Autism spectrum disorder (henceforth ‘autism’) is a lifelong neurodevelopmental condition characterised by differences in social relating, communication, flexibility and sensory processing. Currently, autistic individuals, compared to non-autistic individuals, have a high risk of developing mental health problems, but a low chance of receiving effective help for these difficulties.Reference Mandy1 In part, this is because the current evidence base to guide formulation, prevention and treatment of mental health problems for autistic individuals is limited.Reference Linden, Best, Elise, Roberts, Branagan and Tay2
In recent years it has become clear that autistic girls and women are at increased risk of developing restrictive eating disorders (REDs), defined here as encompassing anorexia nervosa (i.e. restrictive eating behaviours and/or compensatory behaviours resulting in significantly low body weight, driven by weight and shape concerns), atypical anorexia nervosa (i.e. meeting criteria for anorexia nervosa, but without being substantially underweight) and avoidant/restrictive food intake disorder (ARFID) (i.e. clinically significant restrictive eating that is not driven by weight and shape concerns). Around 20% of women with an anorexia nervosa diagnosis meet criteria for autism;Reference Westwood and Tchanturia3 and rates of ARFID are also elevated in autistic individuals.Reference Bourne, Mandy and Bryant-Waugh4 REDs have serious consequences for physical and mental health, functioning, quality of life and mortality.Reference Arcelus, Mitchell, Wales and Nielsen5
Although there is a developing research literature on the overlap between autism and REDs,Reference Kerr-Gaffney, Hayward, Jones, Halls, Murphy and Tchanturia6 thus far, we lack a systematic, empirical description of the clinical characteristics of autistic women with REDs using validated measures of both autistic and eating disorder features. This gap in knowledge constrains quality of care for two reasons. First, it limits our understanding of why autistic women are at elevated risk for REDs, which hinders progress towards developing effective interventions. Autistic women, and those with high autistic traits, benefit less from current eating disorder interventions and care pathways than women with low autistic traits.Reference Tchanturia, Adamson, Leppanen and Westwood7 They have lower recovery rates and longer illness duration, and require more intense treatment.Reference Nazar, Peynenburg, Rhind, Hibbs, Schmidt and Gowers8–Reference Saure, Laasonen, Lepistö-Paisley, Mikkola, Ålgars and Raevuori10 Further, autistic women tend to report that their autism is generally not accommodated in eating disorder services, such that their needs are not met, and they find conventional eating disorder treatments hard to access and/or ineffective.Reference Babb, Brede, Jones, Elliott, Zanker and Tchanturia11,Reference Babb, Brede, Jones, Serpell, Mandy and Fox12 This reduced effectiveness of care for autistic women with REDs may partially reflect the fact that their REDs have autism-related causal and maintaining factors, which are not addressed by treatment models which were developed with and for non-autistic people.Reference Brede, Babb, Jones, Elliott, Zanker and Tchanturia13 Instead, treatments often target ‘traditional’ symptoms, such as underlying weight and shape concerns,Reference Fairburn, Cooper and Shafran14 which may be less prominent in autistic individuals with REDs.Reference Brede, Babb, Jones, Elliott, Zanker and Tchanturia13
Second, the current lack of an account of the clinical presentation of autistic women with REDs limits attempts to identify autistic women in eating disorder services. Usually, autistic women with a RED present to services without an autism diagnosis, and those who are subsequently identified as autistic tend to experience long (~8 years) delays before their diagnosis.Reference Babb, Brede, Jones, Elliott, Zanker and Tchanturia11 When their autism is unrecognised, it impedes their ability to access and benefit from appropriate clinical care.Reference Babb, Brede, Jones, Serpell, Mandy and Fox12 Identifying autistic women with REDs is difficult, because (i) it is challenging to assess autism in adult women,Reference Cumin, Pelaez and Mottron15 and (ii) behaviours superficially presenting as autistic traits (e.g. rigidity, differences in social cognition) can arise as psychological effects of starvation and thus may be misinterpreted by clinicians.Reference Kinnaird and Tchanturia16 A description of the clinical autistic characteristics and disordered eating symptoms of autistic women with REDs can help improve their identification in eating disorder services.
Aims
We aim to describe the autistic characteristics (autistic traits in adulthood and childhood, restricted and repetitive behaviours, camouflaging behaviours) and disordered eating symptoms (traditional eating disorder symptoms, body image issues, pride in eating pathology, autism-specific unusual eating behaviours) of autistic individuals with REDs (Autism + REDs), compared with autistic individuals without REDs (Autism) and women with REDs who are not autistic (REDs).
