As hypertension is associated with both an increased incidence of dementia, Reference Whitmer, Sidney, Selby, Johnston and Yaffe1 and conversion from mild cognitive impairment to dementia, Reference Rozzini, Chilovi, Bertoletti, Conti, Del Rio and Trabucchi2 attention has focused on treatments for hypertension as modifiers of cognitive decline. The influence of a number of antihypertensives has been investigated from mild cognitive impairment2,Reference Hajjar, Hart, Chen, Mack, Novak and Chui3 through to established dementia, Reference Gao, O'Caoimh, Healy, Kerins, Eustace and Guyatt4 with some agents associated with a lowered risk, but negative or inconsistent results from clinical trials. Reference Skoog5–Reference De Oliveira, Bertolucci, Chen and Smith9 Angiotensin-converting enzyme inhibitors (ACEIs) have attracted interest in Alzheimer's disease specifically; however, not all ACEIs cross the blood–brain barrier which may give rise to heterogeneity in effects. In one study of community residents, reduced cognitive decline was found in those receiving centrally active inhibitors (centrally acting angiotensin-converting enzyme inhibitors (C-ACEIs)) compared with non-centrally active inhibitors (non-centrally acting angiotensin-converting enzyme inhibitors (NC-ACEIs)) and calcium channel blockers. Reference Ohrui, Tomita, Sato-Nakagawa, Matsui, Maruyama and Niwa10 Studies of dementia incidence have been mixed, Reference Sink, Leng, Williamson, Kritchevsky, Yaffe and Kuller11–Reference Maxwell and Hogan15 but C-ACEI receipt was associated with slower cognitive decline in dementia over 6 months, Reference Gao, O'Caoimh, Healy, Kerins, Eustace and Guyatt4 and C-ACEIs have recently been recommended for slowing dementia progression in elderly patients with hypertension. Reference Opie16 Given the paucity of evidence in this field, we analysed a large, prospective clinical database of people receiving dementia assessment and acetylcholinesterase treatment, to compare cognitive function trajectories and survival among C-ACEI users with those using NC-ACEIs or neither agent.
Method
Study setting and data source
A retrospective observational study was conducted using data from the South London and Maudsley NHS Foundation Trust (SLaM) Biomedical Research Centre (BRC) case register. SLaM is one of Europe's largest mental healthcare providers, serving a geographic catchment of over 1.2 million residents in four South London boroughs (Lambeth, Lewisham, Southwark and Croydon). In 2007–2008, the Clinical Record Interactive Search (CRIS) application was developed with National Institute for Health Research (NIHR) funding to provide researcher access to anonymised copies of SLaM's electronic health record within a robust governance framework. Reference Fernandes, Cloete, Broadbent, Hayes, Chang and Roberts17 The SLaM BRC case register has been described in detail, Reference Stewart, Soremekun, Perera, Broadbent, Callard and Denis18 and has supported a range of studies, Reference Hayes, Chang, Fernandes, Broadbent, Lee and Hotopf19,Reference Chang, Hayes, Perera, Broadbent, Fernandes and Lee20 including several longitudinal studies of large dementia cohorts. Reference Perera, Khondoker, Broadbent, Breen and Stewart21–Reference Ward, Perera and Stewart23 Data are currently archived in CRIS on over 270 000 cases with a range of mental disorders and the database has full approval for secondary analysis (Oxford Research Ethics Committee C, reference 08/H0606/71+5). Data from CRIS have been extensively supplemented through natural language processing applications using Generalised Architecture for Text Engineering (GATE) software, applying information extraction techniques to derive structured information from the extensive text fields held in the mental health record. Reference Perera, Broadbent, Callard, Chang, Downs and Dutta24
Sample
All cases with Alzheimer's disease (ICD-10 F00.x) diagnosed at any point between 1 January 2000 and 31 May 2014 were ascertained in CRIS using a combination of data from structured fields for primary and secondary diagnoses, and a specific natural language processing application extracting text associated with diagnostic statements in open text fields. Reference Perera, Broadbent, Callard, Chang, Downs and Dutta24 The sample was restricted to patients who received a first diagnosis of Alzheimer's disease during the study period, and who were commenced on treatment with an acetylcholinesterase inhibitor. Those who received angiotensin receptor blockers at the time of Alzheimer's disease diagnosis were also excluded – this was partly to maximise homogeneity of the sample and partly because those receiving this treatment had the most routine data on cognitive decline.
Measurements
The index date for defining exposure and confounding variables was the date of the first recorded Alzheimer's disease diagnosis. Demographic information obtained included age at diagnosis, age at death, gender, ethnicity (European and non-European) and index of multiple deprivation (2010 projections from the UK Census) for each patient's neighbourhood (UK lower super output area) at the time of diagnosis. Health of the Nation Outcome Scales (HoNOS) are routinely administered in UK mental health services and recorded as structured data on the electronic health record. HoNOS item scores and dates were extracted for the period of 6 months before and after the diagnosis date, and the closest scores in time were included in analyses.
Medications received were extracted from structured medication fields supplemented by natural language processing applications applied to open text fields. Reference Perera, Broadbent, Callard, Chang, Downs and Dutta24 C-ACEIs were defined as perindopril, ramipril, trandolapril, captopril, fosinopril, lisinopril, prinivil, monopril; NC-ACEIs were defined as enalapril, imidapril, cilazapril, quinapril, moexipril. Initial and most recent dates were ascertained for when these medications were recorded, and exposure was defined on the basis of recorded medication use at or before the Alzheimer's disease diagnosis. The analysis excluded patients who were recorded as receiving both C-ACEIs and NC-ACEIs (because these groups were too small to analyses separately), and a third group of patients were defined with no recorded ACEI use. Use of antipsychotic or antidepressant medication was also ascertained at the time of the Alzheimer's disease diagnosis. Use of other antihypertensive medication (vasodilators, diuretics, calcium channel blockers, beta-blockers, alpha-blockers) was also ascertained at the time of Alzheimer's disease diagnosis.
