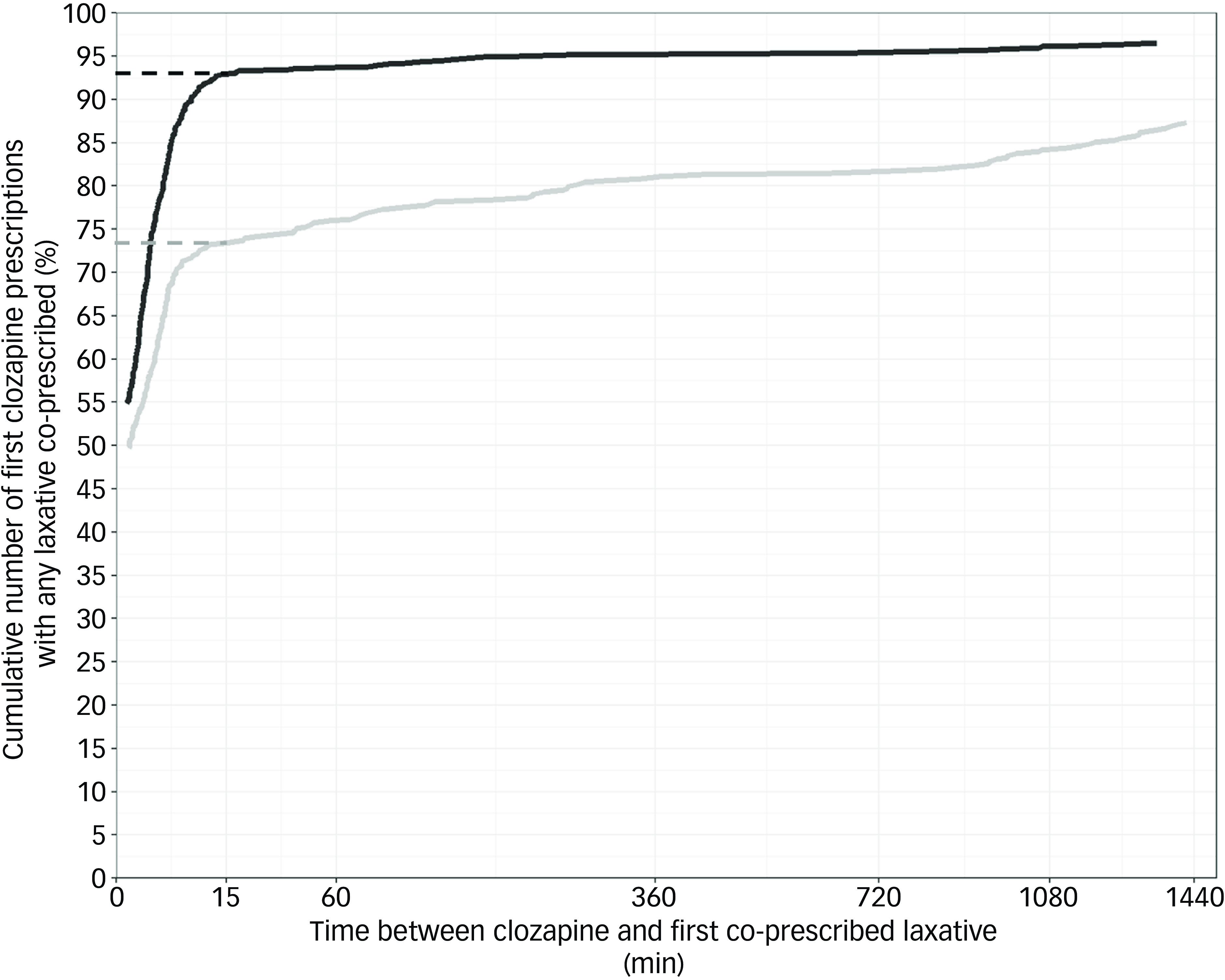
Fig. 3 Cumulative number of first clozapine prescriptions with any laxative co-prescribed within 24 h. Dashed lines indicate the cumulative number of first clozapine prescriptions that had a regular non-bulking laxative co-prescribed within 15 min. Grey: pre-alert, black: post-alert. Plots do not start at x = 0 because earliest co-prescribing of a laxative after clozapine was several seconds later. Time between clozapine and first co-prescribed laxative has been square root transformed.
