The World Health Organization (2018) has recently estimated that schizophrenia affects more than 23 million people worldwide, with an increased risk of earlier mortality. It has been estimated that lifespan is shortened by 15–20 years (Schizophrenia Commission 2012). It is therefore important to keep up to date with current research findings so that effective preventive and supportive strategies can be put in place. We have divided our examination of this area into a three-part series (see also Romain Reference Romain, Eriksson and Onyon2019a,Reference Romain, Eriksson and Onyonb). Different risk factors, not just for schizophrenia but for psychotic symptoms in general, will be addressed in accordance with the timeline shown in (Fig. 1), starting at the beginning of life and considering factors that increase risk on the path towards adulthood. The first two articles discuss risk factors from early life through to adulthood. The third considers common pathological pathways and possible preventative strategies, discussing future targets and ways to improve quality of care. Overall, this series aims to update knowledge of risk factors for psychosis and provide a basis for discussion focused on making positive changes for the future.
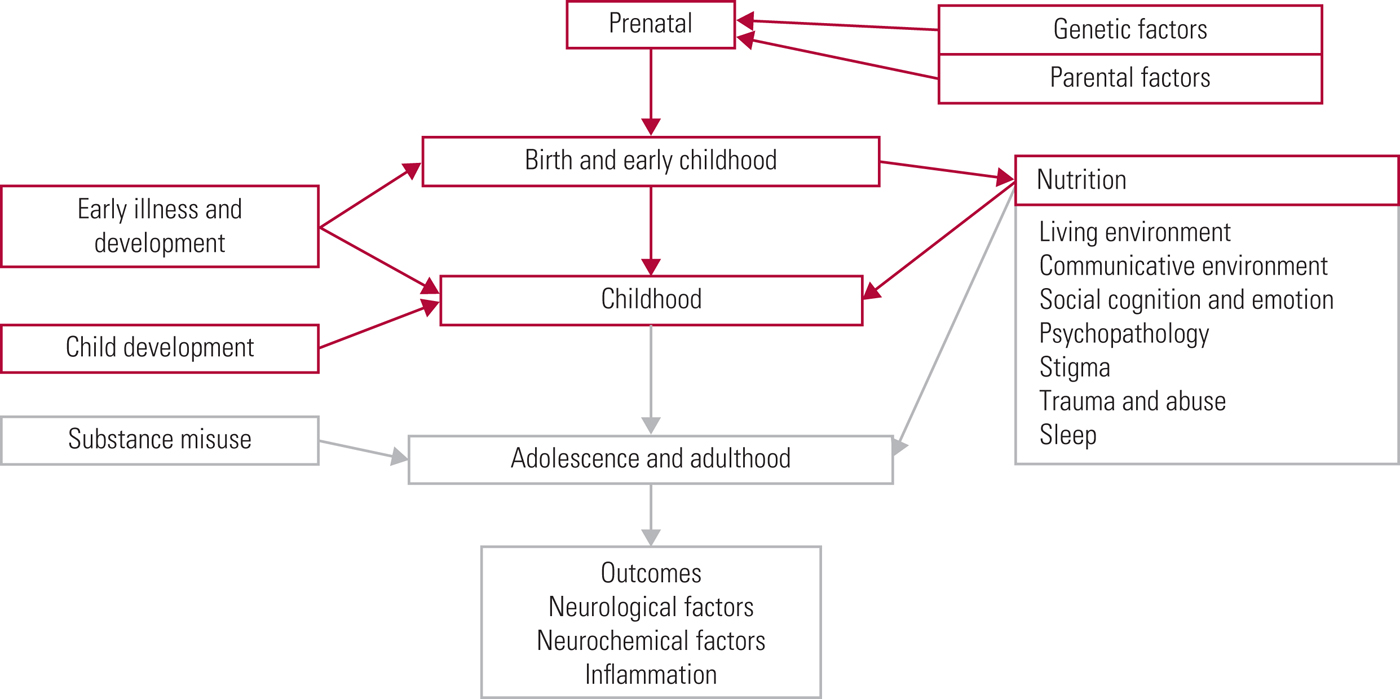
FIG 1 The psychosis risk timeline: factors over the lifespan that can affect the risk for psychosis.
An important concept when considering the literature is the so-called clinical high-risk state for psychosis. This encompasses criteria including the at-risk mental state (ARMS), the prodromal period and the ultra-high-risk state (UHR) (Fusar-Poli Reference Fusar-Poli, Borgwardt and Bechdolf2013). UHR criteria include one or more of: attenuated psychotic symptoms, brief intermittent psychotic symptoms or thirdly a family history of psychosis in a first degree relative if there is an associated decline in psychosocial functioning. Basic symptoms can be assessed alongside this; these are disturbances in perception, thought processing or language, but where insight remains intact (Fusar-Poli Reference Fusar-Poli, Borgwardt and Bechdolf2013). Those at high risk of psychosis have a transition rate estimated at 22% at 1 year, progressing to 36% after 3 years (Fusar-Poli Reference Fusar-Poli, Bonoldi and Yung2012). Throughout our three articles, evidence regarding the risk for psychotic symptom development is considered as a broad concept, covering multiple psychiatric disorders, including schizophrenia and schizoaffective disorder.