The primary outcome was change in cognitive function as estimated from recorded Mini-Mental State Examination (MMSE) scores. In British dementia treatment services, cognitive function continues to be primarily monitored in routine clinical practice using the MMSE, a 30-point measure of global function in wide use. Reference Folstein, Folstein and McHugh25 MMSE numerator and denominator scores and dates of assessment for the cohort were obtained from structured fields and a bespoke natural language processing application. Reference Perera, Broadbent, Callard, Chang, Downs and Dutta24 According to the availability of follow-up data and to minimise the influence of differential attrition, MMSE scores were restricted to those recorded within 3 years after the Alzheimer's disease diagnosis date, and the analysed sample was restricted to patients with more than one MMSE score recorded. Date of death was obtained from death registry information from the Office for National Statistics (ONS) linked to CRIS.
Statistical analysis
Initially, generalised additive models for location, scale and shape (GAMLSS) Reference Rigby and Stasinopoulos26 were used to visualise the shape of MMSE score trajectories for the three cohorts – those who were receiving C-ACEIs, those who were receiving NC-ACEIs and those who were not receiving any ACEIs. An advantage of GAMLSS is that they are not restricted to a linearity assumption: important because of the potential association between cognitive decline and time since Alzheimer's disease diagnosis and acetylcholinesterase inhibitor initiation. GAMLSS are parametric, in that they require a parametric distribution assumption for the response variable, and ‘semi’ in the sense that the modelling of the parameters of the distribution, as functions of explanatory variables, may involve using non-parametric smoothing functions. Although GAMLSS output provided a helpful way of visualising the pattern of cognitive decline within the observation window, it did not permit analyses of predictive covariates, confounding and effect modification. By inspecting the curves derived from GAMLSS, it was concluded that it would be appropriate to use parametric methodology in the form of a two-piecewise linear mixed model to estimate cognitive change and its predictors, using the two time components: Segment 1 for the 0–9 months after diagnosis and Segment 2 for the 9–36 months after diagnosis. Slopes and slope differences were obtained using linear mixed estimation methodology. Two-piecewise model estimates were adjusted for age, gender, ethnicity, other antihypertensives, antipsychotic, antidepressant receipt and HoNOS subcomponents including agitated behaviour, self-injury, problem drinking or drug use, physical illness, hallucinations or delusions, depressed mood, relationship problems, daily living problems, living conditions problems and occupational problems.
Survival analyses were carried out for mortality as an outcome up to a census point of 8 July 2014. Kaplan–Meier survival curves were constructed using STATA 13 software, and a log-rank test was carried out to test associations with ACEI use.
Results
Fourteen patients were excluded who were receiving both C-ACEI and NC-ACEI agents and an additional ten patients who had only one recorded MMSE score since diagnosis. Furthermore, 27 patients receiving C-ACEIs, 2 patients receiving NC-ACEIs and 296 patients receiving neither were excluded as they received angiotensin receptor blockers at the time of Alzheimer's disease diagnosis. The analysed samples comprised 1207 patients receiving C-ACEIs, 143 receiving NC-ACEIs and 3910 receiving neither. Characteristics of the samples are summarised in Table 1. In summary, the group receiving NC-ACEIs were more likely to be to be male, to be of non-European ethnicity and to be rated as having hallucinations or delusions than those receiving C-ACEIs or neither. There were no significant individual group differences in age (ANOVA post hoc test). However, a post hoc test showed a significant difference in mean deprivation score between C-ACEI group and the group receiving neither ACEI (P=0.008) and between the NC-ACEI group and those receiving neither ACEI (P=0.041). Antipsychotic and antidepressant uses were more common in patients receiving C-ACEIs. Both the C-ACEI and NC-ACEI groups were rated as having more severe physical health problems on the HoNOS than those receiving neither. Importantly, although there were differences in the timing and number of some measurement points, there was no difference in MMSE score closest to diagnosis between the three groups.
