A diagnosis of dementia is usually made following specialist review that includes a clinical history and examination involving patients and carers, a dementia blood screen, cognitive assessment, assessment of activities of daily living, behavioural problems and sometimes brain imaging.Reference Rubinsztein, Van Rensberg and Al-Salihy1,2 Although the Mini-Mental State Examination (MMSE) is still the gold standard tool employed in research and clinical trials, the Addenbrooke's Cognitive Examination-III (ACE-III) screening tool is increasingly used in UK memory clinics as an alternative to the MMSE. It is a practical bedside test, easy to use and freely accessible to all, unlike the MMSE and Montreal Cognitive Assessment (MoCA), which are both subject to copyright agreements.Reference Folstein, Folstein and McHugh3,Reference Nasreddine, Phillips and Bedirian4 A shorter version, the Mini-Addenbrooke's Cognitive Examination (M-ACE), has also been developed and is considered helpful where patients struggle with longer cognitive examinations; the M-ACE has validated cut-offs of ≤25/30 and ≤21/30 in screening for dementia versus no dementia.Reference Hsieh, McGrory and Leslie5
There is inconsistent evidence on how to categorise dementia severity. Clinicians and researchers have often used the MMSE to help them in defining clinical staging, with the following recommended ranges and cut-offs: mild: 21–26 (cut-off ≤26); moderate: 10–20 (cut-off <21); severe: <10.2 We examined similar guiding cut-off scores to stage dementia using the ACE-III and M-ACE. We also examined how cognition correlated with everyday functioning and neuropsychiatric questionnaires.
Method
We used a cross-sectional study design.
Participants and data collection
Patients were identified by clinicians in secondary mental health services. We included patients over 50 years of age with a clinician-diagnosed dementia (all subtypes and severity) who were sufficiently clinically stable to undertake the assessment. Patients had to have a study partner (‘carer’, defined as someone who knew the person well, had contact with them for at least 1 h per week) and were happy to complete informed consent and answer questions about the person's functional abilities. All patients and carers received separate study information sheets and signed consent forms before any study activity took place. A mental capacity assessment was undertaken as part of the informed consent process. Patients lacking capacity were included and we used a separate consultee assent process for these individuals.
All study procedures comply with the ethical standards of relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The study took place within the demenTia Research and Care Clinic (TRACC) study at the University of East Anglia (IRAS ID: 205788) with ethics approval.
Description of instruments
Global cognition
The Addenbrooke's Cognitive Examination-III (ACE-III) is a 26-item cognitive scale measuring the following domains: attention, memory, language, fluency and visuospatial. A higher total suggests less cognitive impairment, with a total possible score of 100.Reference Hsieh, Schubert and Hoon6
The Mini-Addenbrooke's Cognitive Examination (M-ACE), which is derived from the ACE-III, assesses the attention, memory, verbal fluency and visuospatial (clock drawing) domains.Reference Hsieh, McGrory and Leslie5 A higher total suggests less cognitive impairment, with a total possible score of 30.
The Mini-Mental State Examination (MMSE) is a brief 30-item screening tool assessing domains similar to those in the ACE-III and M-ACE, with a higher total indicating less cognitive impairment.Reference Folstein, Folstein and McHugh3 Traditionally used widely in clinical practice, its clinical utility is now affected by being subject to copyright. The copyright version (the MMSE-2)Reference Folstein, Folstein and McHugh7 was used for our study with permission of PAR (parinc.com). The MMSE2 is analogous to the original and, in the interest of ease of reading, is referred to as the MMSE throughout this paper.
Behaviour and everyday function
The Cambridge Behavioural Inventory-Revised (CBI-R) is a 45-item scale on which carers are asked to rate the frequency (never to constantly) of changes, cognitive and behavioural (i.e. memory and orientation, everyday skills, self-care), observed over the previous month.Reference Wear, Wedderburn and Mioshi8
The Frontotemporal Dementia Rating Scale (FTD-FRS) is a novel tool assessing neuropsychiatric symptoms (i.e. lack of affection, impulsivity) and everyday functioning (e.g. going out, shopping, household chores, using a telephone, taking medications). The tool has previously been used in determining disease progression and severity stage in the subtype frontotemporal dementia; it is also useful in other dementia subtypes and the semi-structured interview format was considered useful to complement the CBI-R for the purposes of our study.Reference Mioshi, Hsieh and Savage9
Statistical analysis
Data were analysed using SPSS Statistics 25 for Windows.10 Before any analysis, variables were plotted and checked for normality of distribution using the Shapiro–Wilk test. Pearson correlations were conducted to investigate relationships between the total scores on the three scales included in this analysis (ACE-III, M-ACE, MMSE).
