LEARNING OBJECTIVES
After reading this article you will be able to:
• recognise demographic and clinical factors affecting clozapine dose requirement
• know how to best use clozapine TDM services
• interpret plasma clozapine concentrations in relation to clinical response and adverse effect burden and understand factors affecting the plasma clozapine/norclozapine ratio.
Clozapine is the only drug with proven efficacy in schizophrenia that does not respond to other antipsychotics (treatment-refractory schizophrenia). In the UK and in many other parts of the world, clozapine is licensed to treat not only treatment-refractory schizophrenia in people aged 18 years and older, but also Parkinson's disease psychosis. The dose for this latter condition is five- to ten-fold lower than for the former (Lutz Reference Lutz, Sirfy and Wiatr2014).
In treatment-refractory schizophrenia, some 30% of individuals respond to clozapine at 6 weeks, with 60–70% responding at 1 year (Lally Reference Lally and MacCabe2015). However, there is a narrow margin between an effective and a potentially toxic dose (narrow therapeutic range). Dose assessment is complicated because (a) there is a 45-fold inter-individual variation in the rate of clozapine metabolism (Rajkumar Reference Rajkumar, Poonkuzhali and Kuruvilla2012) and (b) alteration in smoking habit, for example, can have a large effect on an individual's clozapine dose requirement (Wagner Reference Wagner, McMahon and Falkai2020). Assessing adherence is also important because in some cases patients may appear asymptomatic even if they have stopped taking the drug.
Many people with treatment-resistant schizophrenia respond at pre-dose (‘trough’) plasma clozapine concentrations (sample taken before a morning dose or in the morning after an evening dose) of 0.35–0.60 mg/L (350–600 μg/L, 350–600 ng/mL, 1.05–1.80 μmol/L), but there is considerable variation in both response and adverse drug reactions (ADRs). It is accepted that 0.35 mg/L is the threshold to ensure a fair trial of the drug (Hiemke Reference Hiemke, Bergemann and Clement2018). However, some individuals may show a good response at pre-dose concentrations of 0.25 mg/L, when inter alia a lower incidence of ADRs is to be expected (Ellison Reference Ellison and Dufresne2015). On the other hand, there is thought to be an increased risk of confusion, constipation, delirium, excess sedation, tonic–clonic seizures, myoclonus and orthostasis if the pre-dose plasma clozapine is >1 mg/L (Hiemke Reference Hiemke, Bergemann and Clement2018).
Clozapine therapeutic drug monitoring (TDM), i.e. the measurement of total (protein-bound and unbound) plasma clozapine and N-desmethylclozapine (norclozapine), the principal plasma metabolite of clozapine, can help check adherence, guide dosage and minimise the incidence of dose-related ADRs (de Leon Reference de Leon2018, Reference de Leon, Ruan and Schoretsanitis2020; Skokou Reference Skokou, Karavia and Drakou2022). Clozapine TDM may also help in investigating suspected drug–drug interactions and in monitoring the effect of changes in smoking habit, for example.
Clozapine TDM results always need to be considered in the context of the clinical situation (e.g. response to clozapine, smoking habit, clozapine-related ADRs). Recording the time of sample collection in relation to the time clozapine was last taken is important when interpreting the results. Plasma norclozapine is commonly measured as well as clozapine if chromatographic methods of analysis are used and this can give information to aid clinical interpretation of results.
Clozapine pharmacokinetics
After oral administration as tablets or suspension, clozapine may be relatively slowly absorbed (t max 1–6 h, possibly longer in the presence of constipation) (Table 1). It has relatively poor (average around 30%) oral bioavailability and is extensively metabolised by N-demethylation, hydroxylation, N-oxidation and conjugation prior to excretion. Only some 14% of the dose is excreted in urine, almost entirely as metabolites (Schaber Reference Schaber, Stevens and Gaertner1998).
TABLE 1 Clozapine and norclozapine: summary data
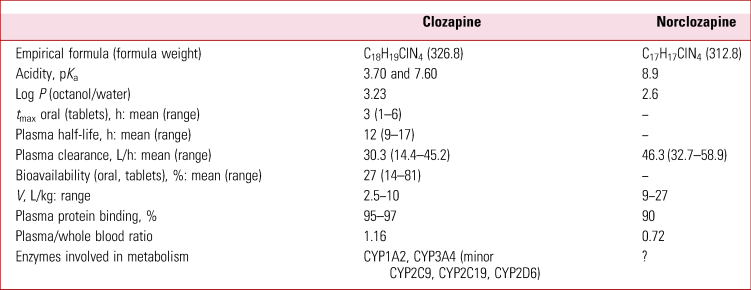
t max, time to reach the maximum plasma concentration; V, apparent volume of distribution.
