Worldwide, an estimated 25% of adults have reported physical abuse in childhood. In the UK, the most comprehensive overview of child protection states that there were 47 008 sexual offences and 10 136 cruelty and neglect offences recorded against children under the age of 16 in 2014/15.1 Childhood maltreatment has been associated with a wide range of negative health consequences, psychosocial outcomes and heightened risk for psychiatric disorders; including anxiety disorder, bipolar disorder, delinquent behaviour, impaired cognitive development and particularly depression.Reference Chapman, Whitfield, Felitti, Dube, Edwards and Anda2–Reference Hovens, Giltay, Spinhoven, van Hemert and Penninx4 Evidence suggests that numerous biological mechanisms become activated in response to maltreatment (for example epigenetic changes, telomere erosion, cortisol dysregulation, inflammation), which alone or in combination may explain the increased vulnerability to disorders such as depression, among adults who have been maltreated.Reference McCrory, De Brito and Viding5
Current understanding
Immunoinflammatory activation, and an increased release of proinflammatory cytokines, is one biological mechanism associated with childhood maltreatment and an area of growing interest in psychiatry. Cytokines play an important role in brain development and affect neurogenesis, synaptic remodelling and neurotransmitter systems to produce behavioural change.Reference Yirmiya and Goshen6, Reference Miller, Maletic and Raison7 Many studies have reported an increase in proinflammatory cytokines such as interleukin (IL)-1, IL-6 and tumour necrosis factor-alpha and increases in the acute phase protein C-reactive protein (CRP) in patients with major depressive disorder (MDD) and among those exposed to maltreatment.Reference Dowlati, Herrmann, Swardfager, Liu, Sham and Reim8 Whereas, others have reported protective effects of anti-inflammatory cytokines such as IL-10.Reference Roque, Correia-Neves, Mesquita, Palha and Sousa9
Aims
The current study investigated the association of inflammatory marker levels in response to childhood maltreatment. Among patients with MDD, we tested whether individuals who had been maltreated had specific differences in levels of inflammatory markers compared with those patients with MDD who had not experienced maltreatment. The rationale for this was to determine if individuals with MDD who had been maltreated represent those with an inflammatory subtype of depression; which could lead to differential diagnoses (for example depression with risk for inflammatory disease) and subsequently novel intervention strategies (such as anti-inflammatory adjuvants).
Among control participants, we tested whether individuals who had been maltreated had altered inflammatory marker levels, compared to those individuals who had not been maltreated. The rationale for this was to identify whether alterations to specific components of the immune system might mark MDD resilience in response to maltreatment, and therefore hint towards a novel treatment strategy.
We addressed these aims by assessing 41 inflammatory markers in a homogeneous treatment-resistant MDD cohort recruited as part of the Antiglucocorticoid augmentation (metyrapone) of antiDepressants in Depression (ADD) study (n = 164), and screened controls recruited as part of the South East London Community Health Study (SELCoH, n = 301). In the case and control groups separately, we investigated the association of inflammatory markers with the presence of childhood maltreatment.
Method
Participants
Peripheral blood samples used in this study were obtained by venepuncture as part of two separate UK studies. Controls were recruited as part of SELCoH and participants with MDD were recruited as part of the ADD study. Childhood maltreatment information was collected within both studies. After collection, serum from both studies were stored at −80°C until required. Participant information relating to each study is detailed in Table 1 and a description of each study is given below.
Table 1 Characteristics of the case and control groups
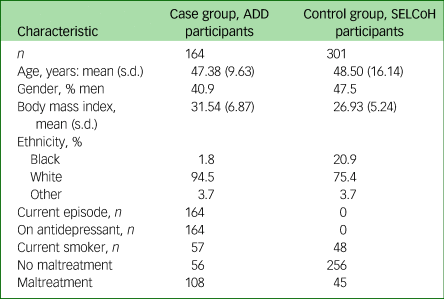
ADD, Antiglucocorticoid augmentation (metyrapone) of antiDepressants in Depression (ADD) study; SELCoH, South East London Community Health Study.