Genetics
Heritability of schizophrenia is estimated at 79%, and for schizophrenia spectrum disorders the estimate is 73% (Hilker Reference Hilker, Helenius and Fagerlund2018). Van der Werf et al (Reference van der Werf, Hanssen and Köhler2012) explain that level of predisposition to psychosis is based on gender, further influenced by age. Men have a 1.15-fold greater lifetime risk of schizophrenia than women (van der Werf Reference van der Werf, Hanssen and Köhler2012). Some key facts regarding genetic risk are summarised in Box 1. Although genetic factors themselves are set before birth, knowledge of these influences and consideration of genetic screening is an important area when looking to identify those who may need further support and in secondary and tertiary preventive strategy development. As knowledge in this area continues to develop, work towards improving the clinical utility of genetic results may significantly alter current practice.
BOX 1 Key facts regarding genetic risk
• 3q29 deletion confers the highest increased risk for schizophrenia, with an odds ratio of 16.98–41.4 (Kotlar Reference Kotlar, Mercer and Zwick2015)
• The most commonly occurring CNV is 22q11.2, which has an odds ratio of 28 (Kotlar Reference Kotlar, Mercer and Zwick2015) and is present in approximately 1% of patients with schizophrenia and 1 in 2000–4000 live births (Baker Reference Baker, Costain and Fung2014; Schneider Reference Schneider, Debbané and Bassett2014)
Some genetic risk factors for schizophrenia have been linked to other disorders, including intellectual disability, autism spectrum disorders and bipolar disorder (Singh Reference Singh, Kurki and Curtis2016; Owen Reference Owen and O'Donovan2017; Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium 2018; Gandal Reference Gandal, Haney and Parikshak2018). Using transcriptomic profiling (which examines the expression level of RNAs in a given cell population) as a way of measuring molecular brain-based phenotypes across five major psychiatric disorders, it has been demonstrated that autism, schizophrenia and bipolar disorder share specific global gene expression patterns, characterised by astrocyte activation and disrupted synaptic processes. This seems to suggest a genetic underpinning to symptomatological links between these conditions, possibly including shared communication difficulties (Gandal Reference Gandal, Haney and Parikshak2018).
Singh et al (Reference Singh, Kurki and Curtis2016) found an example of a genetic overlap between schizophrenia and developmental disorders in the form of rare loss-of-function variants in the SETD1A gene. SETD1A is also known as KMT2F and its product is involved in methylation of histone H3, linked to severe developmental phenotypes, and representing another mechanism under investigation within schizophrenia pathogenesis (Singh Reference Singh, Kurki and Curtis2016). Overlaps in symptomatology, diagnostic boundaries and risk factors have led Owen & O’Donovan (Reference Owen and O'Donovan2017) to propose that, rather than being discrete entities, schizophrenia and developmental disorders lie on a neurodevelopmental continuum. They therefore suggest that the individual clinical syndrome seen reflects the timing and pattern of the abnormal brain development. The Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium (2018) found 114 genome-wide significant loci shared by schizophrenia and bipolar disorder as well as some specific loci that distinguish them, adding to data suggesting some shared genetic risk underlying overlapping symptomatology. One example they discussed was the DCAKD gene, thought to have a role in brain development, which was associated specifically with schizophrenia rather than bipolar disorder.
SNPs, CNVs and epigenetics
There are a great many genetic variables that further alter risk for psychosis. These include single nucleotide polymorphisms (SNPs) and copy number variants (CNVs) (Box 2). Many SNPs, each associated with a small increased risk of psychosis, have been identified. As SNPs alone do not account for a sizeable proportion of heritability, rarer CNVs have also been implicated (Mulle Reference Mulle2012). There are now thought to be more than 128 SNPs, which explain around 3.4% of schizophrenia variance, and more than 15 CNVs which have been implicated in schizophrenia (Kotlar Reference Kotlar, Mercer and Zwick2015).
BOX 2 SNPs and CNVs
Single nucleotide polymorphisms (SNPs)
These are common genetic changes in the general population and individually they are are associated with minimal increases in risk of psychosis (Mulle Reference Mulle2012).