A subsidiary aim is to describe a group of women with high autistic traits and REDs, but no formal autism diagnosis (HATs + REDs), to better understand their presentation and thereby help services to identify clients who might benefit from autism assessments and treatment modifications. In addition, we test whether autistic traits were correlated with body mass index (BMI) in each group to assess whether those with more autistic traits weighed less, which would support the theory that autistic traits in RED populations are in part driven by the effects of starvation.
Ethics statement
Written informed consent was obtained from all participants in this study. Full ethical approval was gained from the UCL Research Ethics Committee (12973/002), the Health Research Authority and Health and Care Research Wales (19/WA/0303).
Method
Procedure
Two autistic women with experience of REDs reviewed the study protocol and materials and advised on how to make the study accessible for potential participants.
Recruitment was via UK National Health Service (NHS) eating disorder and autism services, social media and charities, with a £15 participation incentive. After eligibility screening and written informed consent, participants provided data via an online survey. Data collection started prior to COVID-19, and the protocol was adapted so that the study could continue during the pandemic; please see Supplementary Appendix 1 available at https://doi.org/10.1192/bjo.2024.65 for information on measures taken to minimise the pandemic's impact upon data collection. Data were collected as part of a larger project. Some of the data presented in the current study have also been included in two PhD thesesReference Brede17,Reference Babb18 and a publication on autistic women's eating disorder service experience.Reference Babb, Brede, Jones, Serpell, Mandy and Fox12
Measures
Background measures
BMI was calculated based on participants’ self-reported current height and weight. Conventionally, those with a BMI below 18.5 are categorised as ‘underweight’, between 18.5 and 24.9 as ‘healthy’ and over 25 as ‘overweight’.
Depression and anxiety were measured with the Hospital Anxiety and Depression Scale (HADSReference Zigmond and Snaith19), a self-report questionnaire. The maximum possible score on each subscale (anxiety/depression) is 21. Scores of 0–7 are considered indicative of non-clinical levels of anxiety and depression, of 8–10 indicative of borderline, and of 11 indicative of high levels of anxiety or depression. Internal consistency in the current sample for HADS anxiety (α = 0.81) and depression (α = 0.83) subscales was high.
Social anxiety was measured using the Social Phobia Inventory (SPINReference Connor, Davidson, Churchill, Sherwood, Foa and Weisler20). Spin total scores can range from 0 to 68. Internal consistency in the current sample was excellent (α = 0.91).
Autistic characteristics
The Ritvo Autism Asperger Diagnostic Scale-14 (RAADS-14Reference Eriksson, Andersen and Bejerot21) is an adult autism screening measure. RAADS-14 total scores were used in the current study to split participants with REDs without an autism diagnosis into the REDs group and HATs + REDs group to reduce the chance that the REDs group (which we intended to be without autistic participants) included undiagnosed autistic women. In addition to enhancing the internal validity of our groups, this allowed us to explore the presentation of those with an eating disorder, no autism diagnosis and high autistic traits. RAADS-14 total scores range between 0 and 42. For allocation to the HATs + REDs group, we used the threshold score of 23, which is recommended for psychiatric populationsReference Eriksson, Andersen and Bejerot21 and has been used in previous studies to identify individuals unlikely to meet criteria for an autism diagnosis.Reference Solaris22 In addition, we used the RAADS-14 to calculate the RAADS-14 childhood ratio. For each RAADS-14 item, participants recorded whether it applied only when they were a child, only now, neither or both. The RAADS-14 childhood ratio, ranging from 0 to 1, considers the number of items endorsed to have been present in childhood relative to the total number of items endorsed: higher scores indicate the presence of more autistic characteristics since childhood. Thus, a higher score supports the presence of ‘true autism’, as opposed to a phenocopy arising after the onset of an eating disorder.
The Adult Autism Spectrum QuotientReference Baron-Cohen, Wheelwright, Skinner, Martin and Clubley23 is a 50-item measure of autistic-like traits and behaviours in the general population and has been widely used, including with individuals with eating disorders.Reference Westwood, Eisler, Mandy, Leppanen, Treasure and Tchanturia24 Total adult autism spectrum quotient scores can range from 0 to 50, with a recommended screening threshold score of 26 in clinical populations.Reference Woodbury-Smith, Robinson, Wheelwright and Baron-Cohen25 Only participants who participated after the onset of COIVD-19 completed this measure (see Supplementary Appendix 1). Internal consistency in the current sample was high (α = 0.89).