Control group
The participants in the control group were recruited as part of SELCoH, which is a study in London, UK investigating mental and physical health in the general South East London population.Reference Hatch, Frissa, Verdecchia, Stewart, Fear and Reichenberg10 Participants in this study received detailed and repeated phenotypic assessments as part of three separate phases. The first phase was carried out to assess common health disorders and mental health disorders in South East London; the second phase aimed to examine the roles of historical social context and policy in shaping patterns of health inequalities; and in the third phase, a number of biological specimens were collected from a subset of participants including blood for serum separation. All phases collected information on psychiatric disorder symptoms. Control participants were defined as those who showed no MDD symptoms in any of the three phases of SELCoH, determined using the Clinical Interview Schedule-Revised,Reference Lewis, Pelosi, Araya and Dunn11 and who had no previous diagnosis of depression, determined by a self-report questionnaire.
Case group
The participants with MDD (case group) were recruited as part of the ADD study, which was a clinical trial that aimed to assess the efficacy of metyrapone (a cortisol synthesis inhibitor) as an adjuvant to selective serotonin reuptake inhibitors (SSRIs) in treating MDD in those previously shown not to respond to at least two forms of treatment (treatment-refractory MDD).Reference Ferrier, Anderson, Barnes, Gallagher, Grunze and Haddad12 Participants were recruited from multiple UK centres, which included Manchester, Leeds, Bradford and Newcastle.
Major depression diagnoses were defined using DSM-IV criteria13 and assessed using the Structured Clinical Interview for DSM Disorders research version.Reference First, Gibbon, Spitzer, Williams and Benjamin14 Further eligibility criteria required participants to have a Hamilton Rating Scale for Depression (HRSD) score greater than 18;Reference Hamilton15 have a Massachusetts General Hospital Treatment Resistant Depression staging score of 2–10;Reference Fava16 be currently taking an SSRI; be aged between 18 and 65; not have alcohol or drug dependence; be free of physical comorbidities (untreated hypothyroidism, disorders of steroid production, cardiac failure, angina, myocardial infarction, renal failure in the past 3 years); and not take a medication that would contraindicate metyrapone. We utilised blood serum collected during the screening phase of the study.
Mild and major depression symptoms at the time of blood collection, were differentiated using the HRSD, where HRSD scores of 18–19 were considered mild symptoms (no participants were recruited with a HRSD <18), and HRSD scores of 20 and above were considered moderate–severe symptoms, as described previously.Reference Hamilton15
Childhood maltreatment measure
The presence of childhood maltreatment was determined using the Childhood Trauma Questionnaire (CTQ).Reference Bernstein, Stein, Newcomb, Walker, Pogge and Ahluvalia17 CTQ data in our data-sets were positively skewed and remained non-normal even after log-transformation. Consequently, we generated ordinal mean maltreatment measures,Reference Bernstein, Stein, Newcomb, Walker, Pogge and Ahluvalia17 which, because of the small numbers of individuals with severe maltreatment, we collapsed into ‘no maltreatment’ (0) and ‘maltreatment’ (mild, moderate or severe maltreatment) (1), (Table 1).
Ethics
For the ADD study, clinical trial authorisation was given by the Medicines and Healthcare products Regulatory Agency (MHRA: EudraCT: 2009-015165-31). Ethical approval was granted by the Sunderland Local Research Ethics Committee (REC reference number 10/H0904/9). The SELCoH study received approval from the King's College London research ethics committee, reference PNM/12/13-152. Participants from both studies provided written informed consent.
Inflammatory marker quantification
Upon use, serum was thawed at room temperature and 41 inflammatory markers were quantified simultaneously using multiplex enzyme-linked immunosorbent assay-based technology provided by the Meso Scale Discovery V-PLEX Plus Human Biomarker 40-Plex kit, and a customised human duplex kit assaying brain-derived neurotrophic factor and interferon-alpha, as described previously.Reference Powell, Gaspar, Chung, Keohane, Gunasinghe and Uher18 Seven-point standard curves were run in duplicate on each plate in order to calculate absolute pg/mL values for the 80 samples assayed per plate, and a no-template control was used to correct for background fluorescence. Case and control groups were randomised across batches, and plates were scanned on the Mesoscale Scale Discovery Meso Quickplex SQ 120 reader at the Social, Genetic and Developmental Psychiatry Centre, Institute of Psychiatry, Psychology and Neuroscience, King's College London.
