LEARNING OBJECTIVES
-
Understand the pharmacological properties of ketamine as an anaesthetic, a drug of misuse and a proposed antidepressant
-
Review the current evidence for ketamine in treating depressive disorder and recognise potential risks associated with long-term use
-
Recognise the arguments for and against the use of ketamine as a novel antidepressant
Major depressive disorder (MDD) is a common psychiatric illness that is estimated to affect 120 million people worldwide (Reference Li, Vicknasingam and CheungLi 2011a). The low heritability of MDD (39%) suggests that environmental rather than genetic factors are contributing more to variations in its phenotype. In the past 50 years, there have been significant advances in the treatment of MDD, including the development of various antidepressants, such as tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs) and noradrenergic and specific serotonergic antidepressants (NaSSAs). Psychotherapies such as cognitive–behavioural therapy (CBT) and interpersonal psychotherapy are also evidence-based treatments. Given the low heritability of MDD, it is not surprising that response rates to SSRIs and NaSSAs are around 62% and 67% respectively (Reference Papakostas, Homberger and FavaPapakostas 2008). Environmental factors such as relationship problems, financial difficulties and comorbid substance misuse often lead to poor treatment response, and antidepressants combined with CBT have shown promising results in prevention of mood disorders (Reference Brenner, Madhusoodanan and PuttichandaBrenner 2010). Although 70–90% of patients with depression achieve remission, around 10–30% are refractory to initial treatment but respond to switching or combination of antidepressants, electroconvulsive therapy (ECT) or psychotherapy. Around one-third of patients who are refractory to initial treatment do not respond to other antidepressants and combined treatment (Reference Al-HarbiAl-Harbi 2012). Hence, 9–10% of patients with depression have treatment-resistant depression. Recently, some studies have found that ketamine has rapid antidepressant effects in patients with treatment-resistant depression.
What is ketamine?
Ketamine was first synthesised in 1962, by Calvin Stevens in a Parke-Davis laboratory. It was considered a safer alternative to the hallucinogen phencyclidine (PCP). Parke-Davis patented ketamine as an anaesthetic in 1966. Ketamine was first used as a battlefield anaesthetic in the Vietnam War. The drug induced a dissociative state that was helpful in treating wounded soldiers by keeping them conscious but cognitively separated from pain (Reference Trujillo, Smith and SullivanTrujillo 2011). During the 1970s, young people started to use ketamine as a recreational drug. Ketamine is still used in veterinary medicine, and also as a battlefield anaesthetic in low- and middle-income countries.
Ketamine is a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist. It exists in two optical isomer forms, S(+) ketamine and R(−) ketamine. In human brain, S(+) ketamine binds to the PCP-binding site of the NMDA receptor with fivefold higher affinity than does R(−) ketamine. A pharmaceutical company is currently testing a nasal spray formulation of the S(+) ketamine as a potential antidepressant, although no results have been published. Ketamine is metabolised by hepatic cytochromes CYP 3A4, 2B6 and 2C9 into dehydronorketamine and norketamine, which can be detected in urine (Reference Li, Vicknasingam and CheungLi 2011a). Ketamine has a high first-pass effect (Reference Salvadore and SinghSalvadore 2013) and short half-life (∼3 h) (Reference Lo and CummingLo 1975). Although ketamine can be administered via the oral, intranasal and intramuscular routes, the intravenous route is preferred because it allows precise dosing and dose adjustment if side-effects occur (Canadian Agency for Drugs and Technologies in Health 2014).