Table 1 Sample characteristics at/around Alzheimer's disease diagnosis by exposure status
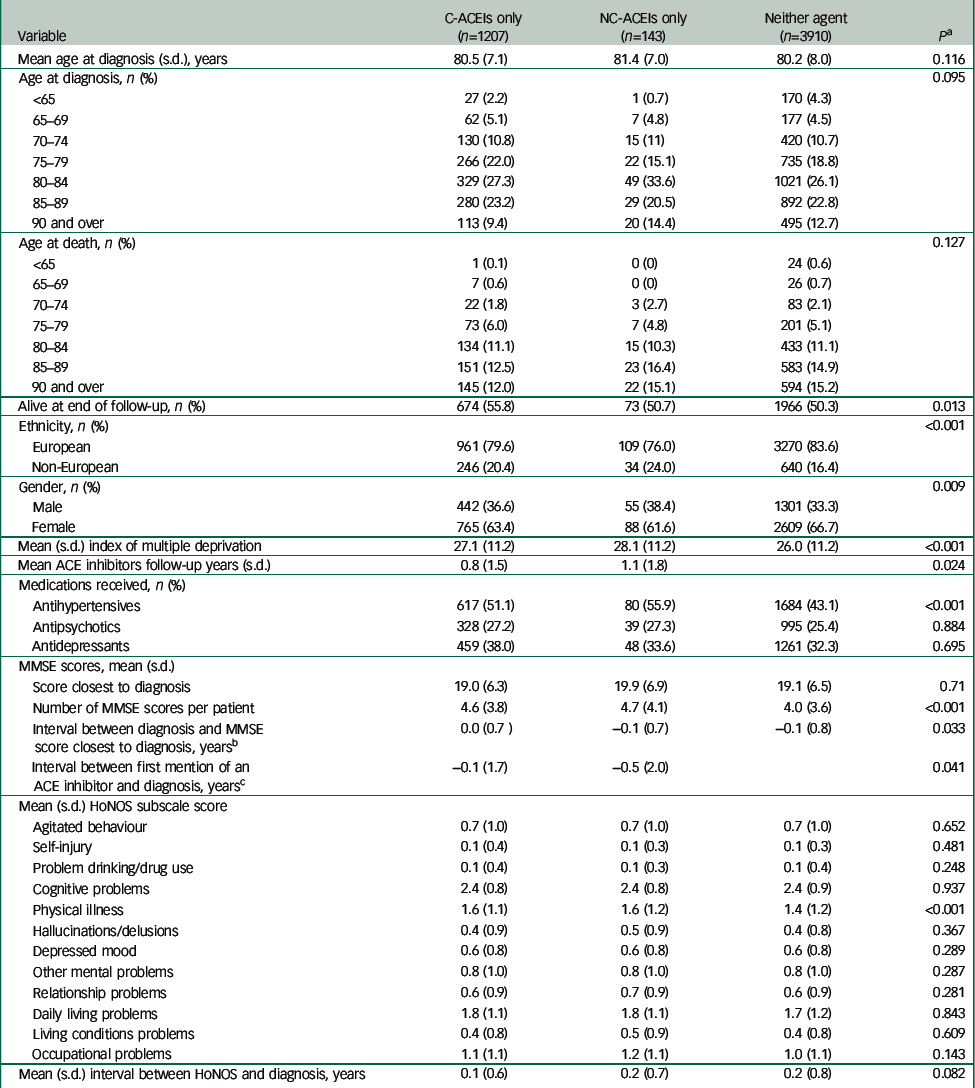
| Variable | C-ACEIs only (n=1207) | NC-ACEIs only (n=143) | Neither agent (n=3910) | P a |
|---|---|---|---|---|
| Mean age at diagnosis (s.d.), years | 80.5 (7.1) | 81.4 (7.0) | 80.2 (8.0) | 0.116 |
| Age at diagnosis, n (%) | 0.095 | |||
| <65 | 27 (2.2) | 1 (0.7) | 170 (4.3) | |
| 65–69 | 62 (5.1) | 7 (4.8) | 177 (4.5) | |
| 70–74 | 130 (10.8) | 15 (11) | 420 (10.7) | |
| 75–79 | 266 (22.0) | 22 (15.1) | 735 (18.8) | |
| 80–84 | 329 (27.3) | 49 (33.6) | 1021 (26.1) | |
| 85–89 | 280 (23.2) | 29 (20.5) | 892 (22.8) | |
| 90 and over | 113 (9.4) | 20 (14.4) | 495 (12.7) | |
| Age at death, n (%) | 0.127 | |||
| <65 | 1 (0.1) | 0 (0) | 24 (0.6) | |
| 65–69 | 7 (0.6) | 0 (0) | 26 (0.7) | |
| 70–74 | 22 (1.8) | 3 (2.7) | 83 (2.1) | |
| 75–79 | 73 (6.0) | 7 (4.8) | 201 (5.1) | |
| 80–84 | 134 (11.1) | 15 (10.3) | 433 (11.1) | |
| 85–89 | 151 (12.5) | 23 (16.4) | 583 (14.9) | |
| 90 and over | 145 (12.0) | 22 (15.1) | 594 (15.2) | |
| Alive at end of follow-up, n (%) | 674 (55.8) | 73 (50.7) | 1966 (50.3) | 0.013 |
| Ethnicity, n (%) | <0.001 | |||
| European | 961 (79.6) | 109 (76.0) | 3270 (83.6) | |
| Non-European | 246 (20.4) | 34 (24.0) | 640 (16.4) | |
| Gender, n (%) | 0.009 | |||
| Male | 442 (36.6) | 55 (38.4) | 1301 (33.3) | |
| Female | 765 (63.4) | 88 (61.6) | 2609 (66.7) | |
| Mean (s.d.) index of multiple deprivation | 27.1 (11.2) | 28.1 (11.2) | 26.0 (11.2) | <0.001 |
| Mean ACE inhibitors follow-up years (s.d.) | 0.8 (1.5) | 1.1 (1.8) | 0.024 | |
| Medications received, n (%) | ||||
| Antihypertensives | 617 (51.1) | 80 (55.9) | 1684 (43.1) | <0.001 |
| Antipsychotics | 328 (27.2) | 39 (27.3) | 995 (25.4) | 0.884 |
| Antidepressants | 459 (38.0) | 48 (33.6) | 1261 (32.3) | 0.695 |
| MMSE scores, mean (s.d.) | ||||
| Score closest to diagnosis | 19.0 (6.3) | 19.9 (6.9) | 19.1 (6.5) | 0.71 |
| Number of MMSE scores per patient | 4.6 (3.8) | 4.7 (4.1) | 4.0 (3.6) | <0.001 |
| Interval between diagnosis and MMSE score closest to diagnosis, years b | 0.0 (0.7) | −0.1 (0.7) | −0.1 (0.8) | 0.033 |
| Interval between first mention of an ACE inhibitor and diagnosis, years c | −0.1 (1.7) | −0.5 (2.0) | 0.041 | |
| Mean (s.d.) HoNOS subscale score | ||||
| Agitated behaviour | 0.7 (1.0) | 0.7 (1.0) | 0.7 (1.0) | 0.652 |
| Self-injury | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.3) | 0.481 |
| Problem drinking/drug use | 0.1 (0.4) | 0.1 (0.3) | 0.1 (0.4) | 0.248 |
| Cognitive problems | 2.4 (0.8) | 2.4 (0.8) | 2.4 (0.9) | 0.937 |
| Physical illness | 1.6 (1.1) | 1.6 (1.2) | 1.4 (1.2) | <0.001 |
| Hallucinations/delusions | 0.4 (0.9) | 0.5 (0.9) | 0.4 (0.8) | 0.367 |
| Depressed mood | 0.6 (0.8) | 0.6 (0.8) | 0.6 (0.8) | 0.289 |
| Other mental problems | 0.8 (1.0) | 0.8 (1.0) | 0.8 (1.0) | 0.287 |
| Relationship problems | 0.6 (0.9) | 0.7 (0.9) | 0.6 (0.9) | 0.281 |
| Daily living problems | 1.8 (1.1) | 1.8 (1.1) | 1.7 (1.2) | 0.843 |
| Living conditions problems | 0.4 (0.8) | 0.5 (0.9) | 0.4 (0.8) | 0.609 |
| Occupational problems | 1.1 (1.1) | 1.2 (1.1) | 1.0 (1.1) | 0.143 |