Pilot studies revealed very high intraplate (r>0.99) and interplate (r>0.97) correlations, suggesting single measurements were acceptably reliable using this methodology. Furthermore, known quantities within the standard curves used on each plate, correlated very highly with quantities predicted by fluorescence intensity (r>0.99).
Statistical analysis
Data processing
Standard curves were used to determine absolute quantities (pg/mL) of each inflammatory marker. Absolute quantities (pg/mL) were then log-transformed to allow for parametric analyses. Subsequently, data points were removed if they exceeded plus or minus 2 standard deviations from the mean. We excluded inflammatory markers where greater than 30% of data was missing.
Maltreatment analyses
For the case and control groups separately, we performed linear regressions with log-protein level as the dependent variable and childhood maltreatment as the independent variable, alongside ethnicity, smoking, antidepressant use, study site (ADD study), plate/batch effects, gender, current depressive episode severity (ADD study), age and BMI as covariates. Within our analyses we applied the Bonferroni method of multiple testing correction, in order to minimise risk for false associations.
Sensitivity analyses
We individually tested the potential mediating/confounding effects of physical illness (type 2 diabetes, arthritis, cardiovascular disease, stroke, high blood pressure and cancer) and socioeconomic status, within the SELCoH study, where this data was available. Physical illness information was obtained via self-report. Socioeconomic status was determined based on an individual's type of employment: manual work, non-manual work, unemployed and economically inactive (such as retired, full-time parents, students or those unable to work because of disability). Based on our previous work (which included SELCoH),Reference Powell, Gaspar, Chung, Keohane, Gunasinghe and Uher18 we also attempted to replicate the effects of BMI on levels of CRP and IL-6 (commonly shown to be associated with maltreatment and MDD) in the ADD study, using the same model as above.
Results
Inflammatory markers adequately detected in serum