Discussion
To the best of our knowledge, this study is the first to evaluate the effectiveness of a CDS alert to promote timely laxative use with clozapine. Alert implementation was associated with significantly increased co-prescribing of laxatives within 24 h of clozapine being first prescribed. This included co-prescribing of regular non-bulking laxatives and any laxatives from 67.0% to 76.1% and 87.3% to 96.5%, respectively. Alert implementation was a significant predictor of both regular non-bulking laxative and any laxative co-prescribing, even after adjustment for potential confounding covariates. No significant differences in constipation and other more serious gastrointestinal complications were found between the pre- and post-alert implementation periods.
There has been limited research into CDS systems to prevent clozapine-related adverse effects, especially CIGH. This is despite the wide-spread acknowledgement that CDS systems have great promise to enhance the delivery and quality of care in psychiatry. Reference Bauer, Monteith, Geddes, Gitlin, Grof and Whybrow34 To date, use of CDS alerts to improve management of clozapine-related adverse effects has primarily focused on metabolic monitoring. Reference DelMonte, Bostwick, Bess and Dalack35–Reference Lai, Chan, Pan and Chen37 Studies conducted in both the in-patient and out-patient settings reported significant improvements in adherence to guideline-recommended metabolic monitoring. Reference DelMonte, Bostwick, Bess and Dalack35–Reference Lai, Chan, Pan and Chen37 Like clozapine, opioids are also highly constipating and prophylactic laxative use is recommended but variably carried out. Reference Twycross, Sykes, Mihalyo and Wilcock38 Implementation of a CDS alert in this clinical context was associated with an increase in laxative co-prescribing rate from 70.0% to 83.0% and reduced opioid-induced constipation from 56.0% to 12.0%. Reference Scheepers-Hoeks, Neef, Doppen and Korsten25 However, fundamental study differences, such as the study period, definitions of study outcomes and completely different clinical contexts, make direct comparison with our study difficult. A 2020 meta-analysis that assessed 122 studies with any form of CDS system interventions showed that absolute improvements in desired care on average were 5.8% (95% CI, 4.0–7.6%) post-implementation. Reference Kwan, Lo, Ferguson, Goldberg, Diaz-Martinez and Tomlinson39 The improvements in both regular non-bulking laxative co-prescribing and any laxative co-prescribing associated with the alert in our study exceeded the typically observed improvements. This could have been because of the simplicity of the action recommended in the alert and the potentially severe consequences of not prescribing a laxative. Other factors that could have increased responsiveness to the alert include a number of highly publicised coroner cases about deaths from CIGH published locally, and a large UK pharmacovigilance study published in February 2022 that estimated that patients with severe CIGH had a 33% risk of mortality. Reference Handley, Every-Palmer, Ismail and Flanagan6,Reference Tokalau40 That said, longitudinal co-prescribing of any laxatives and regular non-bulking laxatives during the study period presented in Supplementary Fig. 2 does not indicate a notable change in laxative co-prescribing after February 2022. Our study adds evidence that CDS alerts can be used to improve timely prevention of clozapine adverse effects in the context of laxative prescribing for CIGH prevention. Because the alert is simple, relatively cheap to implement, effective and had high rates of adherence that appeared to be sustained over time (Supplementary Fig. 2), it compares well with other strategies to improve prescribing, such as educational programmes, which are more resource intensive. If education efforts are not sustained to account for staff turnover and complacency, this may lead to extinction of the desired behaviour over time. Reference Bailey, Duncan, Cunha, Foorman and Yeager41–Reference Nathan, Shelton, Laur, Hailemariam and Hall43
The increases in laxative co-prescribing observed with clozapine between pre- and post-alert implementation periods appeared to be almost exclusively driven by increases in regular laxative use as rates of only ‘as required’ laxatives were similar across study periods (20.0% and 19.7%, respectively). This finding was surprising given the non-specific wording used in the alert for which laxative and what dosing frequency to prescribe (Fig. 1). An explanation for this could be that our institutional clozapine guidelines, implemented in late 2015, have recommended the preventative management of CIGH set out in the Porirua Protocol (Supplementary Fig. 3). Reference Every-Palmer44 The finding that docusate and sennoside combination and macrogol-3350 accounted for the vast majority of laxatives co-prescribed pre- and post-alert implementation further supports this notion.