Copy number variants (CNVs)
CNVs are gains or losses of genetic material which are at least 1 kilobase in size (Kotlar Reference Kotlar, Mercer and Zwick2015). These are rarer but associated with larger effects (Mulle Reference Mulle2012).
Epigenetic implications have been linked to psychosis. These are heritable changes in gene expression that do not involve a DNA sequence change (Keshavan Reference Keshavan, Mehta and Padmanabhan2015; Janecka Reference Janecka, Mill and Basson2017). One example of this is growth arrest and DNA-damage-inducible beta (GADD45b), which promotes DNA demethylation and has been linked to deficits in neurogenesis and dendritic growth of neurons (Keshavan Reference Keshavan, Mehta and Padmanabhan2015). DNA methylation changes have been linked to increases and decreases in gene expression and in other genomic functions. They have been further associated with increased psychosis risk linked to advanced paternal age, discussed further in the section on parental factors later in this article (Janecka Reference Janecka, Mill and Basson2017).
3q29 deletion
3q29 is currently considered the highest-risk genetic factor in schizophrenia, with an odds ratio most recently calculated as up to 41.1 (Kotlar Reference Kotlar, Mercer and Zwick2015). 3q29 contains 22 genes, and of these PAK2, DLG1 and FBXO45 have been suggested as possible candidates, although as yet these have not been conclusively linked and further investigation is ongoing (Kotlar Reference Kotlar, Mercer and Zwick2015).
22q11.2 deletion syndrome
DiGeorge syndrome, caused by a microdeletion of one copy of chromosome 22q11.2, is the most common CNV in psychosis (Vacic Reference Vacic, McCarthy and Malhotra2011; Gothelf Reference Gothelf, Law and Frisch2014). It is otherwise known as velocardiofacial syndrome (Sebat Reference Sebat, Levy and McCarthy2009) and has been associated with intellectual disability, cardiac defects, craniofacial abnormalities and bipolar disorder as well as autism, schizophrenia and other psychotic disorders (Tam Reference Tam, Redon and Carter2009). In children with 22q11.2 deletion syndrome, the most frequently associated disorder is attention-deficit hyperactivity disorder (Schneider Reference Schneider, Debbané and Bassett2014). The same study reported that 41% of men over 25 years of age with the syndrome had a diagnosed psychotic disorder.
But how does this deletion confer increased risk? One of many genes found in the 22q11.2 region is catechol-O-methyltransferase (COMT). This modulates dopamine, particularly in the prefrontal and hippocampal regions. There are a large variety of COMT variants (SNPs) that can modulate its effects (Gothelf Reference Gothelf, Law and Frisch2014). Thomson et al (Reference Thompson, Karelis and Middleton2017) reviewed evidence on this, explaining that the COMT gene produces an enzyme that catabolises catecholamines and therefore influences dopamine metabolism. Other SNPs implicated in schizophrenia, such as rs4680 valine/methionine (Val/Met) substitution, also have effects through COMT by altering the enzyme activity and further altering stress responses (Howes Reference Howes, McCutcheon and Owen2017; Thomson Reference Thompson, Karelis and Middleton2017).
Alongside COMT, other candidates for problem areas linked to 22q11.2 have been proposed, including: proline dehydrogenase 1 (PRODH); DiGeorge syndrome critical region 8 (DGCR8; also known as pasha); putative palmitoyltransferase (ZDHHC8); and guanine nucleotide binding protein beta-subunit-like polypeptide (GNB1L) (Tam Reference Tam, Redon and Carter2009; Thompson Reference Thompson, Karelis and Middleton2017). These and other links and associations with 22q11.2 are further described in supplementary Appendix 1, available at https://doi.org/10.1192/bja.2018.66.
DISC1
Disrupted in schizophrenia 1 (DISC1) is a well-known translocation associated with increased schizophrenia risk. DISC1 is expressed in the brain and is involved in mechanisms including neuronal migration and synaptogenesis (Sebat Reference Sebat, Levy and McCarthy2009; Mulle Reference Mulle2012). One particular translocation interacting with DISC1 has been identified in the form of phosphodiesterase 4B (PDE4B) (Tam Reference Tam, Redon and Carter2009). These are both further linked to psychosis via the modulation of the stress signalling pathways (Howes Reference Howes, McCutcheon and Owen2017).
NMDA Receptor
Kimoto et al (Reference Kimoto, Glausier and Fish2016) investigated the issue of N-methyl-d-aspartate receptor (NMDA) receptor hypofunction as this has been linked to cognitive impairments in schizophrenia. They specifically studied regulator of G protein signalling 4 (RGS4), lower levels of which have been demonstrated in the dorsolateral prefrontal cortex in people with schizophrenia. Higher levels of miR16, a microRNA that regulates RGS4, were also evident. These abnormalities were linked to hypofunction of the NMDA receptor, signalling abnormalities and cognitive impairments. However, current research has not yet reached a conclusion regarding whether these changes represent a cause or consequence of the disease process (Kimoto Reference Kimoto, Glausier and Fish2016).