The Adult Repetitive Behaviours Questionnaire (RBQ-2AReference Barrett, Uljarević, Baker, Richdale, Jones and Leekam26) measured autism-related restricted and repetitive behaviours. Possible total RBQ-2A scores range from 20 to 60. Internal consistency in the current sample was excellent (α = 0.91).
The Camouflaging Autistic Traits Questionnaire (CAT-QReference Hull, Mandy, Lai, Baron-Cohen, Allison and Smith27) measured autism-related camouflaging behaviours, with possible CAT-Q total scores ranging from 25 to 175. Internal consistency in the current sample was excellent (α = 0.93).
Disordered eating symptoms
The Eating Disorder Examination-Questionnaire (EDE-QReference Fairburn and Beglin28) was used to assess eating disorder symptoms across four subscales: dietary restraint, eating concern, weight concern and shape concerns. The current paper reports the global score across subscales, referred to as ‘traditional eating disorder symptoms’. Mean EDE-Q global scores can range from 0 to 6, with a threshold score of 2.5 being recommended for screening purposes.Reference Rø, Reas and Stedal29 Internal consistency was excellent (α = 0.96) in the current sample.
The Body Shape Questionnaire (BSQReference Cooper, Taylor, Cooper and Fairbum30) measured preoccupations with body image and concerns about body shape, with possible BSQ total scores ranging from 34 to 204. Scores of less than 81 suggest little or no worry about body shape, whereas scores of more than 140 suggest extreme worry about body shape, with a total possible score range.Reference Cooper, Taylor, Cooper and Fairbum30 There was a high internal consistency in the current sample (α = 0.89).
The Pride in Eating Pathology Scale (PEP-SReference Faija, Fox, Tierney, Peters and Gooding31) measured pride towards eating disorder symptoms such as food restriction and weight loss. The PEP-S total score can range from 49 to 343. Internal consistency was excellent (α = 0.98).
The SWedish Eating Assessment for Autism Spectrum Disorders (SWEAAReference Karlsson, Rastam and Wentz32) measured unusual eating behaviours and eating disturbances common for autistic adults. The measure is divided into eight subscales, each including 2–11 items (‘perception’, ‘motor control’, ‘purchase of food’, ‘eating behaviour’, ‘mealtime surroundings’, ‘social situation at mealtimes’, ‘other behaviours associated with disturbed eating’, ‘hunger/satiety’), and two single items (‘pica’, ‘simultaneous capacity’). A brief description of each subscale is provided in Supplementary Appendix 2, sTable 1. Each subscale score is transformed into a scale from 0 to 100 to aid interpretability. Internal consistency of subscales was high or acceptable (all α ≥ 0.68), apart from for the SWEAA hunger/satiety subscale (α = 0.32), which may reflect its small number (n = 2) of items.
Participants
There was insufficient existing literature from which to estimate anticipated group difference effect sizes to inform sample size/power calculations. Thus, we chose, a priori, to power this study to be sensitive to detect difference of an effect size estimated to be of clinical importance (medium-large effect size; Cohen's d ≥ 0.6 with two-tailed alpha at 0.05) and identified a minimum target sample size on this basis. Participants were recruited in three distinct groups: (i) those who have an autism diagnosis and do not have REDs (Autism group); (ii) those who have a diagnosis of autism and a RED (Autism + REDs group); and (iii) those with a RED diagnosis, without an autism diagnosis. Autism and eating disorder diagnoses were self-reported. Steps taken to verify diagnostic status and other inclusion criteria are outlined in Supplementary Appendix 3. Prior to analysis, participants with REDs without an autism diagnosis were then split into the REDs group and HATs + REDs group. The final sample comprised 244 participants meeting inclusion criteria (Autism n = 69; Autism + REDs n = 57; HATs + REDs n = 38; REDs n = 80). A post-hoc sensitivity analysis determined that all group comparisons were powered to detect medium-large effects (i.e. Cohen's d ≥ 0.6) (Supplementary Appendix 4, sTable 3).