| Mean (s.d.) interval between HoNOS and diagnosis, years | 0.1 (0.6) | 0.2 (0.7) | 0.2 (0.8) | 0.082 |
C-ACEIs, centrally acting angiotensin converting enzyme inhibitors; NC-ACEIs, non-centrally acting angiotensin converting enzyme inhibitors; MMSE, Mini-Mental State Examination; ACE, angiotensin converting enzyme; HoNOS, Health of the Nation Outcome Scales.
a Difference in means between three groups tested using one-way ANOVA test, difference in means between two medication groups were tested using independent sample t-tests, differences in frequencies tested using chi-squared tests.
b Negative values indicating that MMSE scores recorded before Alzheimer's disease diagnosis.
c Negative values indicating ACE inhibitor medication recorded before Alzheimer's disease diagnosis.
Figure 1 displays GAMLSS curve for trajectories of MMSE scores by ACEI receipt, and Tables 2 and 3 summarise the results of the linear mixed estimations from two-piecewise estimations model comapring slopes at 0–9 months and 9–36 months after Alzheimer's disease diagnosis. Analysis with adjustments showed the NC-ACEI group to have slightly higher MMSE scores at Alzheimer's disease diagnosis compared with the other two groups. In patients who received C-ACEIs, MMSE scores improved over the first 9 months, with a slope coefficient of 0.72 (points per year), whereas they declined in those receiving NC-ACEIs and slightly improved in those that received neither ACEI, with slope coefficients of −0.61 and 0.19 respectively. For patients receiving C-ACEIs, these slopes were significantly different both compared with those receiving NC-ACEIs (slope difference 1.33, P=0.028) and compared with those who received neither ACEI (slope difference 0.53, P=0.037). However, there were no significant group differences in slopes for the 9–36 month period after diagnosis. Figure 2 displays GAMLSS curve for trajectories of MMSE scores by other antihypertensive use for those who did not receive any ACEIs. There were no significant differences in slopes between those who received other antihypertensive medication and not receiving such medication. Likewise, as shown in Fig. 3, there was no observable group difference in survival. Log-rank tests for equality of survivor functions gave P-values of 0.82 and 0.84, respectively, for comparisons between C-ACEIs and NC-ACEIs, and between C-ACEIs and no ACEI.
Table 2 Adjusted MMSE slopes (points/year) after Alzheimer's disease diagnosis comparing those who received C-ACEIs and those who did not receive any ACEI
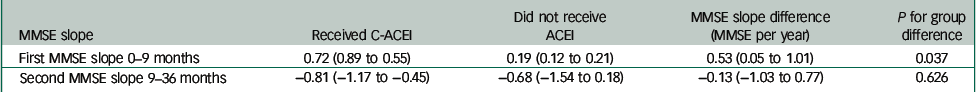
| MMSE slope | Received C-ACEI | Did not receive ACEI | MMSE slope difference (MMSE per year) | P for group difference |
|---|---|---|---|---|
| First MMSE slope 0–9 months | 0.72 (0.89 to 0.55) | 0.19 (0.12 to 0.21) | 0.53 (0.05 to 1.01) | 0.037 |
| Second MMSE slope 9–36 months | −0.81 (−1.17 to −0.45) | −0.68 (−1.54 to 0.18) | −0.13 (−1.03 to 0.77) | 0.626 |
MMSE, Mini-Mental State Examination; C-ACEIs, centrally acting angiotensin converting enzyme inhibitors; ACEI, angiotensin converting enzyme inhibitor.