MMSE scores for mild, moderate and severe dementia were stratified using National Institute for Health and Care (NICE) guidelines (mild: MMSE score ≤26; moderate: <20; severe: <10).2 Scatterplots were calculated to compare the raw scores between the MMSE and the ACE-III and the MMSE and the M-ACE. This exploration was based on similar conversion analyses.Reference Law, Connelly and Randall11 Corresponding conversion scores were calculated for the ACE-III and M-ACE using a regression score derived from the linear relationship (y = a + bx, where x is the independent variable, y is the dependent variable, b is the slope and a is the y-intercept) between the scales from these scatterplots. This created binary variables for logistic regression, which was used to model the probability of mild or moderate cut-offs. Receiver operating characteristic (ROC) analysis was employed to measure the accuracy of the different cut-offs (mild and moderate) on the ACE-III compared with the MMSE and on the M-ACE compared with the MMSE. There was further exploration, using ROC analysis, of the three cognitive scales and the CBI-R and FTD-FRS.
Results
Participants
We recruited 80 patients and their carers over a 2-year period from March 2017 to April 2019. All were of White British or Irish ethnicity, with English as their first language. The majority of participants were educated to secondary school level (60%), with a small proportion (23%) receiving further education. Alzheimer's dementia was the most common diagnosis, followed by dementia with Lewy bodies (DLB), mixed dementia and vascular dementia; one individual had a diagnosis of Parkinson's disease dementia. Mean scores on the three cognitive instruments were: MMSE, 20.7 (s.d. = 5.7); ACE-III, 63.7 (s.d. = 15.7); and M-ACE, 12.5 (s.d. = 6.3).
Of the 80 participants recruited into the study, 15 were removed from the ACE-III analysis and 9 were removed from the M-ACE analysis because of missing data.
Relationship of MMSE scores with ACE-III and M-ACE scores
Regression equations for determining ACE-III and M-ACE cut-offs based on the MMSE NICE guideline cut-offs were based on the linear relationship between these scales using a scatterplot.
The regression equations were:
The scatterplot in Fig. 1 shows the relationship between raw scores on the MMSE and the ACE-III and on the MMSE and the M-ACE.
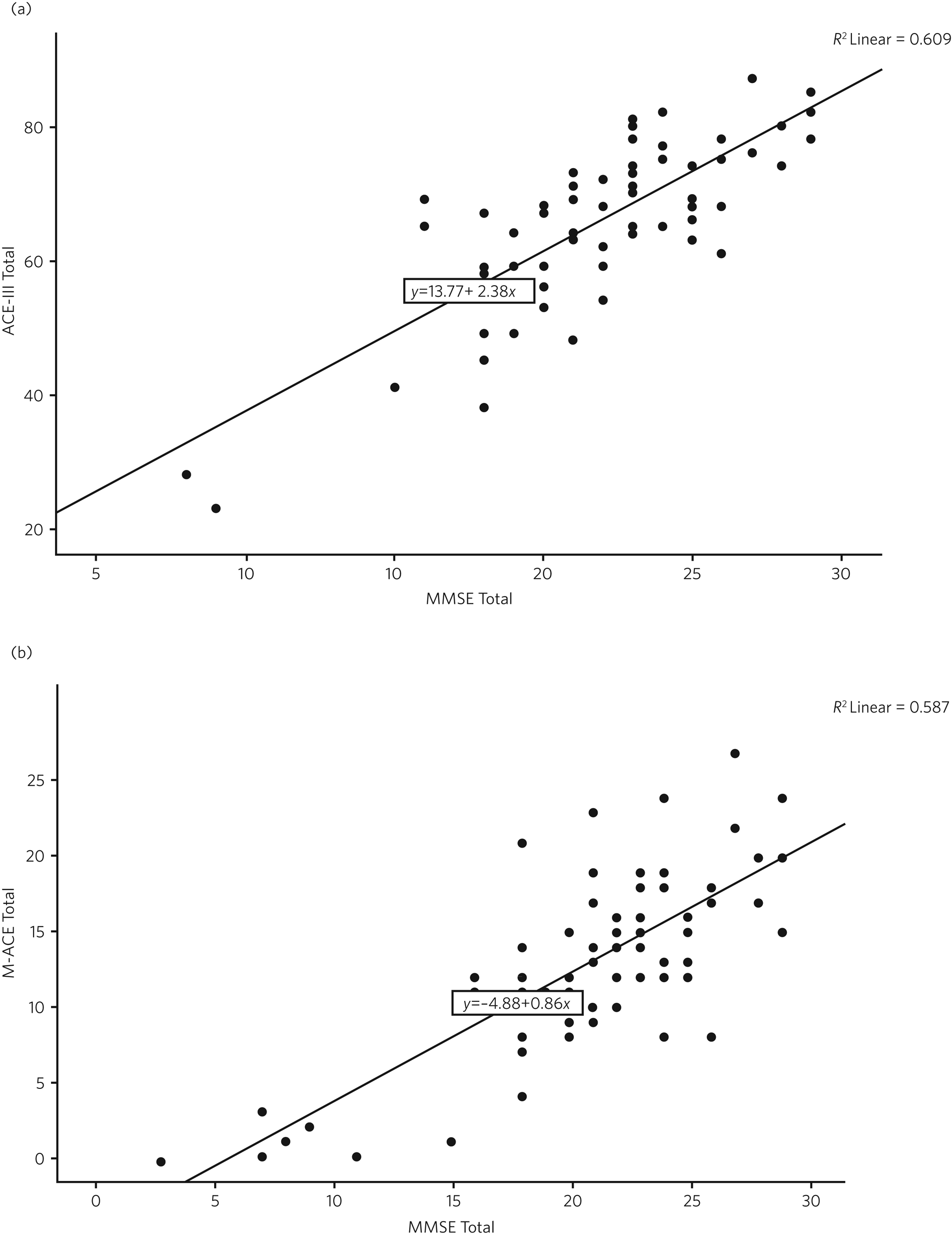
Fig. 1 (a) Scatterplots of raw scores for the Mini-Mental State Examination (MMSE) and Addenbrooke's Cognitive Examination-III (ACE-III). (b) Scatterplot of raw scores for the MMSE and the Mini-Addenbrooke's Cognitive Examination (M-ACE).
Examining the raw scores, a score of 76/100 on the ACE-III approximately equates to the highest ‘mild dementia’ score on the MMSE (26), a score of 59/100 on the ACE-III equates with the top of the moderate range on the MMSE (<20). The Pearson correlation coefficient between the ACE-III and the MMSE total scores was 0.78 (P = 0.001).
For the M-ACE according to the raw scores, a score of 19/30 on the M-ACE is approximately equal to or less than 26 (mild) on the MMSE, a total M-ACE score of 13/30 is approximately equal to or less than 20 (moderate) on the MMSE. The Pearson correlation coefficient between M-ACE and MMSE total scores was 0.77 (P = 0.001).
There were insufficient data on both scales to calculate meaningful scores for severe dementia.
Sensitivity and specificity analysis
Sensitivity and specificity analyses were performed using logistic regression and ROC analysis to determine the effectiveness of the ACE-III and M-ACE cut-off scores derived from the MMSE NICE cut-off scores using the regression equations determined above. Mild and moderate groups determined by these new cut-off scores were explored using MMSE performance.
ACE-III
Twelve patients were removed from the analysis exploring ACE-III mild and moderate groups because they scored above the threshold for the mild group, as determined by the conversion analysis above based on the MMSE (ACE-III score <76). Logistic regression for the mild and moderate groups determined by the new ACE-III cut-offs indicated that the regression model based on the MMSE score predictor was statistically significant (χ2(1) = 18.86, P < 0.001). The model explained 42.4% (Nagelkerke R 2) of the variance in ACE-III group severity and correctly classified 77.4% of participants with mild and moderate dementia scores (33/37 (89.2%) with mild; 8/16 (50.0%) with moderate) into their respective cohorts.