Analysis
The raw data were inspected for missing responses. Levels of missing were generally low for both scale- and item-level data. For none of the questionnaires were more than 2.4% of the data missing, BMI and adult autism spectrum quotient excepted. For BMI there were likely reasons related to their eating disorder why participants did not report their height or weight. For the adult autism spectrum quotient scores there were methodological reasons for missing data (see Supplementary Appendix 1). Non-available data for these variables were treated as missing and pairwise deletion was employed. Little's Missing Complete at Random (MCAR) test was carried out on all other variables. The tests were non-significant for all measures, indicating no pattern to missing data. Given that data were likely to be missing completely at random and less than 5% were missing, we opted to impute missing data to retain statistical power. We followed the Treatment and Reporting of Missing Data in Observational Studies framework,Reference Lee, Tilling, Cornish, Little, Bell and Goetghebeur33 using multiple imputation and pre-specified sensitivity analyses to compare imputed and complete case analyses. Item-level missing data were addressed using multiple imputation by chained equations in R, drawing on all available variables as statistical predictors. We imputed five data-sets, combining estimates using Rubin's rules.Reference Rubin34 Winsorising was used to substitute outliers. To identify outliers, each variable, split by group, was assessed using the outlier-labelling rule, which proposes an interquartile range multiplier approach to detect outliers.Reference Hoaglin and Iglewicz35 The current study employed a multiplier of 2.2, which is considered most sensitive.Reference Hoaglin and Iglewicz35 Results are based on the imputed data-set, with any differences in the senstivity analysis being reported.
Spearman's rank-order correlations between autistic traits (RAADS-14 total) and BMI were calculated for each group to assess the relationship between autistic traits and low weight, which was used as an indicator of starvation.
Groups were compared on each dependent variable using one-way analysis of covariances (ANCOVAs), adjusting for group differences in age. Non-parametric alternatives were used where assumptions had been violated. To assess group differences in the pattern of subscale scores on the SWEAA, we conducted mixed-design ANCOVAs on nine of the ten subscales. The SWEAA pica subscale was not included as its data varied widely from a normal distribution.
Results
Descriptive
Demographics and clinical background variables for each group are presented in Table 1. Participants in the Autism group were significantly older than participants in the other three groups and were on average diagnosed as autistic later than Autism + REDs participants. On the HADS, the Autism group scored significantly lower for both depression and anxiety than the other three groups, who all scored similarly. Regarding social anxiety, the Autism + REDs and HATs + REDs groups both scored significantly higher than the other two groups.
Table 1 Means (s.d.) and frequencies (%) for demographic and clinical background variables for each group

a. Tests of main effects used standard parametric tests, depending on whether there were 2 groups (T-test) or ≥3 groups (analysis of variance), unless otherwise stated.
b. Assumption of homogeneity of variance not met.
c. Reduced sample sizes due to missing BMI data: Autism (n = 64), Autism + REDs (n = 51), REDs (n = 77), HATs + REDs (n = 33).
d. Covariate is evaluated at the following value: Age (years) = 33.04.
ARFID, avoidant/restrictive food intake disorder; ED, eating disorder; REDs, restrictive eating disorders; GCSE, General Certificate of Secondary Education; HATs, high autistic traits; BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; SPIN, Social Phobia Inventory.
Correlation between autistic traits and BMI
Spearman's rank-order correlations showed no significant correlations between any autistic traits and BMI in each group (see Supplementary Appendix 5, sTable 4).
Autistic characteristics
Table 2 shows group comparisons of mean total scores on measures of autistic characteristics (autistic traits in adulthood and childhood, restricted and repetitive behaviours, camouflaging behaviours), adjusting for differences in mean age between the groups. There was a significant effect of the group on each measure of autistic characteristics. For each measure, participants in the Autism + REDs and Autism groups scored higher than REDs participants. Both autistic groups showed similar levels of autistic characteristics, with one exception: Autism + REDs participants reported higher levels of restricted and repetitive behaviours than Autism participants. Overall, those with a RED who did not have an autism diagnosis but did have high autistic traits (HATs + REDs) showed a similar pattern of autistic characteristics, including childhood traits, to the diagnosed autistic participants. The only exception was on the adult autism spectrum quotient, where they scored significantly lower than autistic participants with and without REDs. HATs + REDs participants scored significantly higher than REDs participants on all autistic characteristics measures.