Source: Schaber et al (Reference Schaber, Stevens and Gaertner1998); Albitar et al (Reference Albitar, Harun and Zainal2020); Flanagan et al (Reference Flanagan, Yusufi and Barnes2003).
The principal enzymes involved in clozapine metabolism are CYP1A2 and CYP3A4, with CYP2C9, CYP2C19 and CYP2D6 playing minor roles (Thorn Reference Thorn, Müller and Altman2018). CYP1A2 is activated by exposure to the polycyclic aromatic hydrocarbons in cigarette and also in cannabis smoke and on average the clozapine dose requirement of smokers is decreased by some 30% if that same person stops smoking, and vice versa (Wagner Reference Wagner, McMahon and Falkai2020; Flanagan Reference Flanagan, Hunter and Obee2023). On the other hand, during infection evidence suggests downregulation (phenoconversion) of CYP1A2, CYP3A4 and CYP2C9 synthesis (Thorn Reference Thorn, Müller and Altman2018) via release of pro-inflammatory cytokines, i.e. the opposite effect to upregulation of CYP1A2 by cigarette smoke. Inhibitors of CYP1A2 and CYP3A4 such as fluvoxamine, cimetidine and ciprofloxacin (Guo Reference Guo, Zhu and Badawy2021) can also significantly increase clozapine exposure at constant dose.
For people prescribed clozapine the usual maintenance dose lies between 300 and 600 mg/day (regulatory maximum 900 mg/day), but there is wide variation. Usually, a greater proportion if not all of the daily dose is given at night. Owing to high inter-individual variability in clozapine metabolism, people may experience potentially serious toxicity such as constipation with daily doses as low as 100 mg. On the other hand, young male smokers especially have sometimes been given doses above the maximum licensed dose in order to maintain effective plasma clozapine concentrations (Table 2). Patient factors such as adherence, age, constipation and infection/inflammation, and environmental factors, such as cigarette smoke, that affect clozapine dose requirement must be taken into account when considering the value of undertaking genetic testing in an attempt to identity fast clozapine metabolisers.
TABLE 2 Plasma clozapine and norclozapine concentrations and prescribed dose in male and female smokers and non-smokersa
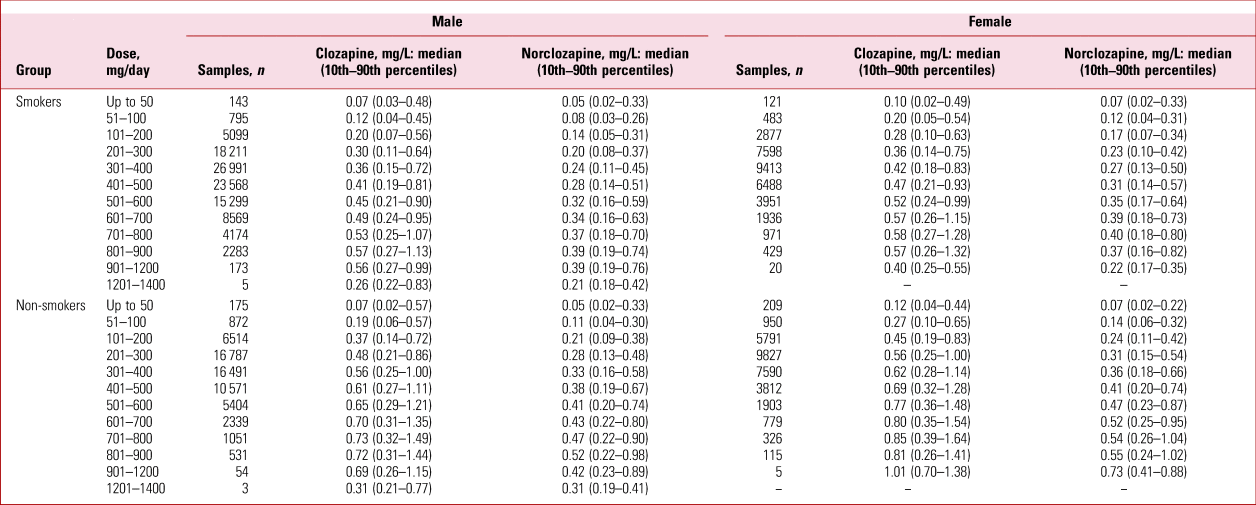
a Data from a clozapine therapeutic drug monitoring service, 1993–2017: see Flanagan et al (Reference Flanagan, Hunter and Obee2023) for details of the service.