Receptor hypofunction, owing to its effects on glutamatergic pathways, has been associated with schizophrenia. These effects include deletions in genes coding for the GluN2A subunit of the NMDA receptor and deletion of the gene encoding for cAMP response element-binding protein (CREB), which is related to transcription (Keshavan Reference Keshavan, Mehta and Padmanabhan2015). SNPs in the D-amino acid oxidase activator (DAOA, formerly G72) and the DAO genes have been shown to have interactions that may be related to the development of schizophrenia, and again these influence glutamatergic signalling through the NMDA receptor pathway (Chu Reference Chu, Chow and Cohen-Woods2017).
Neurexins
Neurexins are presynaptic cell adhesion proteins encoded by three genes (NRXN1–3), which are located in different chromosome loci (Tam Reference Tam, Redon and Carter2009; Owen Reference Owen and O'Donovan2017). As with many genes thought to increase risk of schizophrenia, NRXN1 has been linked to autism (Tam Reference Tam, Redon and Carter2009). Neurexin-1 primarily acts as a neuronal cell surface receptor on the presynaptic membrane and here it is involved with synapse formation and neurotransmission (Tam Reference Tam, Redon and Carter2009). 2p16.3 encompasses the NRXN1 gene and deletions here are associated with increased psychosis risk, with an odds ratio of 8.97 (Kotlar Reference Kotlar, Mercer and Zwick2015).
Within the CYFIP1 gene the resulting proteins regulate mRNA stability for proteins associated with the neurexin-1 gene (NRXN1) (Tam Reference Tam, Redon and Carter2009). CNV microdeletion within NRXN1 and contactin-associated protein-like 2 (CNTNAP2), a member of the neurexin family, and a further CNV in APBA2, which encodes the neuronal adapter protein A2 (also called Mint2) that binds to neurexins, have been linked to schizophrenia (Sebat Reference Sebat, Levy and McCarthy2009; Tam Reference Tam, Redon and Carter2009; Mulle Reference Mulle2012).
Deletion 17q12
Moreno-De-Luca et al (Reference Moreno-De-Luca, Mulle and Kaminsky2010) considered the CNV deletion 17q12 further. It confers an elevated risk of schizophrenia, but is rare and most often arises de novo. This deletion is important as it is one of the 10 most frequent recurrent genomic deletions identified in children who have unexplained neurodevelopmental problems and it is linked to both schizophrenia and autism. This area includes 15 genes, one of which is the gene HNF1B (hepatocyte nuclear factor 1 homeobox B, also known previously as transcription factor 2 (TCF2)), which is linked to renal cysts and diabetes syndrome and phenotypic variants including neurocognitive impairment. Research by Moreno-De-Luca et al (Reference Moreno-De-Luca, Mulle and Kaminsky2010) has demonstrated that one or more of the genes in this deleted region is essential for normal brain development.
Other syndromes
Schizophrenia has been associated with other well-established genetic syndromes. One such is Klinefelter syndrome, characterised by an extra X chromosome (most commonly, the 47 XXY karyotype). This affects 1 in 670 males (Cederlöf Reference Cederlöf, Ohlsson Gotby and Larsson2014). Klinefelter syndrome is associated with a 3.6–3.8 times higher risk of having a diagnosis of schizophrenia or bipolar disorder (Cederlöf Reference Cederlöf, Ohlsson Gotby and Larsson2014).
A CNV deletion at 15q11.2 is also associated with schizophrenia and partially overlaps with areas associated with Prader–Willi syndrome and Angelman syndrome. Interestingly, within this region there is the cytoplasmic FMR1-interacting protein 1 isoform (CYFIP1) gene, which encodes for a protein linked to the cognitive effects of fragile-X syndrome (Stefansson Reference Stefansson and Rujescu2008; Tam Reference Tam, Redon and Carter2009).
7q11.23 duplication is also linked to schizophrenia. This has sparked enthusiasm because of the role of its reciprocal deletion in causing Williams–Beuren syndrome. Those with Williams–Beuren syndrome are highly social, which contrasts with symptoms often seen in schizophrenia and autism spectrum disorders. Work to replicate findings and further investigate this is ongoing (Kotlar Reference Kotlar, Mercer and Zwick2015).
Other genetic evidence
Many additional genetic links are known to be associated with an increased risk of psychosis; further examples are detailed in Table 1, although this list is far from exhaustive.