Ketamine can relieve pain through the mu opioid receptors. Its action on the monoaminergic system is similar to that of amphetamine or cocaine (Reference SchatzbergSchatzberg 2014), and the drug is liable to misuse owing to its dopaminergic, opiate and stimulant effects (Reference Moghaddam, Adams and VermaMoghaddam 1997; Reference SchatzbergSchatzberg 2014). Other pharmacodynamic mechanisms include prevention of the influx of calcium ions and alteration of limbic system functioning (Reference BergmanBergman 1999). Calcium influx into mitochondria may lead to neurotoxicity in the frontal cortex (Reference Xu and LipskyXu 2015). Ketamine is associated with memory impairment (Reference Morgan, Dodds and FurbyMorgan 2014). By blocking NMDA receptors, ketamine exacerbates the psychotic and cognitive symptoms of schizophrenia (Reference Malhotra, Pinals and AdlerMalhotra 1997).
At low doses, ketamine causes euphoria, sensory distortions, impairments in set-shifting and heightened feelings of empathy (Reference Krystal, Karper and SeibylKrystal 1994; Reference Jansen and Darracot-CankovicJansen 2001; Reference Dillon, Copeland and JansenDillon 2003). At high doses, it causes dissociative effects, hallucination, intoxication and frightening experiences (Reference Trujillo, Smith and SullivanTrujillo 2011). Reference Lai, Katalinic and GlueLai et al (2014) have demonstrated that the psychotomimetic effects of ketamine are dose-related and that its antidepressant effects may also be. Ketamine misuse is associated with amnesia, dependence, dissociation, lower urinary tract dysfunction and poor impulse control (Reference Li, Vicknasingam and CheungLi 2011a). The worldwide incidence of ketamine seizures significantly increased between 2003 and 2006 (Reference Xu and LipskyXu 2015).
In this review, we will examine recent evidence regarding the use of ketamine as a rapid antidepressant and its potential for misuse (Reference Yang and HashimotoYang 2014). In arguing that the evaluation of antidepressant ketamine must go beyond clinical trials, we hope to give psychiatrists and patients a better understanding of the drug, its antidepressant efficacy and side-effects, and the controversies surrounding its use in psychiatry.
Current legal status and prevalence of ketamine misuse
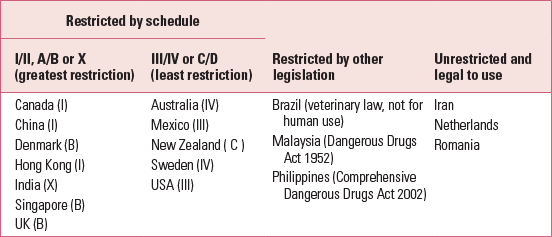
Ketamine misuse is common worldwide, under street names such as K and Special K. Recreational users snort ketamine powder or inject liquid ketamine. Table 1 shows the current legal status of ketamine in different countries. In the UK, ketamine is now a class B drug because the prevalence of ketamine misuse among recreational users increased from 25% to 40% between 2002 and 2007 (Reference McCambridge, Winstock and HuntMcCambridge 2007). The British Crime Survey of 2010–2011 reported that there were 125 000 recreational users of ketamine in the UK (Reference Smith and FlatleySmith 2011). In the USA, ketamine is a schedule III drug (Reference SchatzbergSchatzberg 2014), and emergency room visits due to ketamine misuse increased by 2000% between 1995 and 2002 (Substance Abuse and Mental Health Services Administration 2003). In Hong Kong, ketamine is a schedule I drug. It is the most common drug of misuse and consumed by more than 80% of drug users (Reference Li, Vicknasingam and CheungLi 2011a). Ketamine is the second most commonly misused drug in Taiwan (Reference Lua, Lin and TsengLua 2003), where the age at onset of ketamine misuse is as young as 15 years (Reference Lee, Yeh and YangLee 2012). There is pressure to reschedule ketamine to limit its use in Taiwan (Reference Li, Vicknasingam and CheungLi 2011a). Ketamine has become the second most common drug of misuse in central rural China (Reference Deng, Tang and SchottenfieldDeng 2012). Ketamine misusers have a false perception that ketamine is relatively safe, and this has made it the preferred drug. Some Asian countries have very strict laws on drugs, imposing harsh punishment. In Malaysia and Singapore, anyone caught with ketamine faces imprisonment or strokes of the cane (Central Narcotics Bureau 2013). Repeat offenders may face the death penalty in Malaysia.