Factors affecting clozapine dose requirement
In clinical practice, male smokers and non-smokers are prescribed significantly higher median doses of clozapine than females, but attain lower median plasma clozapine and norclozapine concentrations (Table 2). Moreover, significantly higher plasma clozapine and norclozapine concentrations have been observed in older as compared with younger patients (Bowskill Reference Bowskill, Couchman and MacCabe2012). In a large survey (78 431 samples, 23 516 patients) the dose predicted to attain a pre-dose plasma clozapine concentration of 0.35 mg/L in a non-smoking White European male aged 40 years, at weight 70 kg and plasma clozapine/norclozapine ratio 1.32 was 344 mg/day (95% CI 227, 526). The predicted dose was 33% higher and 20% lower in otherwise analogous Afro–Caribbean and Asian patients, respectively. In all cases the predicted dose was increased by 36% in smokers and decreased by 22% in females. Overall, the predicted dose decreased by 20.4% between age 20 and 80 years (Flanagan Reference Flanagan, Hunter and Obee2023). There is thus a need to review the dose as people get older to minimise the risk of dose-related ADRs.
In one survey of TDM data, dose, sex, age, body weight, smoking habit and the plasma clozapine/norclozapine ratio accounted for 48% of the observed variability in pre-dose plasma clozapine (Rostami-Hodjegan Reference Rostami-Hodjegan, Amin and Spencer2004). The remaining variation was probably accounted for by (a) problems with adherence, (b) the use of crushed tablets suspended in water or another appropriate medium, or proprietary suspension rather than tablets (Flanagan Reference Flanagan, Hunter and Obee2022), (c) the effect of co-ingested drugs, (d) changes in smoking habit/intensity, (e) covert smoking/inadvertent exposure to cigarette smoke, (f) samples that were not ‘trough’ samples, (g) erratic clozapine absorption due to the effects of the drug on the gastrointestinal tract, (h) errors in the information provided on request forms, (i) genetic variation in the expression of drug metabolising enzymes and (j) the effects of infection/inflammation.
One observation that is not understood is that clozapine gradually accumulates in some individuals over time, other factors such as stopping smoking seemingly notwithstanding. This may be because the capacity to metabolise (eliminate) the drug becomes saturated. Sometimes this may go unrecognised until the person suffers a seizure, paralytic ileus or other serious ADR, but it can be detected by clozapine TDM (Couchman Reference Couchman, Morgan and Spencer2010).
Smoking habit
The effect of smoking on clozapine dose requirement is often difficult to manage. If a non-smoker showing a good response to clozapine starts smoking regularly, they will begin to lose the benefit of the drug in 48 h or so (Faber Reference Faber and Fuhr2004). On the other hand, if someone, especially a young male smoker, suddenly stops smoking and the dose is not cut promptly they will be at risk of serious, possibly life-threatening toxicity within a few days or weeks. Stopping smoking may be a consequence of admission to a smoke-free unit. The problem is compounded if in-patients in smoke-free units go on leave, as they may smoke again while outside the unit and, of course, dose adjustments will need to be made after discharge if the person starts smoking again. Indeed, the individual may have to be readmitted (Qurashi Reference Qurashi, Stephenson and Nagaraj2019).
Respiratory infections in clozapine-treated patients who smoke pose especial problems because (a) they may be admitted to a non-smoking hospital, (b) they may not want or be able to smoke as normal, hence the effective dose of ‘smoke’ may be reduced and (c) they may be treated with an antibiotic that inhibits clozapine metabolism. The simple fact of the acute illness may also exacerbate the effect of clozapine on gastrointestinal motility, which may in turn affect clozapine pharmacokinetics.
It is thought that smoking 7–12 cigarettes per day is sufficient for maximal induction of clozapine metabolism (Haslemo Reference Haslemo, Eikeseth and Tanum2006). Plasma clozapine concentrations should be monitored 7 and 14 days after any change in smoking status and clozapine dose. Of course, people vary in the way and intensity with which they smoke and when asked tend to understate the number of cigarettes smoked. This being said, there could be an increasing effect on clozapine dose requirement up to about 7 or so cigarettes a day.
Nicotine replacement therapy (NRT), including ‘vaping’, is not thought to effect clozapine dose requirement. The same applies to the use of snuff, ‘snus’ or chewing tobacco. As to passive smoking, there are anecdotal reports of clozapine toxicity in non-smokers when a partner has stopped smoking, so this possibility should not be neglected. The converse may apply where non-smokers taking clozapine start spending time in spaces where people smoke (Grubisha Reference Grubisha, Wilson and Gopalan2015).
Drug–drug interactions
The most clinically significant drug–drug interactions with clozapine are those with ciprofloxacin and fluvoxamine. Clearly fluvoxamine co-prescription with clozapine should be avoided in normal circumstances. However, if a patient refuses to take an adequate dose of clozapine, if the maximum licensed dose proves inadequate or exceeding the maximum licensed dose is unpalatable to either prescriber or patient, then cautious co-prescription might be considered (Gee Reference Gee and Howes2016).