The decision to use the within 24 h time-frame to represent timely laxative co-prescribing was both guided by expert clinician consultation and by other studies that have utilised similar outcome measures. Reference Scheepers-Hoeks, Neef, Doppen and Korsten25,Reference DelMonte, Bostwick, Bess and Dalack35,Reference Liu, Gnjidic, Patanwala, Rubin, Nielsen and Penm45 Visual inspection of cumulative laxative co-prescribing with first clozapine prescriptions highlighted inflection points at 15 min post-clozapine being first prescribed (Figs. 2 and 3). Comparison of the rate of laxative co-prescription at 15 min post-clozapine being first prescribed showed 14.8% and 19.5% proportional increases in regular non-bulking laxative and any laxative co-prescribing post-alert implementation, respectively. This finding further supports that laxatives were being co-prescribed closer to when clozapine was first prescribed post-alert implementation. That said, we could not differentiate between prophylactic laxative co-prescribing and laxative co-prescribing to treat existing constipation (e.g. patient was constipated on admission), so some of the early laxative prescribing may have been treatment related rather than prophylaxis.
The co-prescribing of regular non-bulking laxatives pre-alert implementation (67.0%) was similar to general laxative use with clozapine reported previously at other in-patient settings (50.9–87.1%). Reference Boyd and de Zuniga20,Reference Sazhin and Pushkal21,Reference Nakamura and Nagamine23 The co-prescribing of any laxatives pre-alert implementation was higher but comparable to the laxative co-prescribing rate reported previously. However, these other studies have reported estimates of laxative prescribing with clozapine by patient, rather than per admission as in our study. Reference Boyd and de Zuniga20,Reference Sazhin and Pushkal21,Reference Nakamura and Nagamine23 While 76.1% of admissions had a regular non-bulking laxative co-prescribed within 24 h of when clozapine was first prescribed post-alert implementation, there were still a significant minority of admissions where only an ‘as required’ laxative was initiated within 24 h of clozapine being first prescribed (Table 2). Similar rates of only ‘as required’ laxative co-prescribing shown in our study (∼20.0%) have been reported by others (25.8%). Reference Boyd and de Zuniga20 While this scenario meets the alert specified criteria of a laxative being co-prescribed, ‘as required’ laxatives have been shown to be prone to under-utilisation, Reference Boyd and de Zuniga20 resulting in patients receiving inadequate prophylaxis or treatment for constipation. We acknowledge that 100% co-prescription of laxatives, as recommended by the alert, is not clinically desirable as some patients will have contraindications for being prescribed laxatives (e.g. present with acute abdominal conditions or diarrhoea). Hence, the clinically appropriate rate of co-prescribing will be <100%, like the 96.5% co-prescribing of any laxatives shown post-alert implementation in this study. We recommend that the alert should not be blindly followed and always considered in the context of available patient information.