TABLE 1 Restriction of ketamine use in different countries
Controversies surrounding ketamine
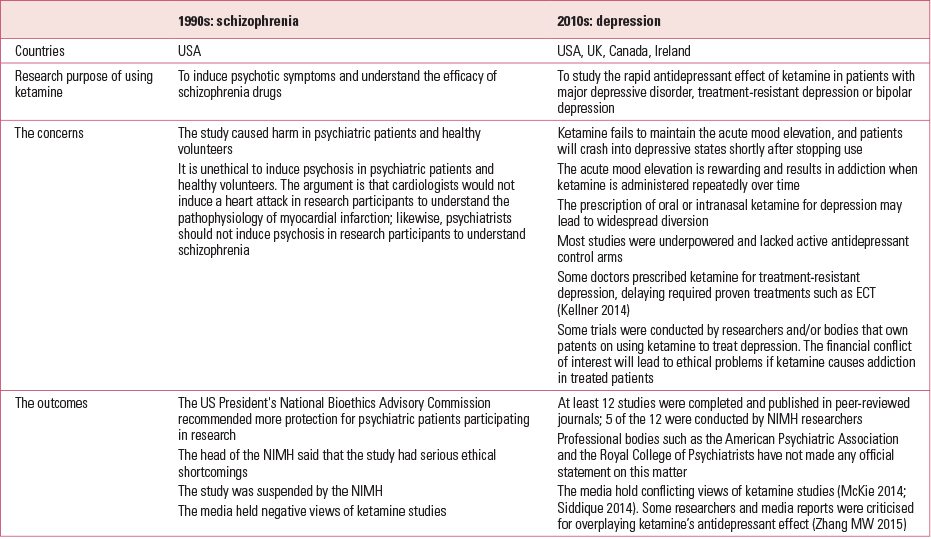
The use of ketamine in psychiatric research has been the subject of controversy over recent decades (Table 2).
TABLE 2 The use of ketamine in psychiatric research in the 1990s and 2010s
Ketamine and schizophrenia
The first debate occurred in 1999 (Reference MarshallMarshall 1999), when the National Institute of Mental Health (NIMH) suspended a US study in which ketamine was used to induce psychosis in patients with schizophrenia. Researchers argued that such ketamine challenge studies would generate important new knowledge about the pathophysiology of schizophrenia and improve patients’ well-being (Reference CarpenterCarpenter 1999). However, after 15 years, ketamine trials have neither changed the treatment nor improved the outcome of schizophrenia.
Ketamine and depression
Controversy regarding ketamine has arisen in the current decade. This time, researchers have shifted the focus to depression and propose using ketamine as a rapid antidepressant to treat MDD, treatment-resistant depression or bipolar depression. Their trials try to convince psychiatrists that low-dose ketamine is a rapid antidepressant and does not cause addiction or memory impairment. Some researchers have proposed repeated ketamine infusions to maintain its antidepressant effect. Yet here is the problem. In the past two decades, we have gained more knowledge about the misuse potential of ketamine and its harmful psychological and physical consequences (Reference Morgan and CurranMorgan 2012). Many governments have rescheduled ketamine to exert tighter control over the drug. Claims that low-dose ketamine is a non-addictive and safe antidepressant are based on just short periods of observation: cross-sectional (Reference Tang, Morgan and LauTang 2015) and longitudinal studies (Reference Morgan, Muetzelfeldt and CurranMorgan 2010) have found that regular consumption of ketamine leads to addiction, depression and cognitive impairment. The proposed repeated infusions of ketamine may lead to addiction, just as repeated low-dose intake of other common substances of misuse, such as nicotine, can. There is no evidence to suggest that repeated infusions of ketamine at subanaesthetic doses would not cause addiction.