Selective serotonin reuptake inhibitors other than fluvoxamine (escitalopram, sertraline, paroxetine, fluoxetine) do not affect clozapine dose requirement markedly at the doses seen in clinical practice (Couchman Reference Couchman, Morgan and Spencer2010). Likewise, the dose of caffeine (400 mg/day or more) required to possibly inhibit CYP1A2 is unlikely to be attained. However, cimetidine, oral contraceptives, theophylline, verapamil and quinolone antibiotics such as ciprofloxacin can inhibit CYP1A2 activity at the doses used clinically (Guo Reference Guo, Zhu and Badawy2021). On the other hand, carbamazepine, phenytoin and rifampicin enhance clozapine dose requirement and should not normally be co-prescribed. Moreover, carbamazepine is itself a risk factor for neutropenia.
Clozapine TDM
Except in emergencies such as suspected overdose, a plasma clozapine and norclozapine assay is usually only useful to guide dosage after at least 2 to 3 weeks of treatment, and only then if the prescribed dose of clozapine and smoking habit, for example, have been stable over the preceding week or two (Box 1).
BOX 1 Treatment-refractory schizophrenia: when to do clozapine therapeutic drug monitoring
• In the early stages of treatment, to identify poor metabolisers of the drug (e.g. female non-smokers, the elderly)
• In the later stages of dose titration, to monitor dose/response and to ensure a fair trial of the drug (leave at least a week between samples if the effect of a dose change on plasma clozapine is to be monitored)
• During apparently successful treatment, to provide baseline results in case problems occur later
• At any stage if poor adherence is suspected
• To help adjust dosage if smoking habit changes, or in the presence of severe infection or other medical problem(s)
• To investigate suspected drug–drug interactions or clozapine ADRs
• Every 3–4 months if the pre-dose plasma clozapine concentration is >0.6 mg/L, especially in smokers, to guard against dose-related toxicity, otherwise annually if there are no clinically apparent problems
Samples and sampling
The optimal time to take a blood sample (use ethylenediaminetetraacetic acid (EDTA) anticoagulant) for plasma clozapine and norclozapine assay is in the morning before any morning dose. If planned to coincide with a full blood count (FBC), additional venepuncture can be avoided (Box 2). Indeed, residue from an FBC sample can be used to derive plasma for clozapine TDM if necessary. However, samples taken 1–6 h post-dose or if the patient suffers from constipation may make the result difficult to interpret because clozapine absorption and distribution into tissues might not be complete when the sample is taken. Attempting to standardise sampling time to 12 h post-dose confers no advantage and indeed is impractical in most situations (Jakobsen Reference Jakobsen, Larsen and Svensson2017).
BOX 2 Protocol for plasma clozapine and norclozapine assay
• Take blood by venepuncture (2.7 mL ethylenediaminetetraacetic acid (EDTA) S-Monovette® without gel separator or 5 mL EDTA tube) immediately before a morning dose or, if dosage is once daily, in the morning after the previous evening's dose
• Mix gently and send (a portion) to the laboratory together with a completed assay request form
• Be sure to record the dose, the time and date of last dose, and time and date of sampling
• If the result is required urgently, contact the laboratory in advance to arrange to prioritise the assay
• Ensure that an address/telephone number for the report is provided
Standard provisions for consent to treatment should be followed. This includes informing the patient that the assay results may be held on a database. Blood samples should be packed carefully according to sample transport regulations. Hazardous samples (e.g. from individuals known or suspected to have hepatitis C) require special provision. Advice can be obtained from a local hospital pathology laboratory.
Delayed (by more than 1 week) analysis of samples should be undertaken with caution, especially with serum and with haemolysed whole blood. In human serum, norclozapine is unstable after 5 days at ambient temperature, 3 weeks at 2–8°C and 9 months at −20°C. In whole blood, clozapine and norclozapine are stable for up to 4 weeks at −20°C (Fisher Reference Fisher, Partridge and Handley2013). Clozapine and norclozapine concentrations are significantly higher in plasma than in serum when obtained from blood collected into VacuetteTM serum separator collection tubes, but the difference (5% or so) is insignificant in the clinical interpretation of clozapine and norclozapine TDM results (Handley Reference Handley, Silk and Fisher2018).
Dried blood spots (DBS), usually collected from a ‘finger-prick’ sample onto a purpose-designed card or other sampling device, present an alternative to conventional sampling for clozapine TDM (Geers Reference Geers, Cohen and Wehkamp2017). However, there are many problems in relating the results obtained to those obtained from plasma, in addition to the obvious fact that finger-prick capillary blood is not venous plasma (Francke Reference Francke, Peeters and Hesselink2022). Collection directly onto DBS cards means that taking an accurate volume of liquid blood for analysis is not always possible. Moreover, analyte distribution on the collection card may be affected by chromatography of the analyte on the card during collection, the viscosity of the sample, which is related to haematocrit, which affects the amount of blood collected within a given area of the card, and so on. Analyte stability on the DBS card is a further consideration, although if the analyte is stable on the card at ambient temperature, transport and storage of DBS is easier than with liquid samples.