Using our methodology, 34 inflammatory proteins passed our quality control criteria. Seven inflammatory markers were found to have greater than 30% missing data from across the whole sample and were removed from any downstream analyses (macrophage inflammatory protein-1a, granulocyte-macrophage colony-stimulating factor , IL-1a, IL-13, IL-1b, IL-2, IL-4). See Fig. 1 for a summary of detectable inflammatory markers. Known quantities within the standard curves used on all plates, correlated very highly with quantities predicted by fluorescence intensity (r>0.99), results also showed acceptably low levels of intraplate and interplate variability based on coefficient of variation metrics. For further details on correlations between different inflammatory markers, and assay variability metrics, see supplementary Tables 1 and 2 available at https://doi.org/10.1192/bjo.2018.80.
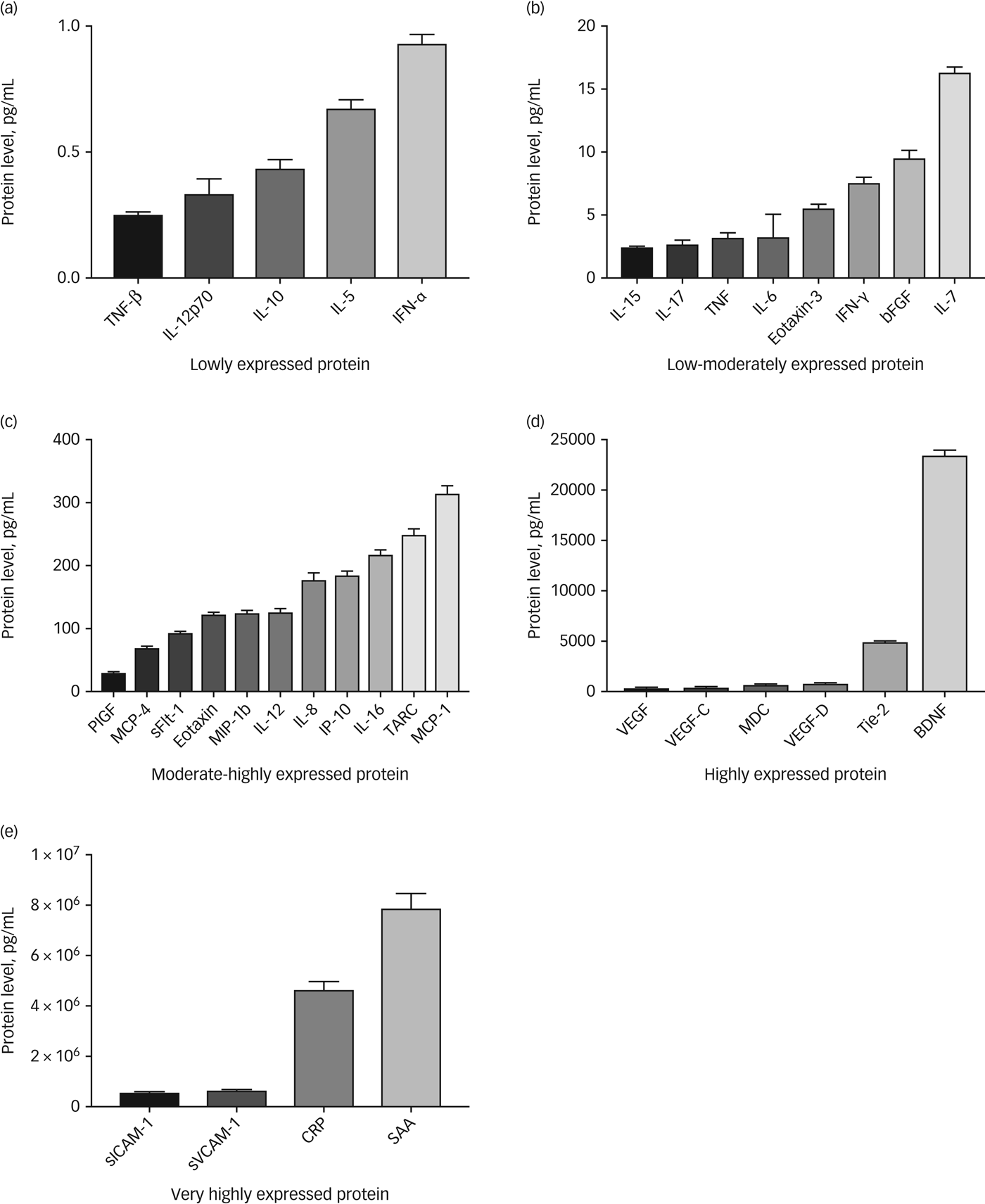
Fig. 1 Detectable inflammatory markers.
(a) Lowly expressed protein, < 1 pg/mL; (b) low–moderately expressed protein, 1 – 20 pg/mL; (c) moderate–highly expressed protein, 21 – 400 pg/mL; (d) highly expressed protein, 401 – 25,000 pg/mL; (e) very highly expressed protein, 25,001 – 100,000,000 pg/mL. Bars represent the mean and error bars represent the standard error of the mean. TNF, tumour necrosis factor; IL, interleukin; IFN, interferon; bFGF, basic fibroblast growth factor; PIGF, phosphatidylinositol glycan biosynthesis class F protein; MCP, monocyte chemoattractant protein 4; sFLT, soluble fms-like tyrosine kinase; MIP, macrophage inflammatory protein; IP, induced protein; TARC, chemokine (C-C motif) ligand 17 (also known as CCL17); VEGF, vascular endothelial growth factor; MDC, macrophage-derived chemokine; Tie, tyrosine kinases with immunoglobulin-like and EGF-like domains; BDNF, brain-derived neurotrophic factor; sICAM, soluble intercellular adhesion molecule; sVCAM, soluble vascular cell adhesion molecule; CRP, C-reactive protein; SAA, serum amyloid A.
Effect of maltreatment on inflammatory marker levels in case and control groups
We found no significant associations between childhood maltreatment and levels of inflammatory markers in controls (Table 2). We found one nominally significant association between maltreatment and levels of an inflammatory marker in the MDD case group, whereby, childhood maltreatment was associated with a reduction in circulating levels of serum amyloid A in adulthood (F(1, 143) = 4.837, P = 0.029, variance explained, 3.3%). This effect did not remain significant following multiple testing correction (Table 2).