Recent reviews discuss the potential of ketamine as an antidepressant (Reference Salvadore and SinghSalvadore 2013; Reference DeWilde, Levitch and MurroughDeWilde 2015), but their authors include employees and advisors of the pharmaceutical company that owns the manufacturer of intranasal ketamine. These reviews give minimal coverage to ketamine misuse and potential complications of chronic use. They may underestimate the risks of repeated administration of ketamine to maintain its antidepressant effects (Reference Salvadore and SinghSalvadore 2013).
Some researchers recommend integrating ketamine into in-patient antidepressant treatment (Reference Naughton, Clarke and O'LearyNaughton 2014). By contrast, others emphasise that two-thirds of patients relapsed within 1 week and not all patients with MDD respond to intravenous ketamine (Reference Katalinic, Lai and SomogyiKatalinic 2013). Reference RasmussenRasmussen (2016) stresses that the safety of prolonged ketamine use has not been established and that enthusiasm must be tempered by consideration of its addiction potential and tolerance effects.
The rapid antidepressant effect of ketamine is not unique, and most psychedelic drugs, such as amphetamine and cocaine, demonstrate an acute mood-elevation effect (Fig. 1). Indeed, amphetamine was found to be a powerful antidepressant in the 1930s (Reference HirschfieldHirschfield 2012). However, this acute mood elevation is short-lived, and recipients will crash into depressive states after stopping use (Reference Barr, Markou and PhillipsBarr 2002). The recipient tries to avoid the crash or withdrawal by taking more of the drug. The acute mood elevation is rewarding and leads to addiction.
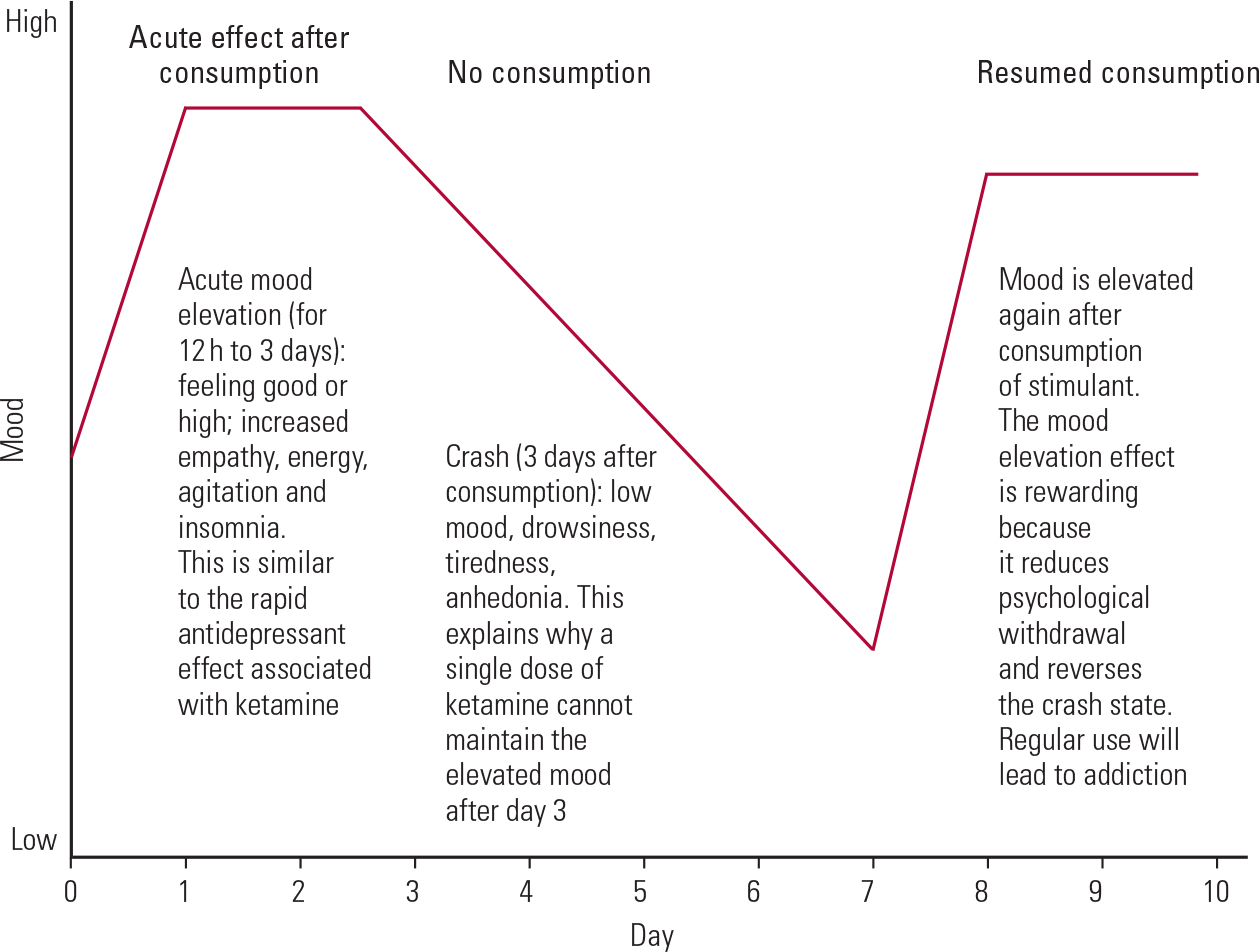
FIG 1 The effect of stimulants (e.g. amphetamine, cocaine) on mood.