Figure 2(a) shows the ROC analysis for mild dementia on the ACE-III according to scores on the MMSE. For an ACE-III cut-off for mild dementia of 76/100, area under the curve analysis (AUC) was estimated at 0.85 (s.e. = 0.05, 95% CI 0.75–0.95), which is significantly different from AUC = 0.5 (indicating no discrimination at P < 0.001). For a cut-off for moderate dementia of 59/100, the AUC was estimated at 0.15 (s.e. = 0.05, 95% CI 0.05–0.25), which showed poor sensitivity and specificity to detect the moderate disease stage (Supplementary Fig. 3(a) available at https://doi.org/10.1192/bjb.2023.27).
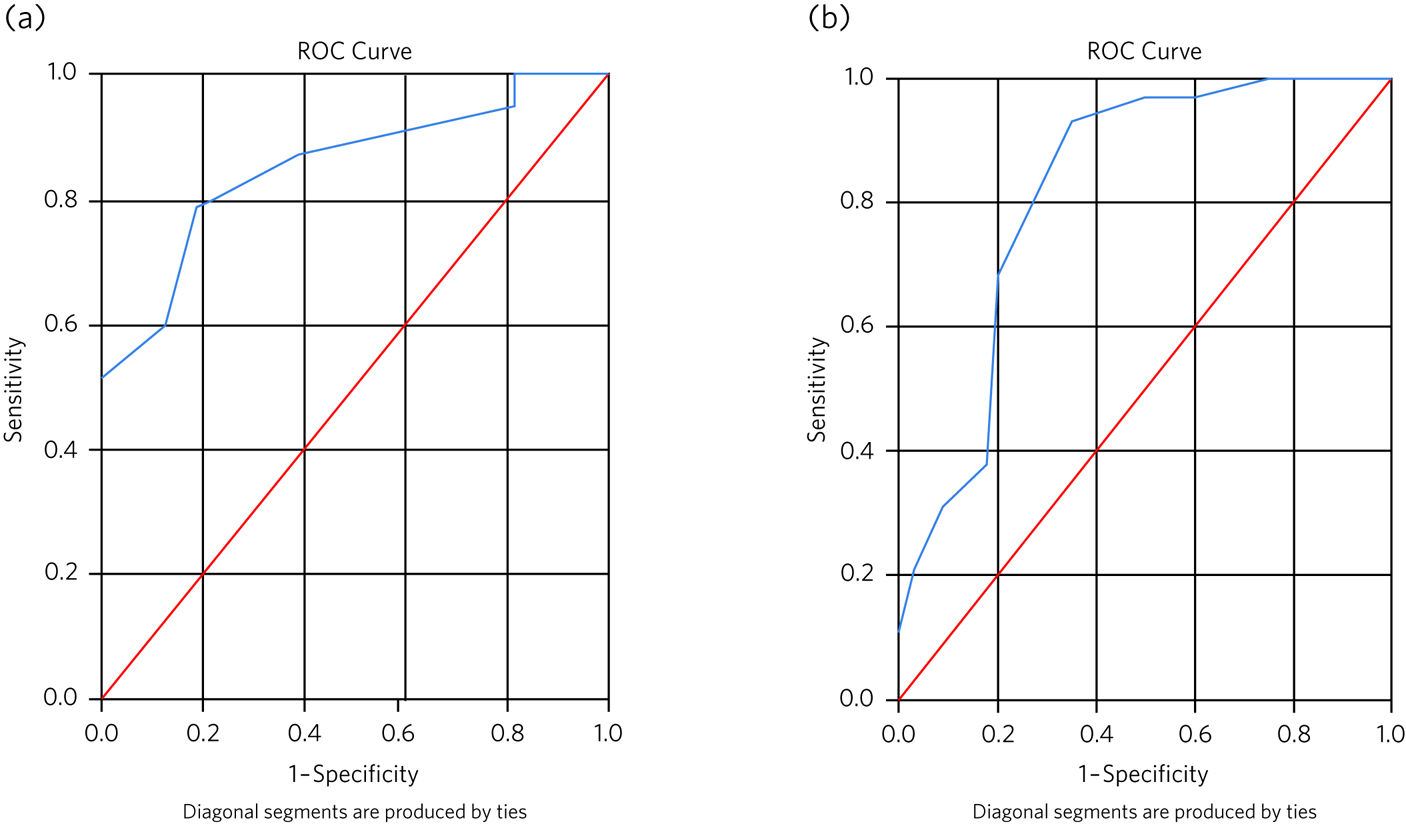
Fig. 2 (a) The receiver operating characteristic (ROC) curves for the Addenbrooke's Cognitive Examination-III (ACE-III) with a cut-off of 76 (mild dementia) when associated with the Mini-Mental State Examination (MMSE). (b) The receiver operating characteristic curves for the Mini-Addenbrooke's Cognitive Examination (M-ACE) with a cut-off of 19 (mild dementia) when associated with the MMSE.