Table 3 Adjusted MMSE slopes (points/ year) after Alzheimer's disease diagnosis comparing those who received C-ACEIs and those who received NC-ACEIs

| MMSE slope | Received C-ACEIs | Received NC-ACEIs | MMSE slope difference (MMSE per year) | P for group difference |
|---|---|---|---|---|
| First MMSE slope 0–9 months | 0.72 (0.89 to 0.55) | −0.61 (−0.21 to −1.02) | 1.33 (0.25 to 1.41) | 0.028 |
| Second MMSE slope 9–36 months | −0.81 (−1.17 to −0.45) | −0.63 (−1.36 to 0.10) | −0.18 (−1.50 to −1.14) | 0.531 |
MMSE, Mini-Mental State Examination; C-ACEIs, centrally acting angiotensin converting enzyme inhibitors; NC-ACEIs, non-centrally acting angiotensin converting enzyme inhibitors.
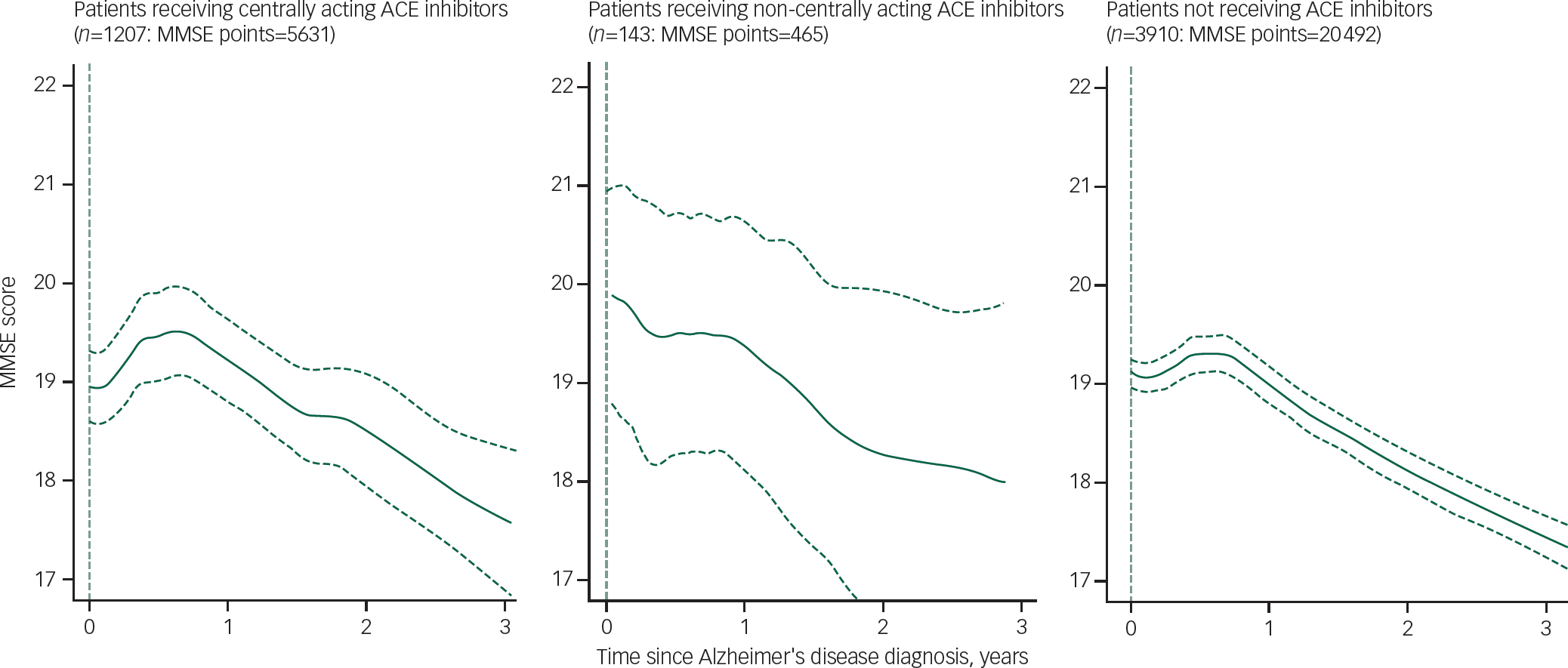
Fig. 1 Comparison of longitudinal change in Mini-Mental State Examination (MMSE) in the samples using generalised additive models for location, scale and shape (GAMLSS) methodology. ACE, angiotensin converting enzyme.