In addition, recent animal studies have shown how ketamine could successfully reverse the hypodopaminergic state following withdrawal from amphetamine. This finding suggests that ketamine has a stimulant effect (Reference Belujon, Jakobowski and DollishBelujon 2016). This stimulant effect may be responsible for the rapid antidepressant effects that have been reported in most clinical trials conducted on people with depression. As yet, there is no scientific evidence to prove that the rapid antidepressant effect associated with ketamine is different from the ‘drug high’ of stimulants. Consequently, ketamine is not safe for regular use.
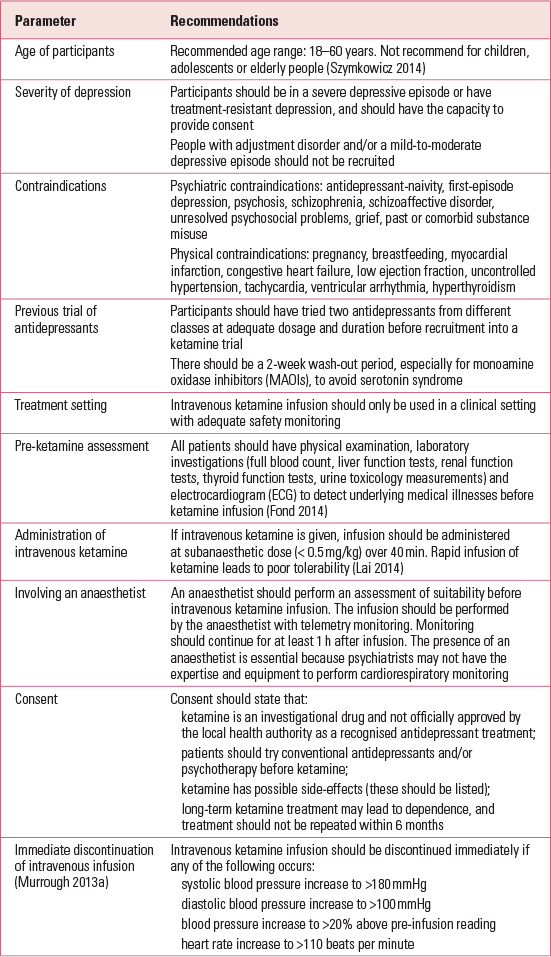
There are other concerns related to ketamine use in the treatment of MDD. Aan het Reference TorreRot et al (2010) recommend that ketamine should not be administered outside the hospital. Reference TorreTorre (2010) urges that knowledge of cognitive impairment and reduction in well-being associated with frequent ketamine use be disseminated to both clinicians and the general public. Reference Li, Vicknasingam and CheungLi et al (2011a) highlight the risks of ketamine, including urinary tract dysfunction, risk of diversion of prescribed ketamine and the fact that ketamine may cause more harm than some of the drugs scheduled in the United Nations international drug control conventions. Most reviews end inconclusively, suggesting that more research needs to be done, but none of the reviews attempts to explore other issues, including ethics, conflict of interest and motives behind ketamine research. No evidence-based guidelines recommend intravenous ketamine in the treatment of MDD. However, a report by the Canadian Agency for Drugs and Technologies lists four important conclusions and implications (Box 1), and The Maudsley Guidelines (Reference Taylor, Paton and KapurTaylor 2015) list the pros and cons of ketamine prescription.