With clozapine, clozapine N-oxide degrades to clozapine on treated and untreated DBS cards, hence precautions must be taken to prevent interference from this metabolite prior to application to the card (Temesi Reference Temesi, Swales and Keene2013). Use of volumetric absorptive microsampling and microfluidic-generated dried blood spot technology may present viable alternatives to conventional DBS sampling (Marasca Reference Marasca, Mandrioli and Sardella2022), although again care must be taken to avoid artefactual elevation of the clozapine result from degradation of clozapine N-oxide. In any case, however, the dried sample still has to go to a laboratory to be analysed.
Oral fluid (‘saliva’) has the advantage that it is a non-invasive sample, although collection is not straightforward and requires a cooperative patient. Although qualitative identification of clozapine is easily possible, the relationship between oral fluid and plasma clozapine and norclozapine concentrations does not permit the calculation of one from the other with any degree of reliability (Fisher Reference Fisher, Beyer and van Schalkwyk2017).
Assay request form
It is important to differentiate clozapine TDM (plasma clozapine and norclozapine assay or clozapine point-of-care/point-of-contact testing (POCT)) in finger-prick whole blood from mandatory ‘clozapine blood monitoring’ (FBC, white cell count) – use of a separate TDM request form is advisable. The sample time and date, the time and date of the last clozapine dose, and the last dose change should be recorded. This will aid interpretation and ensure prompt availability of results. The request form should also record the patient's name, date of birth, sex, height, body weight and smoking status (smoker/non-smoker), the clozapine provider registration number, clozapine dose (mg/day) and co-prescribed medications, and an address (and telephone number) for the report. Electronic requesting is preferable.
Units of measurement
All measurements have errors. Duplicate or even triplicate analysis improves accuracy provided sufficient sample is available. In the case of plasma clozapine and norclozapine, an analytical error of ±20% at the limit of analytical measurement (generally taken as 0.01 mg/L) is reasonable and allows differentiation of 0.01 from 0.02 mg/L. To cite results in ng/mL, e.g. 350 ng/mL, implies that the method used can reliably tell the difference between, for example, 350 and 351 ng/mL, which is an extremely unlikely situation given the assay range needed to measure plasma clozapine and norclozapine in clinical samples (0.01–2.0 mg/L, 10–2000 ng/mL for both compounds). Use of mg/L is thus a sensible compromise. If the top calibrator is 2.0 mg/L, samples containing clozapine at concentrations >2.0 mg/L require dilution with analyte-free sample matrix and re-assay, assuming sufficient sample is available.
Laboratory analysis
Clearly, to be clinically relevant the results of an analysis need to be communicated as quickly as possible to the person requesting it. However, there are also issues of accuracy, accountability, traceability and cost. Nowadays the laboratory will normally use liquid chromatography-tandem mass spectrometry (LC-MS/MS). It is important to ensure that the analytical procedure used does not degrade clozapine N-oxide to clozapine (Lin Reference Lin, McKay and Hubbard1994). Sending the sample (EDTA whole blood, plasma or serum) to a well-organised, properly funded, accredited laboratory by post, or courier if necessary, for clozapine and norclozapine assay should facilitate electronic reporting of the authorised results of the analysis within 24–48 h of receipt of the sample. LC-MS/MS with isotopic internal calibration offers the flexibility given by random access analysers in clinical chemistry, hence rapid turnround (<2 h) of results can be achieved (Couchman Reference Couchman, Belsey and Handley2013).
The laboratory should provide clinical interpretation as appropriate. The laboratory will take care of other important matters such as safe handling of potentially hazardous samples, staff training, external quality assurance (EQA, also known as proficiency testing, PT), storage and disposal of samples once analysed and archiving of results. All this costs money.
In circumstances where an LC-MS/MS-based clozapine assay service is either not available or the turnaround of results is so slow as to render them of marginal value clinically, alternatives are becoming available. A laboratory-based clozapine turbidimetric homogenous competitive immunoassay may improve turnaround. However, only clozapine is measured (assay range 0.068–1.500 mg/L) and it is unclear whether plasma, serum or whole blood are used (Clarke Reference Clarke, Salyer and Hussey2021).
Point-of-contact testing
POCT promises a result without having to send a sample to a laboratory and await a report. However, the fact that POCT devices are designed for use by non-laboratory staff does not mean that such devices are foolproof and therefore that rigorous quality assurance (QA) measures are not needed. Some of the considerations surrounding POCT are addressed in an ISO standard (International Organization for Standardization 2016) and have been discussed by Kalaria & Kelly (Reference Kalaria and Kelly2019). A major QA issue is staff training (Box 3).