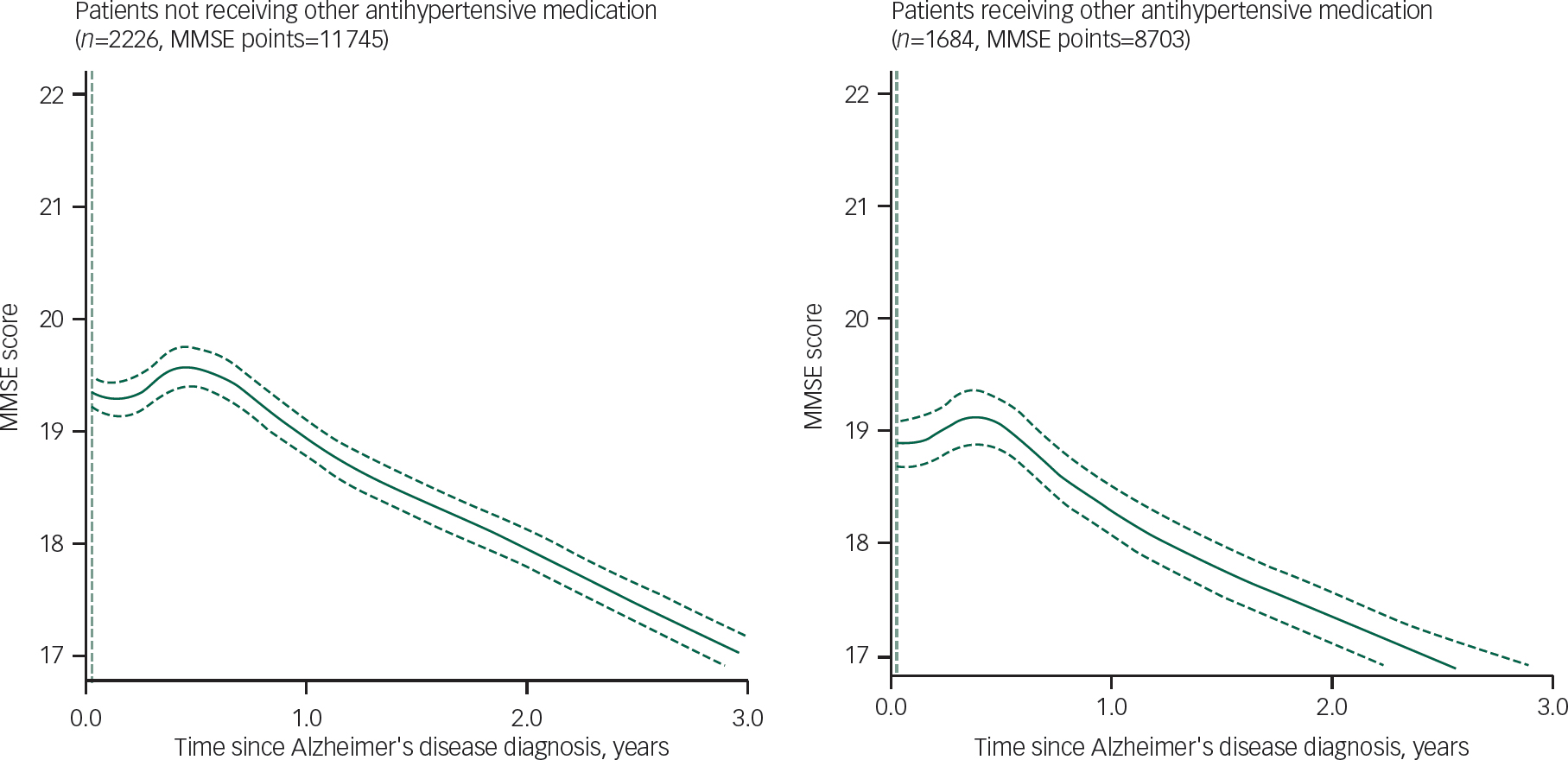
Fig. 2 Comparison of longitudinal change in Mini-Mental State Examination (MMSE) among patients not receiving angiotensin converting enzyme inhibitors stratified by other antihypertensive use.
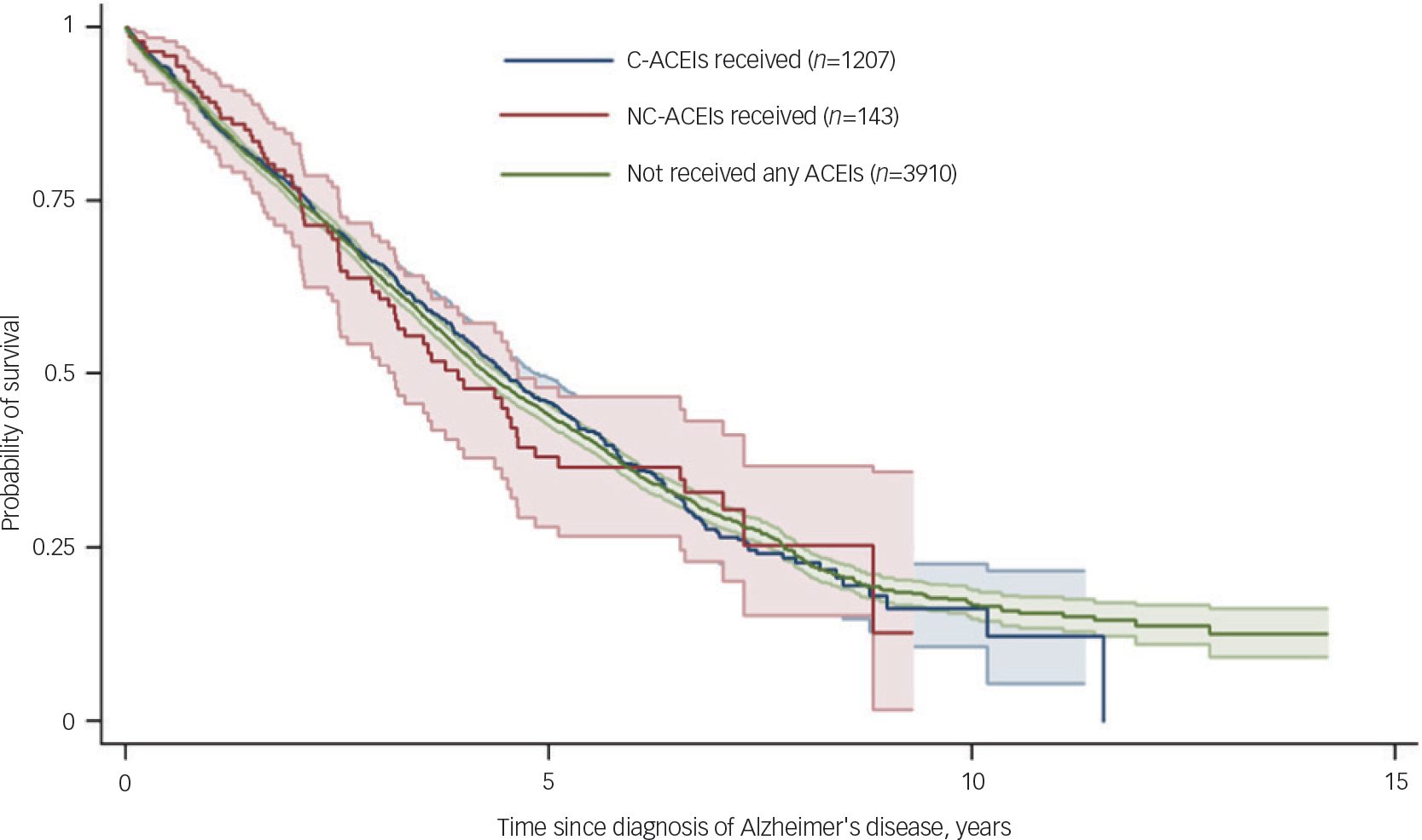
Fig. 3 Kaplan–Meier survival curves comparing exposure groups. ACEIs, angiotensin converting enzyme inhibitors; C-ACEIs, centrally acting angiotensin converting enzyme inhibitors; NC-ACEIs, non-centrally acting angiotensin converting enzyme inhibitors.