TABLE 1 Genetic variables associated with an increased risk of psychosis

The polygenic risk score
As the genetic implications for psychosis risk are complex, attempts have been made to collate information in a meaningful way. The polygenic risk score is the sum of risk alleles for schizophrenia in an individual, with each being weighted via complex calculations based on large-sample genetic data (calculations further explained by Agerbo et al, Reference Agerbo, Sullivan and Vilhjálmsson2015). It has been demonstrated that those in the highest decile of polygenic risk have an odds ratio of 8.01 for schizophrenia. However, it is worth remembering that, at this stage, these scoring systems have low clinical utility (Agerbo Reference Agerbo, Sullivan and Vilhjálmsson2015).
The process of polygenic risk scoring is improving in its discriminative power and ability to separate risk for schizophrenia from other psychosis and symptoms across different disorders (Vassos Reference Vassos, Di Forti and Coleman2017; Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium 2018). Continued developments in this area aim to improve understanding of the scoring in relation to illness severity, transition rates and treatment response rates (Vassos Reference Vassos, Di Forti and Coleman2017). These scores have also had some success in helping to predict cognitive ability and some psychopathology. For example, polygenic risk scores have predicted social and behavioural impairments in children as young as 4 years old (Riglin Reference Riglin, Collishaw and Richards2017).
Parental factors
Parental age
Various studies have considered the role of parental age, with variable outcomes. Miller et al (Reference Miller, Messias and Miettunen2010) demonstrated an increased risk of schizophrenia in offspring of fathers aged over 30 and under 25. Another meta-analysis, by Laurens et al (Reference Laurens, Luo and Matheson2015), corroborated evidence of an increased risk with higher paternal age, but not with younger paternal age. Fountoulakis et al (Reference Fountoulakis, Gonda and Siamouli2018) showed a higher risk of schizophrenia in those born to fathers aged over 25 or mothers aged over 22. This relationship was not found to be ‘dose-dependent’, as risk did not increase with combined higher paternal and maternal age (Miller Reference Miller, Messias and Miettunen2010; Laurens Reference Laurens, Luo and Matheson2015; Fountoulakis Reference Fountoulakis, Gonda and Siamouli2018). This demonstrates the difficulty in research of finding the correct age cut-offs. Further studies looking at maternal age have demonstrated an increased risk of schizophrenia spectrum disorder in the offspring of both younger and older mothers (Laurens Reference Laurens, Luo and Matheson2015; Byars Reference Byars and Boomsma2016; Janecka Reference Janecka, Mill and Basson2017). It is possible that increased paternal age may have a greater effect on female than male offspring, but data here are not conclusive (Janecka Reference Janecka, Mill and Basson2017; Fountoulakis Reference Fountoulakis, Gonda and Siamouli2018).
Byars & Boomsma (Reference Byars and Boomsma2016) took a different approach and demonstrated increased risk when parents were dissimilarly aged. They did not demonstrate the same risks from higher parental ages (maternal or paternal) in schizophrenia development but did demonstrate this for autism. Interestingly they therefore viewed schizophrenia and autism at opposite ends of a gradient, with young maternal age being associated with schizophrenia and older age with autism (Byars Reference Byars and Boomsma2016).
Other suggested mechanisms linking parental age and risk of psychosis include psychosocial, cultural and resource-related factors (Byars Reference Byars and Boomsma2016): for example, the possible psychological impact of having an older parent may include the increased risk of experiencing early parental death (Fountoulakis Reference Fountoulakis, Gonda and Siamouli2018). From a biological perspective, in males, spermatogenesis is ongoing and so is associated with an age-related increase in mutations within the gametes (Janecka Reference Janecka, Mill and Basson2017). Maternal high body mass index (BMI) during or prior to pregnancy also confers further increased risk for a schizophrenia spectrum disorder in the offspring, as does lower parental, particularly maternal, education levels (Laurens Reference Laurens, Luo and Matheson2015).
Maternal exposure to trauma during pregnancy
Weinstein et al (Reference Weinstein, Levav and Gelkopf2018) investigated maternal exposure to terror attacks during pregnancy and found an increased risk of schizophrenia in offspring. In their sample, schizophrenia was diagnosed in 0.25% of controls and 0.64% of the offspring with maternal terror attack exposure. As a potential mechanism they suggested a neurodevelopmental critical period that is particularly sensitive to stress and to possible alterations to the fetal immune system, glucocorticoid response and amygdala activity.