Table 2 Analysis of associations between childhood maltreatment and levels of inflammatory markers in the case and control groups
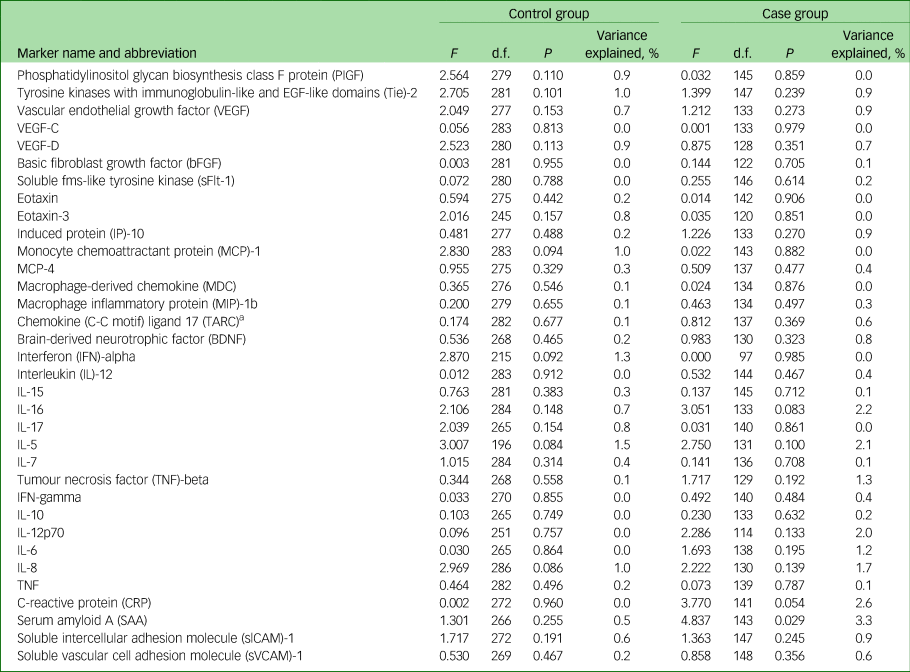
a. Also known as CCL17.
Sensitivity analyses
We found no significant effects of physical illness, or socioeconomic status on inflammatory markers (P>0.05). We replicated our previous work, showing strong positive correlations between BMI and CRP levels (F(1, 86) = 29.489, P = 5.137 × 10–7, variance explained, 25.5%), and between BMI and IL-6 levels (F(1, 85) = 32.994, P = 1.403 × 10–7, variance explained, 28%), in the ADD study.
Discussion
Main findings
Inflammation is one putative risk mechanism linking childhood maltreatment to adulthood depression. This study sought to investigate whether childhood maltreatment evokes differential effects on inflammatory markers among participants with MDD and controls. We studied the effects of maltreatment on 41 inflammatory markers in a UK sample of individuals with MDD and screened controls while accounting for a broad range of confounding factors.
We found no significant associations between childhood maltreatment and inflammatory markers in the control group, suggesting that inflammation may not represent a mechanism conferring resilience to MDD in response to maltreatment. Likewise, we did not find a significant association between childhood maltreatment and inflammatory markers among the case group, suggesting there may not be an inflammatory subtype of MDD related to childhood maltreatment exposure.
Interpretation of our findings
Our negative results differ from the more common reports of higher CRP and IL-6 levels in those with a history of childhood maltreatment.Reference Danese, Moffitt, Pariante, Ambler, Poulton and Caspi3, Reference Gouin, Glaser, Malarkey, Beversdorf and Kiecolt-Glaser19 A lack of replication here could relate to the fact that not all studies have covaried for a broad range of confounding factors as we do. For instance, studies have revealed that childhood maltreatment is associated with higher adulthood BMI, and consequently this could partially mediate previously reported associations.Reference Widom, Czaja, Bentley and Johnson20–Reference Power, Pinto Pereira and Li22 Indeed, we have recently reported major influences of BMI (as well as other factors) on inflammatory marker levels, namely CRP and IL-6, which once covaried for, removes MDD case–control differences in inflammatory marker levels.Reference Powell, Gaspar, Chung, Keohane, Gunasinghe and Uher18 Here, we replicate the effects of BMI on CRP and IL-6 levels in the ADD study, confirming the necessity to appropriately covary for BMI and other confounders in statistical models relating to inflammatory measures.