Table 2 Mean total scores, F statistic and post-hoc comparisons (adjusted for differences in age) for each autistic characteristic measure
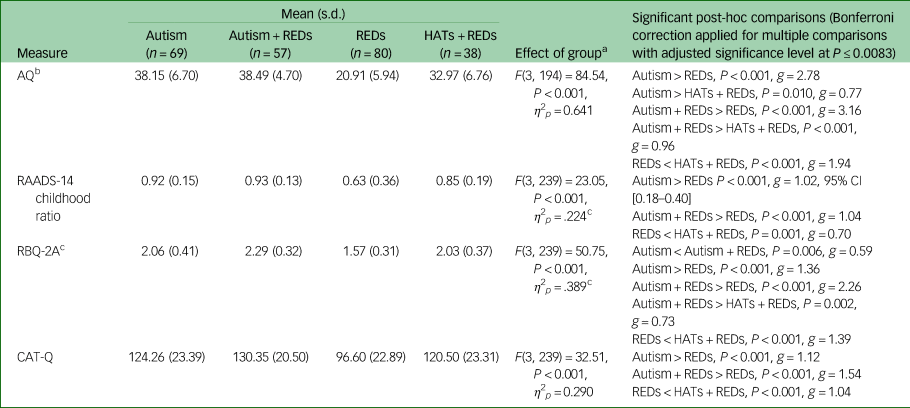
a. Covariate is evaluated at the following value: Age (years) = 33.04.
b. Reduced sample size for AQ comparison due to missing data: Autism (n = 40), Autism + REDs (n = 39), REDs (n = 79), HATs + REDs (n = 36).
c. Assumption of homogeneity of variance not met.
REDs, restrictive eating disorders; HATs, high autistic traits; AQ, Adult Autism Spectrum Quotient; RAADS-14, Ritvo Autism Asperger Diagnostic Scale; RBQ-2A, Adult Repetitive Behaviours Questionnaire; CAT-Q, Camouflaging Autistic Traits Questionnaire.
Disordered eating symptoms
Age-adjusted group comparisons on measures of disordered eating symptoms (traditional eating disorder symptoms, body image issues, pride in eating pathology, autism-specific unusual eating behaviours) are presented in Table 3. There was a significant effect of the group on each measure indicating differences in traditional eating disorder symptoms, body image issues, pride in eating pathology and autism-specific unusual eating behaviours. The Autism + REDs group, compared with the Autism group, scored higher across all measures of disordered eating symptoms. In terms of traditional disordered eating symptoms, Autism + REDs participants scored similarly to the REDs group. However, although not significant, there was a consistent pattern of Autism + REDs participants presenting with lower levels of eating disorder symptoms compared to the REDs group, with small to medium effect sizes: traditional eating disorder symptoms (P = 0.095; g = 0.40; 95% CI [−0.05–1.13]), body image issues (P = 0.173; g = 0.38, 95% CI [−2.73–28.79]) and pride in their eating disorder (P = 0.66; g = 0.26; 95% CI [−4.57–18.45]). Notably, in the sensitivity analysis using only complete cases, the difference between Autism + REDs participants (mean = 3.51, s.d. = 1.43) and REDs participants (mean = 4.10, s.d. = 1.18) for traditional eating disorder symptoms was significant (P = 0.041, g = 0.46; 95% CI [0.016–1.192] with Bonferroni correction applied for multiple comparisons).
Table 3 Unadjusted mean total scores, F statistic and post-hoc comparisons (adjusted for differences in age) for disordered eating-related measure.
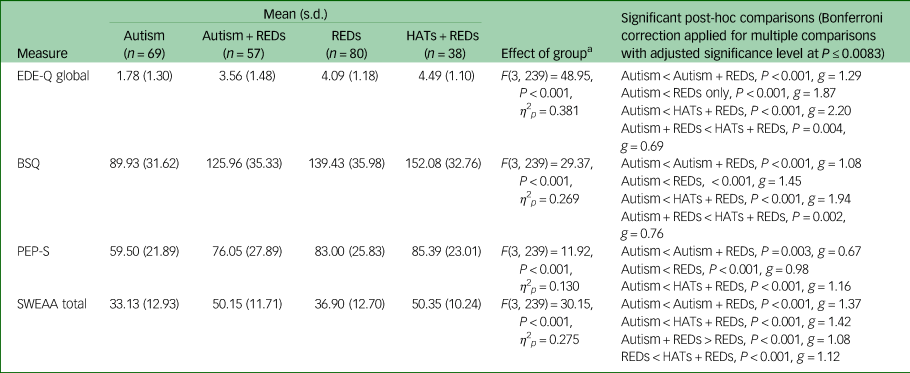
a. Covariate is evaluated at the following value: Age (years) = 33.04.