Prenatal development and birth
Premature birth and obstetric complications
The neurodevelopmental theory of schizophrenia proposes that abnormalities in fetal brain development can lead to disorders of brain function in early adulthood (Fusar-Poli Reference Fusar-Poli, Borgwardt and Crescini2011). Increased risk for affective psychosis and schizophrenia spectrum disorders has been linked to premature birth and haemorrhage during pregnancy. For schizophrenia spectrum disorders, risks included a high obstetric complication index score (a score based on complications of pregnancy, abnormalities of fetal growth and development, and complications of delivery), fewer than 10 antenatal visits and hypoxia-related complications. For affective psychosis, abnormal fetal presentation, non-spontaneous delivery and being small for gestational age were also described as risk factors. It is worth noting that these factors are generally considered a collective risk, and there is limited evidence for each individually (Laurens Reference Laurens, Luo and Matheson2015). Interestingly, offspring at familial high risk of psychosis experienced higher rates of obstetric complications during birth and were more likely to have had more severe developmental difficulties during childhood (Hameed Reference Hameed and Lewis2016).
Prenatal brain insults
One marker of early neurological injury is fluctuating dermatoglyphic asymmetry. This is the pattern of folds in the skin on fingers, palms and feet, which forms during the second trimester of pregnancy. Russak et al (Reference Russak, Ives and Mittal2016) suggested that asymmetries here may represent an early prenatal brain insult, as this skin is formed from the same germinal layer as the brain itself, and they further demonstrated greater fluctuating dermatoglyphic asymmetry in those at high risk for psychosis.
Keshavan et al (Reference Keshavan, Mehta and Padmanabhan2015) described how early life brain insults and early life adversity can predispose to abnormal reorganisation of neural circuits. This may lead to reduced cortical glutamatergic function. Reduced glutamatergic tone leading to decreased grey matter and synaptic density, alongside increased stress exposure, and poor cognitive adaptivity, leads to a maladaptive plasticity cascade. This links in with stress responses, affective dysregulation and therefore psychosis risk (Keshavan Reference Keshavan, Mehta and Padmanabhan2015).
Bao et al (Reference Bao, Ibram and Blaner2012) found an increased risk of schizophrenia spectrum disorders in individuals exposed to low retinol during the second trimester. Retinol is a peripheral tissue form of vitamin A, a source of retinoic acid, which is important in neurodevelopment. Low levels during the second trimester have been associated a greater than threefold increased risk of schizophrenia spectrum disorders. Interestingly, disruption to the genetic coding for some retinoid receptors has been associated with schizophrenia, as have maternal markers of low folate (high homocysteine) and low iron (low haemoglobin) in the prenatal period (Bao Reference Bao, Ibram and Blaner2012; Laurens Reference Laurens, Luo and Matheson2015).
Maternal substance misuse during pregnancy
Maternal substance misuse (for example alcohol use) in the prenatal period can lead to dysmorphic development in the brain (Keshavan Reference Keshavan, Mehta and Padmanabhan2015). Smoking has also been linked to increased risk of affective psychosis (Laurens Reference Laurens, Luo and Matheson2015). Looking at the number and timing of births, there is no clear evidence for an effect of twin birth on risk. However, some evidence suggests that being the first born, having more than three siblings and a short interval between births are associated with risk for schizophrenia spectrum disorders (Laurens Reference Laurens, Luo and Matheson2015). Data from epidemiological studies suggest that those born in winter or spring have higher prevalence of schizophrenia and there is a suggestion that this could be related to low vitamin D, particularly in early life (Kočovskà Reference Kocˇovskà, Gaughran and Krivoy2017).
Early infection
Considerable evidence suggests an increased risk of schizophrenia spectrum disorders being conferred by prenatal exposure to certain infectious diseases. Evidence also suggests a role for childhood infections or brain damage in increasing the risk of schizophrenia spectrum disorders (Laurens Reference Laurens, Luo and Matheson2015). One infection commonly discussed is gestational influenza, particularly when looking at seasonality-associated risk at birth. A meta-analysis by Cai et al (Reference Cai, Wan and He2015) demonstrated that gestational influenza can increase psychosis risk in adult offspring; one suggested mechanism is prenatal induction of cytokines, promoting inflammation and affecting the developing brain.
Khandaker et al (Reference Khandaker, Zimbron and Dalman2012) conducted a meta-analysis of studies looking at viral central nervous system (CNS) infections in early childhood. They demonstrated an associated risk of later psychosis with cytomegalovirus, mumps infection and coxsackie virus. They further discussed evidence that infections during pregnancy (such as toxoplasma gondii, rubella, cytomegalovirus and herpes simplex virus) have a role in both brain and behavioural development (Khandaker Reference Khandaker, Zimbron and Dalman2012). Schizophrenia, for example, is approximately 2.5 times more frequent in those with a marker of previous T. gondii infection (Gutiérrez-Fernández Reference Gutiérrez-Fernández, del Castillo and Mañanes-González2015). Links have also been documented with many other infections, including chlamydia pneumoniae, which was more commonly found in the blood and brain of those with a diagnosis of schizophrenia (Gutiérrez-Fernández Reference Gutiérrez-Fernández, del Castillo and Mañanes-González2015).