Although our study showed that alert implementation was associated with increased timely laxative co-prescribing for clozapine, we could not show a reduction in in-patient events of constipation and other serious gastrointestinal complications. This may have been because of the relatively long lengths of admissions (Table 1) and under-recording in the discharge diagnoses (e.g. constipation occurring during admission may not be felt to be clinically important enough to mention in the discharge diagnoses, especially if it is well controlled by laxatives at the point of discharge). However, others have shown that prescription of laxatives in clozapine-treated patients significantly reduces objective measures of CIGH such as colonic transit time, and the likelihood of experiencing constipation. Reference Every-Palmer, Ellis, Nowitz, Stanley, Grant and Huthwaite46,Reference Lam and Ip47
Our study had some important limitations and considerations. First, this study was conducted at a single centre in New Zealand, with laxative prescribing practices that are largely influenced by local guidelines and laxatives that are subsidised. Hence, more novel treatments for CIGH such as chloride channel activators (e.g. lubiprostone) were not included in our study. However, given the simplicity of our alert and the way it was configured, we believe that this CDS alert is an intervention that is actionable and applicable to other in-patient institutions. Second, because we did not have access to community prescribing data we could not identify patients who were already prescribed clozapine and/or laxatives before hospital admission. Inclusion of such data could allow stratified analysis for patients that were truly prescribed clozapine for the first time where the initiation of a laxative as re-enforced by the alert is most relevant. We also did not have data on pre-treatment defecation patterns of patients or to assess whether patients had contraindications to laxatives or had to discontinue laxatives because of tolerability issues (e.g. diarrhoea). Third, we decided to analyse all admissions where clozapine was prescribed, rather than only the admissions where clozapine was prescribed and a CDS alert triggered. This was because the focus of our analysis was a global overview of laxative co-prescribing change pre- and post-alert implementation. By showing that laxative co-prescribing increased using this more general method of analysis, rather than just focusing on cases where the alert had been triggered, we likely underestimated the true extent of improvement in laxative co-prescribing. The finding that alert implementation was still associated with an increase in laxative co-prescribing, both regular non-bulking and any laxative, adds to the likelihood that the alert is having a true effect. Fourth, because of a lack of data we could not assess the relationships between clozapine dose schemes or clozapine plasma levels and constipation. A clear relationship between these variables and constipation remains to be shown. Reference Shirazi, Stubbs, Gomez, Moore, Gaughran and Flanagan5 Finally, although we adjusted the results for several confounders using multivariable logistic regression analysis, we acknowledge that we did not include all potential confounders and that these may further predict laxative prescribing. However, we are not aware of any other changes in clinical practice affecting laxative use in patients prescribed clozapine during the study period (A. McKean, senior pharmacist, Specialist Mental Health Services, Health New Zealand, personal communication, 2024).
In summary, this study found that implementation of a CDS alert was associated with increased timely laxative co-prescribing for clozapine. Specifically, the alert was associated with improved regular non-bulking laxative use and any laxative use within 24 h of clozapine being first prescribed. No changes in in-patient events of constipation and other gastrointestinal complications were shown pre- and post-alert implementation. Further, larger studies assessing the impact of a CDS alert on clozapine-induced constipation are needed. While the link between the CDS alert and clozapine-induced constipation remains unclear, promoting timely use of laxatives is an imperative first step for prevention.
Supplementary material
The supplementary material for this article can be found https://doi.org/10.1192/bjo.2025.1
Data availability
Data to support the findings of this study are not publicly available to protect patient privacy and because of ethical restrictions. R codes used to conduct the data analysis and statistical analysis are available from the corresponding author upon reasonable request.
Acknowledgements
We would like to thank Andrew McKean for consultation around mental health clinical practice at Health New Zealand Te Whatu Ora – Waitaha Canterbury for clozapine. We would also like to thank Sandra Pugh and Ray Law for their expertise on clinical coding and the health regions data, respectively, at Health New Zealand Te Whatu Ora – Waitaha Canterbury.
Author contributions
All authors were involved in study conception, design and methodology. Data curation and analysis was performed by M.S. The first draft of the manuscript was written by M.S. and P.K.L.C. and all authors commented on previous versions of the manuscript. All authors have read and approved the final submitted manuscript and agree to be accountable for the work.
Funding
M.S. is funded through a University of Otago Doctoral Scholarship. Open Access funding was enabled through the University of Otago as part of the CAUL (Council of Australian University Libraries) Procurement consortium.
Declaration of interest
None.










eLetters
No eLetters have been published for this article.