M-ACE
Eight patients were removed from the analysis of M-ACE groups based on the new cut-offs because they scored above the upper cut-off for the mild group, as determined by the conversion analysis above based on the MMSE (M-ACE score <19). Logistic regression indicated that the regression model based on the MMSE total score predictor was statistically significant (χ2(1) = 24.01, P < 0.001). The model explained 42.3% (Nagelkerke R 2) of the variance in M-ACE group severity and correctly classified 76.2% of participants with mild and moderate dementia scores (2/29 (79.3%) with mild; 25/34 (76.2%) with moderate) into their respective cohorts.
Figure 2(b) shows the ROC analysis for mild dementia on the M-ACE according to scores on the MMSE. For a M-ACE cut-off for mild dementia of 19/30, the AUC was estimated at 0.81 (s.e. = 0.05, 95% CI 0.71–0.93), which is significantly different from AUC = 0.5 (P < 0.001). For a cut-off for moderate dementia of 13/30, the AUC was estimated at 0.18 (s.e. = 0.05, 95% CI 0.08–0.29), which showed poor sensitivity and specificity to detect the moderate disease stage (Supplementary Fig. 3(b)).
Functional performance at ACE-III and M-ACE cut-offs
There were low to moderate negative correlations between the cognitive scales and the CBI-R and the ACE-III (r = −0.24, P = 0.05), M-ACE (r = −0.29, P = 010) and MMSE (r = −0.34, P = 0.005) and low to moderate positive correlations between the FTD-FRS logit score and the ACE-III (r = 0.35, P = 0.003), M-ACE (r = 0.38, P = 0.001) and MMSE (r = 0.47, P < 0.001). Logistic regression and ROC analysis were not significant at the ACE-III- and M-ACE-derived mild and moderate cut-offs for both functional scales.
Discussion
We found that a score ≤76 on the ACE-III and ≤19 on the M-ACE correlated well with MMSE cut-offs for mild dementia, with a good fit on the ROC analysis for both the ACE-III and the M-ACE. However, with moderate dementia, examining the scatterplots of the raw scores for the MMSE against the ACE-III and the M-ACE (Supplementary Fig. 3(a) and (b)) there is a lot of scatter around moderate cut-off points on the MMSE, suggesting that it will be inherently difficult to compare cut-off points at this stage of disease using the MMSE versus the ACE-III and M-ACE. The ROC analysis showed a poor fit in this model in the moderate range for both the ACE-III and the M-ACE and was not sensitive or specific in moderate dementia stages. This may be due, in part, to problems in performing cognitive testing in patients with more severe symptoms, where some individuals may not be able to cope with completing the cognitive scales at all or may be able to tolerate only partial completion. Therefore we recommend that cut-offs suggested for the moderate dementia stage in this study should be interpreted and used with caution.