Potential causes for these effects include direct pathogen effects, impacts on the inflammatory response and alterations in neurodevelopment. There may also be an effect on pre-existing genetic vulnerabilities (Khandaker Reference Khandaker, Zimbron and Dalman2012). Elevated levels of complement C1q (part of the complement immune response) have been seen in the mothers of infants who went on to develop psychosis. This is significantly correlated with viral antigens and may have a further effect on development and synaptic pruning (Nimgaonkar Reference Nimgaonkar, Prasad and Chowdari2017).
Childhood development
Motor, sensory and cognitive impairment
A systematic review by Hameed & Lewis (Reference Hameed and Lewis2016) shows that children of parents with schizophrenia have differences in developmental patterns, evident in some cases from early childhood. They have higher rates of obstetric complications, as discussed previously, but also of motor, cognitive and behavioural changes. These include lower scores for motor coordination, balance and sensory perception skills (Hameed Reference Hameed and Lewis2016). Motor impairment is linked to delays in childhood milestones and neurological problems and is a risk for schizophrenia spectrum disorders and affective psychosis (Laurens Reference Laurens, Luo and Matheson2015). A review of research by Laurens et al (Reference Laurens, Luo and Matheson2015) concluded that a broad range of cognitive impairments in middle childhood are antecedents of schizophrenia spectrum disorders, but found evidence to be less conclusive in early childhood or adolescence.
Language impairment
Language impairment in childhood has been shown to be a risk for later development of schizophrenia spectrum disorders. Language expression deficits are particularly linked, but receptive deficits and speech abnormalities may be antecedent (Laurens Reference Laurens, Luo and Matheson2015). Hearing impairment (congenital or acquired) is similarly associated with an increased risk of later psychosis. In those with hearing impairment, the odds ratio for schizophrenia is 3.15 and for psychotic symptoms 2.23 (Linszen Reference Linszen, Brouwer and Heringa2016). Risks such as exposure to CNS infection have been noted in both hearing loss and psychosis, and both perinatal and genetic links have been proposed as shared factors (Linszen Reference Linszen, Brouwer and Heringa2016). Further mechanisms have been suggested by Linszen et al (Reference Linszen, Brouwer and Heringa2016), including:
• social isolation (linked to social defeat, loneliness and higher stress-induced dopamine release)
• difficulties in communication
• diminished theory of mind
• impaired source monitoring
• auditory deafferentation (understimulation of the auditory system increases its sensitivity, resulting in perception of sound with no external source).
Visual impairment
Notably, having schizophrenia is associated with an increased risk of having visual impairment (in near or distance vision), possibly compounding problems with perception and social isolation (Viertiö Reference Viertiö, Laitinen and Perälä2007).
Medical and metabolic disorders
A review by Giannitelli et al (Reference Giannitelli, Consoli and Raffin2018) further studied the associations between medical disorders and psychosis in children and adolescents. These included inborn errors of metabolism such as disorders of folate, cobalamin (vitamin B12) and copper transport or metabolism. Other evidence for a metabolic component to psychosis development comes from Perry et al (Reference Perry, Upthegrove and Thompson2018). They demonstrated that insulin resistance was linked to early psychotic experiences even before the onset of a psychotic disorder. They further suggested that these metabolic changes may interact with inflammatory processes in childhood, increasing the risk of these psychotic experiences (Perry Reference Perry, Upthegrove and Thompson2018).
Nutrition
Polyunsaturated fatty acid (PUFA)
Nutrition, both pre- and postnatal, has implications for later psychosis. For example, deficits in total polyunsaturated fatty acid (PUFA) consumption have been demonstrated in those at ultra-high risk of psychosis compared with controls (Rice Reference Rice, Schäfer and Klier2015). PUFA metabolism is linked to monoamine transmission, cytokines and oxidative stress, and so fits in with theories of schizophrenia development that we discuss further in the third article in this series. Pawelczyk et al (Reference Pawelczyk, Trafalska and Kotlicka-Antczak2016) concluded that significant differences in types of PUFA consumed were present between high-risk groups who converted to psychosis, those who did not convert and healthy controls. They suggested that dietary patterns may have a role in altering transition risk (Pawelczyk Reference Pawelczyk, Trafalska and Kotlicka-Antczak2016). One type of PUFA under ongoing investigation is docosahexaenoic acid, which is needed for brain development. Studies are not yet conclusive here and data have shown possible associations with high and low levels, so work continues to clarify this (Laurens Reference Laurens, Luo and Matheson2015; Sun Reference Sun, Simonyi and Fritsche2018).