Discussion
In what we believe to be the largest investigation to date of antihypertensive agents and dementia progression, we compared trajectories of routinely measured cognitive function between patients receiving C-ACEIs and NC-ACEIs at the time of Alzheimer's disease diagnosis and those receiving neither type of medication. An initial improvement in cognitive function was found in patients receiving C-ACEIs over the first 9 months after diagnosis compared with deteriorations in the NC-ACEIs group. However, there was no evidence of longer-term slope differences and no differences in survival between the groups.
As previously summarised, several studies have suggested that C-ACEIs may influence cognitive function trajectories in people without dementia. The Cardiovascular Health Study Cognition Sub-Study, which followed up people with hypertension for 6 years, found no significant differences in cognitive decline between ACEIs and other antihypertensives, but did find significantly reduced cognitive decline in people receiving C-ACEIs: reduced by 65% per year of exposure in this group compared with those receiving NC-ACEIs. Reference Sink, Leng, Williamson, Kritchevsky, Yaffe and Kuller11 A Japanese study found no difference in Alzheimer's disease incidence associated with antihypertensive drugs as a whole, but did find a significantly lower risk in a subgroup analysis of captopril and perindopril (C-ACEIs) compared with enalapril and imidapril (NC-ACEIs). Reference Ohrui, Matsui, Yamaya, Arai, Ebihara and Maruyama13 Also, in secondary analyses of the Perindopril Protection Against Recurrent Stroke Study (PROGRESS), there was a reduced risk of cognitive decline in the treatment group. Reference Tzourio, Anderson, Chapman, Woodward, Neal and MacMahon27 There has been less investigation of associations between ACEIs and dementia progression, although one study found that C-ACEIs, compared with other antihypertensive drugs, were associated with a reduced rate of cognitive decline in hypertensive patients with mild to moderate Alzheimer's disease, although all participants had stable and comparable blood pressure levels, Reference Ohrui, Tomita, Sato-Nakagawa, Matsui, Maruyama and Niwa10 suggesting a mechanism other than lowered blood pressure. Our findings of initial improvement in cognition function in patients with C-ACEIs are also consistent with findings from two smaller research cohorts of reduced 12-month functional decline in patients with Alzheimer's disease, Reference O'Caoimh, Healy, Gao, Svendrovski, Kerins and Eustace28,Reference Kehoe, Davies, Martin and Ben-Shlomo29 with reduced 6-month cognitive decline in patients with Alzheimer's, vascular and mixed dementia, Reference Perera, Khondoker, Broadbent, Breen and Stewart21 and with reduced cognitive decline in patients with Alzheimer's disease with specific ACE haplotypes. Reference De Oliveira, Bertolucci, Chen and Smith9
The mechanisms by which C-ACEIs might influence dementia outcomes remain unclear. As previously described, ACEIs have been highlighted as potentially detrimental for cognitive function because of ACE degrading amyloid and observations of an increase of Aβ1-42 in mice associated with ACEI administration. Reference Zou, Liu, Watanabe, Hiraga, Liu and Tanabe30 However, there are also a number of potential reasons why these agents may be protective. First, reduced cerebral blood flow has been observed in Alzheimer's disease, Reference Ruitenberg, den Heijer, Bakker, van Swieten, Koudstaal and Hofman31 and C-ACEIs may improve this by inhibiting the production of angiotensin II, a vasoconstrictor; this is supported by perivascular staining of both angiotensin II and ACE seen in patients with Alzheimer's disease. Reference Savaskan, Hock, Olivieri, Bruttel, Rosenberg and Hulette32 Second, the renin angiotensin system within the brain has an effect on neural plasticity and long-term potentiation, Reference Wright, Reichert, Davis and Harding33 and it has been proposed that C-ACEIs through preventing activation of this system inhibits the release of inflammatory cytokines which may be involved in the neurodegenerative process. Reference Tuppo and Arias34,Reference Yaffe, Lindquist, Penninx, Simonsick, Pahor and Kritchevsky35 The renin angiotensin aldosterone system (RAAS) acts throughout the body, including the central nervous system, Reference Xue, Zhang, Roncari, Guo and Johnson36 and there is growing evidence of its importance in neurodegenerative disorders including Alzheimer's disease and Parkinson's disease. Reference Wright, Kawas and Harding37 Perindopril, but not other ACEIs, significantly inhibited hippocampal ACE, preventing cognitive impairment in mouse models of Alzheimer's disease, Reference Dong, Kataoka, Tokutomi, Nako, Nakamura and Toyama38,Reference Tota, Nath, Najmi, Shukla and Hanif39 and a study in hypertensive rats found that lifelong treatment with captopril (C-ACEI) significantly reduced impairment in learning and memory compared with hydralazine, despite equal blood pressure control in both groups. Reference Wyss, Kadish and van Groen40 Third, angiotensin II inhibits release of acetylcholine, Reference Barnes, Barnes, Costall, Horovitz, Ironside and Naylor41 and C-ACEIs might increase acetylcholine activity (and thus improve cognitive function) by reducing angiotensin II levels. Reference Sink, Leng, Williamson, Kritchevsky, Yaffe and Kuller11 Yamada et al demonstrated an enhancing effect of perindopril (C-ACEI) on extracellular acetylcholine levels in the perirhinal cortex in rats with chronic cerebral hypoperfusion. Reference Yamada, Horita, Takayama, Takahashi, Takaba and Nagata42