Comparison with the literature
A Cochrane review highlights an overall lack of evidence on the detection of dementia using the ACE-III and M-ACE. The review authors concluded that there was insufficient evidence to recommend the ACE-III and M-ACE for screening dementia and mild cognitive impairment. Interestingly, they found a lack of evidence of efficacy in primary care but found that these tools may be useful in secondary services when, using the lower thresholds of 82 for the ACE-III and 21 for the M-ACE, fewer false-positive dementia diagnoses (versus no dementia) were reported.Reference Beishon, Batterham and Quinn12 Additionally, evidence suggests that the optimal cut-offs for distinguishing dementia from mild cognitive impairment using the M-ACE are one point lower than those reported in the original validation study for both the high sensitivity (≤24/30 v. ≤25/30) and high specificity (≤20/30 v. ≤21/30) cut-offs.Reference Larner13 Another study suggested that an ACE-III cut-off of 61 may be helpful in differentiating between mild and moderate dementia, but results were regarded as exploratory as the number of patients included was small.Reference Giebel and Challis14 Our study, as well as consolidating previous ACE-III findings, provides, for the first time, cut-offs for the M-ACE for mild and moderate dementia. This is important as the M-ACE is now used routinely in clinical practice. We have established cut-offs to be used as a guide, clinically and in research, to define mild and moderate dementia, by comparing with the established MMSE cut-offs.
Cut-offs for severe dementia
Owing to the small number of patients with severe dementia, we were not able to robustly establish the cut-offs for the severe stage on either the ACE -III or the M-ACE. It is particularly challenging to recruit individuals with severe dementia to research studies and they can find it difficult to complete cognitive rating scales. Arguably, in clinical practice it is easier to classify the advanced/severe dementia stage as these patients may be unable to complete cognitive scales and have lost many functional skills. This emphasises the need for clinicians to combine clinical judgement with interpretation of rating scale scores in dementia diagnosis and staging.
Correlations between cognition, behaviour and functioning
Additionally, we explored correlations between patients’ cognition and their behaviour and functioning using two functional scales: the CBI-R (self-completed) and the FTD-FRS (semi-structured interview). Both completed by the carer, the self-completion aspect of the CBI-R may facilitate honest expression and the interview process of the FTD-FRS may encourage open reflection. It may be that using a combination of both scales will be of benefit in a clinical assessment. The low to moderate correlations between the cognitive scales (ACE-III and M-ACE) and the functional scales (CBI-R and FTD-FRS) are to be expected and replicate previous findings.Reference Hsieh, Schubert and Hoon6 Cognitive scales have an emphasis on cognition and language, whereas the functional scales assess behaviour and function; therefore a high score on a cognitive scale does necessarily exclude moderate or severe dementia. It is recognised that functional impairments are linked to cognitive deficits, but precise understanding of this complex link is lacking.Reference Wear, Wedderburn and Mioshi8,Reference Giebel and Challis14
Combining a cognitive tool with a functional tool is likely to produce a more accurate picture of dementia severity. A clinician will interpretate the cognitive score and the functional abilities of the patient in order to stage the severity of a dementia. This process is important as it allows patients, carers and clinicians to consider the most appropriate treatment options and it informs decision-making and advance planning.2 Our findings add to the current body of evidence and are in accordance with NICE guidance and recent Cochrane evidence.2,Reference Beishon, Batterham and Quinn12
Patient sample
The sample of patients engaged in our study is very typical of those seen in old age psychiatric memory clinics in the UK in terms of diagnostic range and age.Reference Rubinsztein, Van Rensberg and Al-Salihy1,Reference Cook, Nichol and Isaacs15 The DLB subtype was the second most common diagnosis, which may have been raised in our sample as we were a recruiting site for the DIAMOND-Lewy study, which focuses on identification of DLB using a toolkit.Reference O'Brien, Taylor and Bamford16 The mean age was 78 years (range 58–90), with 46% (n = 37) in the older old age range (80–90 years). Increasing age is the main risk factor in the development, and in the worsening, of dementia, and the average age of 78 in our sample may partly explain the lower ACE-III cut-off of 76 in the mild group found in our study compared with the previous evidenceReference Hsieh, Schubert and Hoon6 (where the commonly employed ACE-III cut-off to delineate mild dementia was below 82). Our study was also not a comparison study against normal controls as in the previous work.Reference Hsieh, Schubert and Hoon6 The cut-off for moderate dementia in our study is lower than in previous evidence,Reference Giebel and Challis14 which may be explained by the larger sample size, more severely affected patients and the slightly older group of patients in our study. Furthermore, the inclusion of participants who lacked capacity in our study adds to the overall practical clinical value of our findings.