REDs, restrictive eating disorders; HATs, high autistic traits; EDE-Q, Eating Disorder Examination-Questionnaire; BSQ, Body Shape Questionnaire; PEP-S, Pride in Eating Pathology Scale; SWEAA, SWedish Eating Assessment for Autism Spectrum Disorders.
Compared to the REDs and the Autism group, Autism + REDs participants had more autism-specific unusual eating behaviours, as measured by the SWEAA.
The HATs + REDs group attained the highest score on each disordered eating measure, indicating both severe traditional eating disorder symptoms and high levels of autism-specific unusual eating behaviours.
SWEAA subscales
To better understand the nature of autism-specific risk factors for REDs, we looked at the pattern of group differences for each SWEAA subscale. The results of this analysis are shown in Supplementary Appendix 6 with group compositions being presented in Table 4. We were particularly interested to see on which subscales the Autism + REDs group scored significantly higher than both the Autism and the REDs group, given that such a pattern could give clues to autism-specific risk factors for REDs. This pattern was observed for the following SWEAA subscales: perception, purchase of food, eating behaviour, mealtime surroundings and social situations at mealtimes. By contrast, on the motor control and simultaneous capacity subscales, the Autism and the Autism + REDs group were indistinguishable, but both scored significantly higher than the REDs group; and on the disturbed eating behaviour subscale, Autism + REDs participants scored similarly to REDs participants, with the Autism group scoring significantly lower. Overall, the Autism + REDs and the HATs + REDs group presented similarly across SWEAA subscales.
Table 4 Unadjusted mean SWEAA subscale scores and post-hoc comparisons for the model adjusted for differences in age
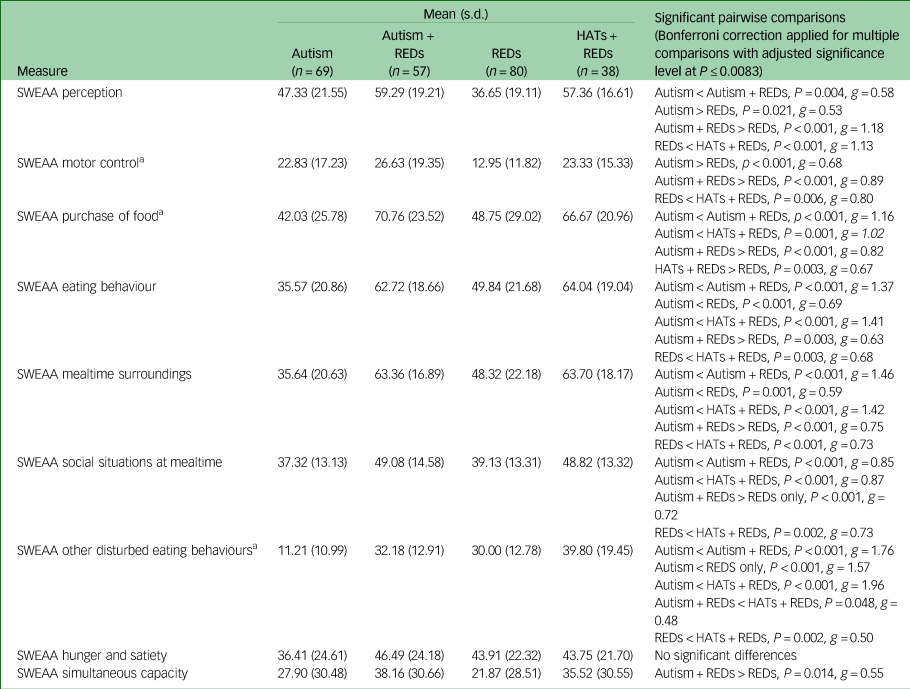
a. Assumption of homogeneity of variance not met.
REDs, restrictive eating disorders; HATs, high autistic traits; SWEAA, SWedish Eating Assessment for Autism Spectrum Disorders.
Discussion
The current study is the first to quantitatively describe the clinical presentation of autistic individuals with REDs compared with autistic individuals without REDs and non-autistic women with REDs. We aimed to increase understanding of what underpins REDs in autistic women and to support recognition of these individuals in eating disorder services. In addition, given issues of diagnostic overshadowing, a group of women with REDs who had no autism diagnosis but did report high autistic traits was included to explore their similarities to individuals with REDs and an autism diagnosis.