BOX 3 Some considerations in clozapine point-of-care testing (POCT)
• Capillary blood (plasma/whole blood distribution 1.16), risk of contamination with tissue fluid during collection
• Norclozapine not measured
• Needs samples for internal quality control (IQC) and external quality assurance (EQA) (adds to costs)
• Potential interferences?
• Hook effect?a
• Archiving of results
• Kit-to-kit reproducibility (you cannot always rely on a manufacturer over time)
• Kit storage/shelf-life
• Possibility of assay discontinuation by the manufacturer
• Staff training
• Sample storage/disposal
• Collection/analysis/disposal of potentially hazardous samples (e.g. hepatitis C)
• Duplicate analysis?
• Accreditation/assay certification
Unlike automated immunoassay-based laboratory methods, for example, the steps in POCT may require operator intervention, and may include sample application, reading/interpreting a visual end-point, and recording and documenting the result. With finger-prick (capillary) blood, care must be taken in collecting an accurate volume of blood. The site of collection must not be squeezed to encourage the flow of blood because this will result in dilution of the sample with extracellular fluid.Footnote a
The management of the use of POCT devices is of vital importance to ensure that reliable results are obtained within the limits of the devices employed. Internal quality control (IQC) samples should be analysed when a new batch of test kits arrives and at specified intervals thereafter, for example every 30 days or as otherwise directed by the manufacturer. This is important to ensure batch-to-batch consistency of results. The kits should be stored under appropriate conditions and will have a defined shelf-life. All sites where POCT is used should be included in EQA schemes, for example that provided by LGC Standards (LGC 2022). In essence, use of POCT means setting up a mini-laboratory with all its associated costs. Continued operation is of course dependent on a reliable supply of kits from the manufacturer.
A whole blood (finger-prick, capillary blood) clozapine assay using automated homogenous immunoassay has been reported (Buckley Reference Buckley, Kitchen and Vyas2020; Taylor Reference Taylor, Atkins and Harland2021; Boland Reference Boland and Dratcu2022). The evaluation reported by Buckley et al (Reference Buckley, Kitchen and Vyas2020) used 117 stored serum samples. Neither the storage conditions nor the time in storage were given, and analyte degradation may have occurred (Fisher Reference Fisher, Partridge and Handley2013). A national reference laboratory using LC-MS/MS to analyse matching samples obtained 18 false-positive clozapine results (range 0.02–0.16 mg/L), whereas immunoassay showed none, but that might be because they came up as false-negatives in the immunoassay. There was relatively poor concordance (r = 0.84) between the results of the samples that did contain clozapine. Moreover, the immunoassay gave results on average 16% higher than those obtained by LC-MS/MS.
Taylor et al (Reference Taylor, Atkins and Harland2021) compared results from finger-prick blood samples obtained using the POCT analyser with laboratory values obtained using venous blood samples by LC-MS/MS (whether whole blood or plasma/serum was assayed is not specified). They reported a correlation of 0.89 between the two sets of values (n = 309), but five ‘outliers’ were excluded from this calculation. Even after excluding the outliers the relationship between the two sets of values was not good enough to accurately predict one from the other. Boland & Dratcu (Reference Boland and Dratcu2022) simply reported POCT results in four patients.
Interpretation of clozapine TDM data
In all cases perform a common sense check (sample from the right patient/compare with previous results from the patient, pre-dose sample, smoking habit constant, no constipation, no features of inflammation/infection) before assessing the results. For example, if there is doubt as to sample timing in relation to the last dose look at the norclozapine result as well (Box 4).
BOX 4 Plasma clozapine/norclozapine ratio: clinical interpretation
• In a pre-dose sample, the ratio increases at higher plasma clozapine concentrations, suggesting saturation of clozapine N-demethylation (Couchman Reference Couchman, Morgan and Spencer2010)

Cautions
• If the ratio is very high (3 or more), but the plasma clozapine is 1 mg/L or so, this suggests (a) inhibition of clozapine N-demethylation by a drug such as fluvoxamine, (b) possible downregulation of clozapine N-demethylation as a result of infection/inflammation or (c) that the sample was taken before absorption/distribution of the last dose was complete
• If the ratio is very high (3 or more), but the plasma clozapine is 0.2–0.3 mg/L or so, this suggests that the patient may not have been taking clozapine regularly
• If the ratio is very low (0.5 or so) this suggest either (a) that the patient is a very fast metaboliser of clozapine (e.g. a young, male smoker) or (b) that clozapine has not been taken for day or two, possibly longer, prior to sampling
Adherence
If clozapine is absent from a sample (normal assay limit of sensitivity 0.01 mg/L) this suggests that clozapine had not been taken for at least a week or so prior to sample collection, except that at low dosage (≤100 mg/day) early in treatment, failure to detect clozapine could be due to rapid metabolism of the drug (in a young male smoker, for example). Some 1% of samples submitted for clozapine TDM to one service had no clozapine in them (prescribed clozapine doses up to 900 mg/day; Couchman Reference Couchman, Morgan and Spencer2010). If non-adherence in the community is identified, a thorough search for the missing tablets must be instituted promptly because of the risks posed by suddenly restarting clozapine after a period of non-adherence. There is also the possibility of self-poisoning, which could prove fatal.