Vitamin D
Access to sunlight and adequate nutrition have a significant role in vitamin D metabolism. Vitamin D is important in bone metabolism as well as calcium homeostasis and has been further shown to have significant effects on the immune system and in neural processes. Given this link, it has been suggested that vitamin D may have a role in schizophrenia via the immune system. People with schizophrenia have demonstrated lower vitamin D levels than controls. Evidence suggests that lower vitamin D levels could affect not only early brain development, but also later growth and maturation (Kočovskà Reference Kocˇovskà, Gaughran and Krivoy2017).
Folate and vitamin C
Levels of folate and vitamin C have been shown to be reduced in some studies of first-episode psychosis. However, work is ongoing to establish the direction of this association and risk impact. Folate in particular is linked to neurological health. Many other vitamins and minerals have been studied, for example B12, calcium and magnesium, with no clear differences concluded (Firth Reference Firth, Carney and Stubbs2018).
Maternal nutrition during pregnancy
The effects of poor maternal nutrition on the risk of psychosis in subsequent offspring have also been investigated. He et al (Reference He, Chen and Guo2018) examined the population exposed to the Chinese famine between 1959 and 1961 and demonstrated that prenatal malnutrition significantly increased the risk of schizophrenia, with an odds ratio of 1.84. However, this was only found in rural, not urban, populations, suggesting possible variations in the severity of famine between areas. Roseboom et al (Reference Roseboom, Painter and van Abeelen2011) similarly concluded that there was an increased risk of schizophrenia in those whose mothers were affected by a Dutch famine. They found that the most vulnerable period was early in gestation, with those conceived during the famine the most affected. Alongside this they noted data showing an association between maternal hyperemesis gravidarum and development of behavioural and psychological problems in offspring during adulthood.
Conclusions
In this first article of a three-part series, we have given an overview of early-life risk factors for psychotic symptoms. Potential risk factors are summarised in Box 3, but it should be noted that the evidence for many of these is equivocal. In part 2 (Romain Reference Romain, Eriksson and Onyon2019a), this risk timeline is continued into later childhood, adolescence and adulthood, discussing substance misuse, psychological and other environmental factors in preparation for the final article (Romain Reference Romain, Eriksson and Onyon2019b), which considers final common pathways and primary, secondary and tertiary preventive options for the future.
BOX 3 Potential early-life risk factors for psychotic disorders
Genetics
There is thought to be high heritability: 79% for schizophrenia and 73% for schizophrenia spectrum disorders
Specific genes have been linked to increased risk of psychosis:
• 3q29
• DISC1
• 22q11.2 DS
• NMDA receptor
• Neurexins
• 17q12 deletion See Table 1 for futher genes
Parental risk factors
• Factors related to parental age
• Maternal high body mass index during or prior to pregnancy
• Lower parental, particularly maternal, education
• Maternal exposure to severe emotional trauma during pregnancy
Prenatal development and birth
• Abnormalities in fetal brain development
• Premature birth
• A high obstetric complication index score
• Low retinol during the second trimester
• Maternal substance misuse during pregnancy
• Birth during the winter or spring
• Prenatal exposure to infectious diseases, e.g. gestational influenza
Childhood development
• Language impairment
• Hearing impairment (congenital or acquired)
• Visual impairment
• Metabolic disorders, e.g. insulin resistance and disorders of folate, cobalamin and copper transport or metabolism
• Childhood CNS infection
Nutrition
• Deficits in total polyunsaturated fatty acid (PUFA), folate, vitamin D
• Very poor maternal nutrition (e.g. famine) during pregnancy
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bja.2018.66.
MCQs
Select the single best option for each question stem
1 The genetic risk factor with the highest odds ratio regarding psychosis is:
a 7q36.3 duplication
b 15q13.3 deletion
c homocysteine SNPs
d 3q29 deletion
e 16p13.1 duplication.
2 The CNV known as velocardiofacial syndrome is:
a 16p13.1 duplication
b 22q11.2 deletion
c 15q12.3 deletion
d 1q21.2 deletion
e 3q18 deletion.
3 Increased risk of psychosis has been linked to nutritional deficiency of:
a potassium
b calcium
c magnesium
d folate
e sodium.
4 Fluctuating dermatoglyphic asymmetry:
a is a pattern of folds thought to be a marker of late neurological injury
b is pattern of folds thought to be a marker of early neurological injury
c is a pattern of folds unrelated to neurological changes
d is formed from a different germinal layer than the brain
e refers to patterns of folds on the surface of the scalp.
5 Mechanisms suggested to underlie the increased risk of psychosis in hearing impairment include:
a diminished theory of mind
b social isolation
c auditory deafferentation
d impaired source monitoring
e all of the above.
MCQ answers
1 d 2 b 3 d 4 b 5 e




eLetters
No eLetters have been published for this article.