There were several similarities between autistic and non-autistic individuals with REDs in terms of BMI, levels of general anxiety, depression and some disordered eating symptoms. An important difference, however, was that the former scored substantially higher on a measure of autism-specific eating behaviours (i.e. the SWEAA). Crucially, the Autism + REDs group also had higher levels of these behaviours compared with the Autism group. This suggests that such autism-specific eating difficulties are not simply a general feature of autism but, rather, are characteristic of that subset of autistic women who have developed clinical REDs. In particular, autistic individuals with REDs presented with higher scores on SWEAA subscales related to sensory-driven food restriction; insistence on sameness and intolerance of uncertainty; inflexible, ritualistic behaviour at mealtimes; as well as the environment and social interactions during mealtimes. Similarly, autistic individuals with REDs reported higher levels of social anxiety than both autistic individuals without REDs and non-autistic women with REDs. In addition, of the autistic characteristics measured in the current study, restricted and repetitive behaviours were the only characteristics endorsed more strongly by autistic participants with REDs than those without REDs, thus suggesting that these could be implicated in REDs development and/or maintenance. This profile fits with qualitative findings based on the lived experience of autistic women with REDs.Reference Brede, Babb, Jones, Elliott, Zanker and Tchanturia13,Reference Kinnaird, Norton, Stewart and Tchanturia36 It is also consistent with a previous study on the nature of autistic traits in young people with anorexia nervosa;Reference Pooni, Ninteman, Bryant-Waugh, Nicholls and Mandy37 and the observation that a range of conditions associated with rigid behaviour and unusual sensory processing are associated with REDs.Reference Drakes, Fawcett, Rose, Carter-Major and Fawcett38
It is noteworthy that autistic individuals with REDs also presented with high levels of camouflaging behaviour (mean = 130.34), although there was no significant difference from the group of autistic women without REDs (mean = 124.26), with this group also scoring high compared with other autistic women from community samples (e.g. mean = 114 inReference Hull, Levy, Lai, Petrides, Baron-Cohen and Allison39). Nonetheless, future research should consider the role of camouflaging and associated distress,Reference Bargiela, Steward and Mandy40,Reference Cage and Troxell-Whitman41 as well as other autism-related factors not explored in this study (e.g. differences social relating and executive functioning) for REDs in autistic women.
This is, to our knowledge, the first study to test the idea, suggested by qualitative research,Reference Brede, Babb, Jones, Elliott, Zanker and Tchanturia13 that autistic women with REDs have fewer weight and shape concerns compared with non-autistic women with REDs. We found that the Autism + REDs group showed a pattern of lower scores on measures of traditional eating disorder symptoms related to weight and shape concerns, body image issues and pride in eating pathology, although this did not reach significance. Further, compared with autistic women without REDs, Autism + REDs participants did score significantly higher on these measures. Therefore, based on the current findings, there is evidence that weight and shape concerns have a role in the onset and/or maintenance of REDs for at least some autistic women. Future research should explore this further, including whether weight and shape concern-related eating disorder symptoms in autistic women with REDs might be qualitatively different, e.g. their onset might be secondary to more typical autistic eating difficulties, or whether there is a subgroup without overt weight and shape concerns.Reference Korn, Vocks, Rollins, Thomas and Hartmann42
This study offers insights into the identification and understanding of autistic women in eating disorder settings. As discussed above, the presence of unusual eating behaviours in addition to more traditional eating disorder symptoms appears to be a good indicator that someone might be autistic. Another obvious difference between autistic and non-autistic individuals with REDs is that the autistic participants scored much higher across measures of autistic characteristics. Yet, camouflaging behaviour might make them seem less autistic, but could be an additional source of stress and exhaustion for these individuals.Reference Bargiela, Steward and Mandy40
Concerns have been raised that having a RED might cause non-autistic women to erroneously score highly on autistic trait measures.Reference Treasure43 However, the fact that women with REDs identified through scores above a conservative cut-off on an autism screening measure (HATs + REDs) presented similarly to individuals with a formal autism diagnosis in terms of other clinical characteristics, suggests that even brief self-report measures can measure autistic traits as distinct from RED and other clinical characteristics in eating disorder populations. This is further supported by the lack of correlation between BMI and autistic traits, which is in line with findings from other research,Reference Leppanen, Sedgewick, Halls and Tchanturia44 as a significant negative correlation would have been consistent with the idea that these are owing to starvation rather than autism. In addition, the fact that higher levels of autistic traits in this group have been present in childhood suggests that they likely pre-dated the onset of the eating disorder, although the limitation of self- rather than informant-reported childhood traits should be noted here.