The effect of BMI on inflammatory levels likely results from the fact that adipose tissue is known to release adipokines and proinflammatory cytokines.Reference Fain23–Reference Fantuzzi25 Therefore, regardless of what causes heightened inflammation among the participants with MDD or individuals who were maltreated, weight management via balanced diets and regular exercise, may be one method to reduce excessive inflammation.
Strengths and limitations
The strengths of the current study include the fact we assessed a broad range of inflammatory proteins using validated electrochemiluminescence methods; we used a well-characterised method of assessment for childhood maltreatment subtypes; we statistically corrected for a number of confounding factors and performed appropriate sensitivity analyses; and we performed analyses in a screened control group and a homogeneous treatment-resistant MDD cohort.
However, the study also has a number of limitations that should be acknowledged. First, and foremost, our study is of a cross-sectional design capturing inflammation levels only in adulthood. A longitudinal design would allow one to determine the temporal ordering of events, and measure how maltreatment has an impact on inflammation immediately, during adolescence and then in adulthood; identifying acute and persistent effects, if any, on inflammatory markers. Second, maltreatment was captured as a binary variable and we were underpowered to assess the effects of maltreatment severity. As the majority of a individuals who had been maltreated in our two samples experienced mild–moderate maltreatment as opposed to severe maltreatment, it is possible that the biological embedding effects of stress are less penetrant in our sample, which is why we did not observe broad effects on inflammatory marker levels.
Third, all the participants with MDD were currently on antidepressant treatment, as per the recruitment criteria. As antidepressants are known to possess some anti-inflammatory properties, it is possible this may be masking the long-term effects of maltreatment on immunoinflammatory function.Reference Hashioka, McGeer, Monji and Kanba26 Fourth, the CTQ measure of childhood maltreatment is widely used and although reliable, arguably lacks validity and is subject to the biases of retrospective recall, especially in individuals with high neuroticism.Reference Frissa, Hatch, Fear, Dorrington, Goodwin and Hotopf27 Despite this, it is important to note that recent research shows a moderate–strong positive correlation between CTQ and prospective measures of maltreatment, collected as part of longitudinal studies, validating the usefulness of the CTQ as a tool for assessing maltreatment severity.Reference Liebschutz, Buchanan-Howland, Chen, Frank, Richardson and Heeren28
Fifth, there may be other confounding factors influencing inflammatory marker levels that we were not able to include here (such as time and season of blood collection) that may have affected our results. Finally, despite representing one of the larger single studies to date assessing disorder-specific effects of maltreatment, we may still be underpowered; therefore, future studies with even larger sample sizes may be able to confirm our largely negative findings.
In conclusion, our study does not support previous research revealing associations between childhood maltreatment and inflammatory markers in either participants with MDD or controls. Instead, our findings suggest that other factors such as BMI may be more pertinent in influencing inflammatory marker levels.
Funding
A.B.P., R.C., S.L.H., R.S., M.H., A.H.Y., A.J.C. and G.B. are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and (Institute of Psychiatry, Psychology & Neuroscience) King's College London. A.B.P.'s PhD studentship is funded by a Rayne Foundation grant awarded to T.R.P., G.B. and S.T. T.R.P. is funded by a Medical Research Council Skills Development Fellowship (MR/N014863/1). The current study was funded by an Eli Lilly LRAP grant awarded to G.B., H.W., D.A.C. and T.R.P. The ADD study was supported by the Efficacy and Mechanism Evaluation (EME) Programme (reference number 08/43/39), funded by the Medical Research Council (MRC) and managed by the NIHR on behalf of the MRC–NIHR partnership. SELCoH was supported by the Biomedical Research Nucleus data management and informatics facility at South London and Maudsley NHS Foundation Trust, which is funded by the NIHR Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London and a joint infrastructure grant from Guy's and St Thomas' Charity and the Maudsley Charity. Phase 3 of the SELCoH study was also funded by the Maudsley Charity. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The funding sources had no role in the study the design, in the collection, analysis, and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Acknowledgements
We would like to thank all those involved in the collection and participation of the SELCoH and ADD studies.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2018.80.






eLetters
No eLetters have been published for this article.