Limitations
Despite our important findings, there are certain limitations to our study. Our sample included patients at all stages of disease severity, resulting in missing data as some individuals were unable to complete all the rating scales. More spread of data across the moderate to severe stages would have added to the analysis and interpretation.
Our sample, although representative of the local population in our memory clinics in East Anglia, did not include any individuals from an ethnic minority background. Additionally, English was the first language of all participants. We acknowledge the inherent cultural bias in all cognitive tests and recommend that our findings are validated in other populations.
Finally, our findings cannot be generalised beyond the four dementia subtypes included in our study. The absence of any patients in our sample with behavioural or language-variant frontotemporal dementia means that the correlations between cognitive instruments shown here cannot be presumed to apply in these disorders.Reference Hsieh, Schubert and Hoon6 In addition, DLB is overrepresented in our cohort compared with typical English memory service populations.Reference Cook, Souris and Isaacs17 Future studies should examine cut-off scores by dementia subtype to better define the performance of the ACE-III and M-ACE in different dementia syndromes.
Table 1 Demographics for the patient sample (n = 80)
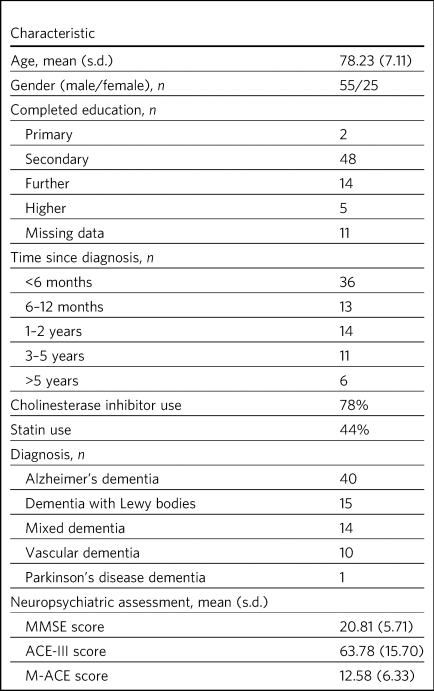
MMSE, Mini-Mental State Examination; ACE-III, Addenbrooke's Cognitive Examination-III; M-ACE, Mini-Addenbrooke's Cognitive Examination.
About the authors
Louise McCarthy is a senior nursing research lead with the Research and Development Team at Norfolk and Suffolk NHS Foundation Trust, Norwich, UK. Judy Rubinsztein is a consultant psychiatrist with Cambridge and Peterborough NHS Foundation Trust (formerly Norfolk and Suffolk NHS Foundation Trust), Cambridge, UK. Ellen Lowry is a lecturer in medical education at Norwich Medical School, University of East Anglia, Norwich, UK. Emma Flanagan is a clinical trials manager at Norwich Medical School, University of East Anglia, Norwich, UK. Vandana Menon is a consultant psychiatrist with Cambridge and Peterborough NHS Foundation Trust (formerly Norfolk and Suffolk NHS Foundation Trust), Cambridge, UK. Silvia Vearncombe is a consultant psychiatrist with Cambridge and Peterborough NHS Foundation Trust (formerly Norfolk and Suffolk NHS Foundation Trust), Cambridge, UK. Eneida Mioshi is Professor in Dementia Care Research in the School of Health Sciences, University of East Anglia, Norwich, UK. Michael Hornberger is Professor of Applied Dementia Research at Norwich Medical School, University of East Anglia, Norwich, UK.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjb.2023.27.
Data availability
Data are available from the corresponding author, L.M., on reasonable request.
Author contributions
L.M., J.R. and M.H. contributed to the study concept and design. L.M., J.R., V.M., S.V., E.F., E.M. and M.H. contributed to data collection. L.M., J.R., E.L., E.F., E.M. and M.H. contributed to data analysis and interpretation. All authors contributed to the writing and revision of the manuscript.
Funding
This research received no specific grant from any funding agency, commercial or not- for-profit sectors.
Declaration of interest
None.







eLetters
No eLetters have been published for this article.