Among participants initially recruited as women with REDs (n = 118) without a formal autism diagnosis, around one-third (n = 38) presented with very high autistic traits (HATs + REDs). Although online recruitment might have induced some bias, this is in line with prevalence estimates from other studies with more representative samplesReference Westwood and Tchanturia3 and qualitative accounts of delay in autism diagnosis in women with REDs.Reference Babb, Brede, Jones, Elliott, Zanker and Tchanturia11 As a group, these women showed high levels of: autistic traits, including in childhood, and associated characteristics (i.e. restricted and repetitive behaviours, camouflaging behaviours); distress, as indexed by their high levels of general anxiety, depression and social anxiety; traditional eating disorder symptoms; and autism-specific eating difficulties. In short, they looked very similar to participants with a RED and a formal autism diagnosis. The only exception was that they had lower scores on one of the measures of general autistic traits (adult autism spectrum quotient), although their mean score (mean = 32.97) was still above that measure's screening threshold of 26.Reference Woodbury-Smith, Robinson, Wheelwright and Baron-Cohen25 A plausible explanation for this is that many HATs + REDs participants might be undiagnosed autistic women. This would need to be confirmed with a full diagnostic assessment, including gold-standard measures and a developmental history. It was a limitation of the current study that, because of the COVID-19 pandemic, we had to rely on self-report measures and were unable include in-person observational measures. This would have allowed us to confirm self-reported autism diagnostic status across the sample and would have provided further evidence as to whether participants in the HATs + REDs group are likely to be undiagnosed autistic women. While online recruitment allowed for the largest sample in this area of research thus far, it might have affected representativeness of the sample. It should also be noted that, as unfortunately is often the case in autism and eating disorder research,Reference Maye, Boyd, Martínez-Pedraza, Halladay, Thurm and Mandell45,Reference Burnette, Luzier, Weisenmuller and Boutté46 the current sample is predominantly white and highly educated, thus implicating transferability of results to other patient groups.
Clinical implications
First, our findings are supportive of the use of a portfolio of self-report autism measures (including those assessing camouflaging and autism-specific unusual eating behaviours) to inform decisions about whether a client with RED might be autistic and would benefit from treatment adaptations. Conducting formal autism diagnostic assessments in currently unwell individuals with REDs is challenging, and this challenge is currently compounded by long waiting times in autism diagnostic services.Reference Beresford, Mukherjee, Mayhew, Heavey, Park and Stuttard47 Therefore, it might be sufficient to rely on the recognition of autistic traits to inform treatment adaptations,Reference Field, Fox, Jones and Williams48 with the option to conduct a full diagnostic autism assessment at later stages. This supports recent thinking in the autism field, which favours a more dimensional, heterogeneous characterisation of autistic individuals over a rigid categorical approach,Reference Happé and Frith49 and could make support more accessible for a greater proportion of individuals.
Second, autism-related disordered eating symptoms should be routinely considered for assessment and treatment (see Reference Li, Halls, Byford and Tchanturia50,Reference Tchanturia, Smith, Glennon and Burhouse51 for examples and guidance). When assessing autistic women's RED presentations, a narrow focus on traditional disordered eating symptoms might overlook or underestimate additional significant eating issues. If treatment only targets more traditional disordered eating symptoms, the persistence of other autism-specific eating behaviours might hinder progress towards recovery or increase risk of relapse.
Third, we present the strongest evidence to date for putative autism-specific risk factors for REDs, related to autistic sensory, flexibility and social differences. Longitudinal research is required to test whether these play a causal role in the development of REDs of autistic women.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2024.65
Data availability
The corresponding author will consider requests from researchers for access to the fully anonymised data-set used in this study.
Acknowledgements
We would like to thank the autistic women, women with a restrictive eating disorder, parents and healthcare professionals who participated and generously shared their insights, and the Autistica Discover network for their support with recruitment.
Author contributions
J.B., C.B., C.R.G.J., L.S., H.B., J.R.E.F. and W.M. all contributed to the conception and design of the study. J.B., C.B., J.A. and H.B. collected data. J.B., L.H. and W.M. conducted the analyses. J.B. and W.M. wrote the manuscript. All authors contributed to the manuscript via reading and commenting on drafts.
Funding
This study was funded by Autistica (7252). The funder had no involvement in the study design and analysis, writing of the manuscript or in the decision to submit the manuscript for publication.
Declaration of interest
The authors disclose no relevant conflicts of interest.








eLetters
No eLetters have been published for this article.