Partial adherence is difficult to assess, but reports of unused tablets being recovered when someone has died, for example, indicate that it is a continuing problem, especially in people living in the community. The data presented in Table 2 can be used to guide interpretation. Although rapid metabolism cannot be excluded at very low clozapine doses, results for both clozapine and norclozapine below the 10th percentile suggest recent poor adherence. Previous clozapine TDM results in a given patient may also aid interpretation by indicating trends or highlighting periods of poor adherence and a good laboratory report should either tabulate such results or point to where they can be found.
Norclozapine persists in plasma for longer than clozapine when clozapine dosage is stopped. It is also less likely to change with sample time in relation to the time of the last dose than the plasma clozapine itself, and this may help in interpreting individual patient results over time if adherence is questioned, for example. Study of the clozapine/norclozapine ratio may also help detect recent clozapine ingestion after a period of non-adherence (Box 4).
Plasma clozapine and response
All patients are different. Nevertheless, guidelines based on pre-dose plasma clozapine concentrations have been found generally useful in treatment-refractory schizophrenia (Table 3). At steady-state, plasma clozapine and norclozapine are reproducible between samples obtained at the same time on different days, other factors (time since last dose, adherence, gastrointestinal motility, recent food intake, use of other drugs, exposure to cigarette smoke, infection/inflammation) being equal (Turrion Reference Turrion, Perez and Bernardo2020). If pharmacotherapeutic augmentation is considered it is advisable to maintain the pre-dose plasma clozapine concentration below 1 mg/L, and preferably below 0.6 mg/L (Lally Reference Lally and Gaughran2019), because if augmentation is successful then it will be unlikely that clozapine dosage will be altered even if unnecessarily high.
TABLE 3 Simple guide to the interpretation of pre-dose plasma clozapine concentrations
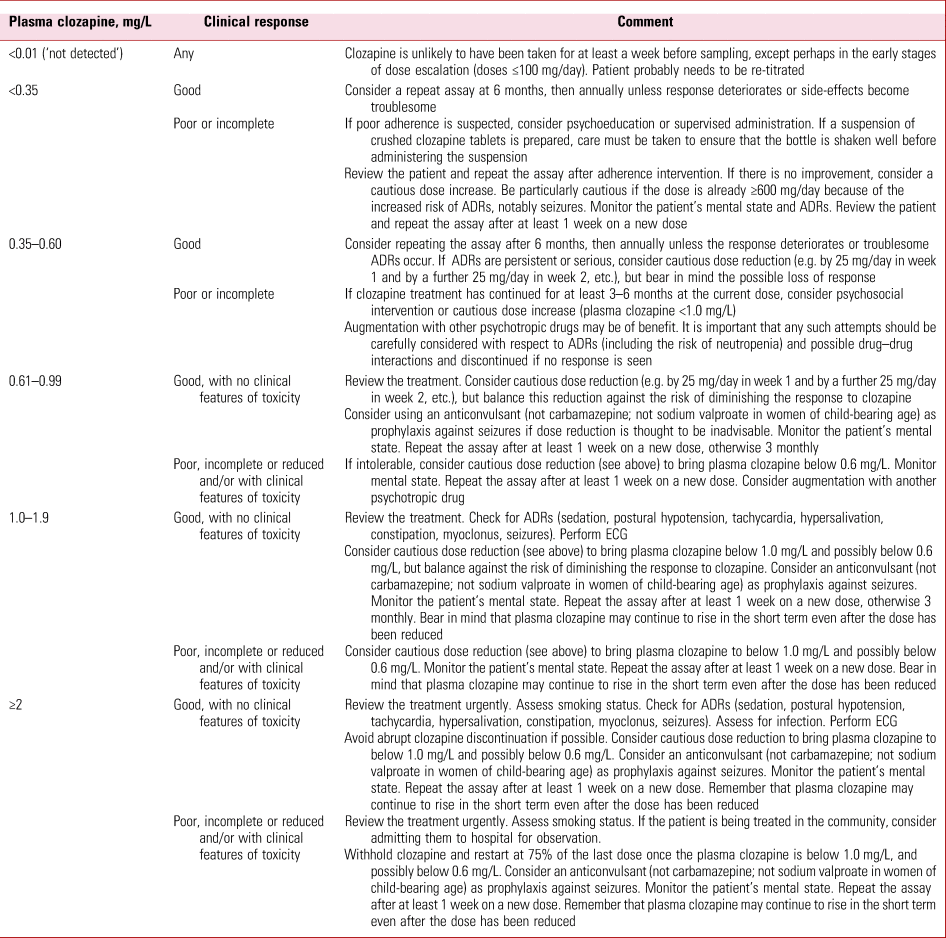
ADR, adverse drug reaction; ECG, electrocardiogram.