The fact that we were only able to observe an improvement over a 9-month period in people with Alzheimer's disease and that the longer-term decline trajectory was unaffected, favours the third and more ‘symptomatic’ of these mechanisms. It should be borne in mind that we only ascertained ACEI use at the time of diagnosis and did not attempt to follow up its continuation. From the same data resource, we have previously reported an improvement in routinely measured cognitive function during the first 6–9 months following initiation of acetylcholinesterase inhibitor medication in dementia. Reference Perera, Khondoker, Broadbent, Breen and Stewart21 All patients included in the study reported here were receiving acetylcholinesterase inhibitor treatment, so that the more marked improvement in MMSE score trajectories in those also receiving C-ACEIs compared with patients who did not receive ACEIs cannot be attributed to cholinesterase inhibition alone. Furthermore, the relative improvement in patients receiving C-ACEIs compared with those receiving NC-ACEIs renders a generic effect of hypertension or antihypertensive treatment less likely, as does the research cited earlier which has tended to report findings specific to C-ACEIs. C-ACEIs and acetylcholinesterase inhibitors may therefore have mutually promoting actions on acetylcholine bioavailability, and consequently cognitive function, but this requires further investigation.
There is evidence that certain C-ACEIs (captopril, trandolapril and ramipril) and NC-ACEIs (enalapril) are associated with reduced all-cause and cardiovascular mortality from 18% to 40% among patients with hypertension. Reference Ferrari and Boersma43 However, ACEIs have been found to be associated with an increased risk of mortality in patients with Alzheimer's disease compared with those receiving angiotensin II receptor blockers or other antihypertensives and also shown that initial protective effect of ACEIs was disappeared after adjusting for protopathic bias. Reference Kehoe, Davies, Martin and Ben-Shlomo29 In our study we found no differences in survival between groups, which might possibly be explained by a balancing of these risk and protective effects.
Regarding strengths of our study, we were able to assemble a large sample of patients with newly diagnosed Alzheimer's disease and homogeneity of the comparison groups, as well as outcome data availability, was maximised by restricting to people receiving acetylcholinesterase inhibitor treatment. We were also able to obtain large numbers of cognitive function recordings and to follow patients up for 3 years after dementia diagnosis: a substantially greater period than most previous studies. Limitations are principally related to the fact that the data-set was derived from routine records-derived information rather than a ‘de novo’ research cohort. For example, MMSE scores were a relatively crude measure of cognitive function, diagnoses were clinical rather than research quality, and confounding factors were limited by available data. In addition, the group receiving NC-ACEIs was relatively small and could not be analysed in detail. Finally, the classification of centrally and non-centrally active agents was based predominantly on animal models because human data have been generally lacking and heterogeneous. Reference Wright, Reichert, Davis and Harding33 Although a compound's ability to cross the blood–brain barrier largely depends on its size, charge and lipophilicity, the integrity of the blood–brain barrier and the dose of the medication are also potentially influential. Reference Sink, Leng, Williamson, Kritchevsky, Yaffe and Kuller11 Importantly, all these limitations would be expected to obscure rather than exaggerate observed associations, and it is noteworthy that positive findings were still found to be present.
In summary, we found some evidence to support a time-limited effect of C-ACEIs on the trajectory of cognitive function in people with Alzheimer's disease receiving acetylcholinesterase inhibitor treatment. Visual inspection of the MMSE score trajectories in Fig. 1 suggests that cognitive function in people receiving C-ACEIs was either stable or improved compared with that at baseline for around 18 months after diagnosis, compared with around 9 months in those not receiving ACEIs. Whether this translates into delayed dependency and other functional outcomes requires further research, which is also required in order for C-ACEIs to be considered as treatment in the early stages of Alzheimer's dementia. Reference Rygiel44 As Rygiel has suggested, in clinical practice, practical suggestions for older people at risk of dementia that have hypertension or other medical conditions with indications for ACEIs, could be started on a C-ACEI instead of an NC-ACEI. Reference Rygiel44
Funding
The data resource, G.P. and R.S. are funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. G.P. additionally was funded by the British cross-research council Lifelong Health and Wellbeing (LLHW) programme under Extending Working Lives as part of an interdisciplinary consortium on Wellbeing, Health, Retirement and the Lifecourse (WHERL) (ES/L002825/1). R.H. is supported by the UCLH NIHR Biomedical Research Centre.









eLetters
No eLetters have been published for this article.