Although seizures feature among the Food and Drug Administration's ‘black box warnings’ for clozapine, this to an extent reflects the situation before the TDM of clozapine was widely available and before the profound effect of stopping smoking on clozapine dose requirement was appreciated. In one audit of a clozapine TDM service (104 127 samples, 26 796 patients), plasma clozapine was 1 mg/L or more in 8.4% of samples. Some individuals appear well at plasma concentrations in the range 2–5 mg/L (Couchman Reference Couchman, Morgan and Spencer2010). In this context, if pre-dose plasma clozapine is found to be very high (>2 mg/L, 0.4% of samples in the audit cited), it may continue to rise for some time after clozapine dosage is significantly reduced or stopped, possibly reflecting continued absorption as gut motility returns.
Norclozapine and clozapine N-oxide
The role of norclozapine in vivo is unclear (Humbert-Claude Reference Humbert-Claude, Davenas and Gbahou2012; Tan Reference Tan, Honarparvar and Falconer2021; Jessurun Reference Jessurun, Derijks and van Marum2022). In isolated rabbit colon, at 20 μmol/L norclozapine had opposite effects to those of clozapine, causing an increase in the frequency of mass peristalsis, with slight increases in basal tone. These slightly augmented contractions were abolished on addition of clozapine, suggesting that interactions between these two compounds and possibly with other clozapine metabolites may also occur at receptors in vivo (Every-Palmer Reference Every-Palmer, Lentle and Reynolds2017). Given this situation, study of plasma norclozapine related to outcome has usually been in respect of the plasma clozapine/norclozapine ratio. Despite conflicting reports (Solomon Reference Solomon, Powell and Sanches2021), the ratio can provide useful information to help interpret plasma clozapine results (Box 4).
The measurement of clozapine N-oxide has also been advocated for TDM purposes. However, this compound is readily reduced to clozapine and seems to be present at appreciably lower concentration (20% or so) than either clozapine or norclozapine in most patient samples. Furthermore, it is relatively polar and (a) is probably eliminated quite quickly via the kidneys and (b) is unlikely to penetrate the blood–brain barrier.
Conclusions
Clozapine TDM used appropriately is a valuable and cost-effective adjunct to the safe and effective use of the drug. There is a clear need to ensure that clozapine TDM services are readily accessible and provide timely results. Clinicians, mental health pharmacists and others concerned in patient treatment must be trained appropriately in sample collection and in the interpretation of results. The availability of a clozapine immunoassay for laboratory use and of clozapine POCT may present further opportunities to improve patient care.
Author contributions
R.J.F. drafted the paper and wrote the final version, S.G. provided input from a clinical pharmacy perspective, S.B. and L.C. provided input from an operational perspective, and J.L. initiated the project and provided critical clinical comment. All authors approved the final version.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
S.B. and L.C. participate in offering laboratory clozapine assay services.
MCQs
Select the single best option for each question stem
1 All of the following are indications for therapeutic drug monitoring in treatment-refractory schizophrenia except:
a assessing adherence
b monitoring for new adverse effects
c investigating pharmacodynamic interactions
d investigating deterioration in clinical symptoms
e ensuring a fair trial of clozapine.
2 Increases in plasma clozapine concentrations are associated with:
a commencing smoking
b starting fluvoxamine
c recovery from viral infection
d missed doses of clozapine in the past 24 h
e delayed time of sampling.
3 A clozapine/norclozapine ratio <1 may be associated with:
a metabolic induction
b metabolic inhibition
c smoking cessation
d not a trough sample
e slow clozapine metabolism.
4 A plasma clozapine concentration of >1 mg/L is reported: all of the following are clinical factors consistent with this except:
a recent dose change
b smoking commencement
c not a trough concentration
d no de novo adverse effects
e rifampicin discontinuation.
5 As regards laboratory analysis of plasma clozapine concentrations:
a norclozapine but not clozapine concentrations are higher in plasma than in serum samples
b samples taken within 6 h of a clozapine dose hinder clinical interpretation
c laboratories use a point-of-contact testing measurement
d an effective laboratory analysis and reporting system would aim to provide results within 48 h
e both clozapine and norclozapine concentrations are higher in serum than plasma samples.
MCQ answers
1 c 2 b 3 a 4 d 5 d







eLetters
No eLetters have been published for this article.