BOX 1 Recommendations of the Canadian Agency for Drugs and Technologies in Health (2014)
-
Psychiatrists need to be mindful that most ketamine trials did not specify which previous treatments patients had received and that the effect of ketamine was compared with placebo or midazolam, but not with active antidepressants
-
The requirement for overnight hospital admission for intravenous ketamine administration makes the logistics of treatment more complicated than with conventional antidepressants
-
The evidence claiming that intravenous ketamine can reduce suicidal ideation is of poor quality; there is no evidence that intravenous ketamine can prevent actual suicide attempts
-
There is a lack of current evidence to recommend ketamine as a standard treatment for common psychiatric disorders (e.g. major depressive disorder, post-traumatic stress disorder)
The current evidence on ketamine as a novel and rapid antidepressant
In the past 15 years, at least 12 studies were conducted to assess the efficacy of ketamine as an antidepressant, but most studies were not adequately powered because of small sample size (online Table DS1). Seven of the 12 that we identified were by authors and/or an institution that reported a financial conflict of interest regarding the use of antidepressant ketamine; four were published in a single journal, Biological Psychiatry. The response rate ranged from 29% (Reference Diamond, Farmery and AtkinsonDiamond 2014) to 79% (Reference Zarate, Brutsche and IbrahimZarate 2012). The relapse rate was as high as 73% (Reference Ibrahim, Diazgranados and Franco-ChavesIbrahim 2012). Not all patients with treatment-resistant depression responded to ketamine. Predictors of poor response included a large hippocampus (Reference Abdallah, Salas and JackowskiAbdallah 2014), homozygous met/met alleles of the brain-derived neutrophic factor (BDNF) gene (Reference Li, Vicknasingam and CheungLiu 2011), lack of initial mood elevation effect (Reference Murrough, Perez and PillemerMurrough 2013a) and old age (age-related brain changes affect sensitivity to ketamine) (Reference Szymkowicz, Finnegan and DaleSzymkowicz 2014).
Owing to low remission rates and the short half-life of ketamine, researchers have advocated multiple ketamine infusions to maintain its antidepressant effect (Reference Aan het Rot, Collins and Murroughaan het Rot 2010; Reference Diamond, Farmery and AtkinsonDiamond 2014). Oral and intranasal ketamine were proposed for convenient administration. This practice is similar to chronic ketamine use by recreational users. Long-term ketamine use activates glutamate neurotransmission in the prefrontal cortex and leads to neurotoxicity (Reference Krystal, Karper and SeibylKrystal 1994).
The researchers emphasise the safety of a subanaesthetic dose of ketamine and distinguish its effects from the high doses used by recreational users. The administration of subanaesthetic doses is not without risk, and a single subanaesthetic ketamine infusion causes side-effects in 2% of participants (Reference Salvadore and SinghSalvadore 2013). The pharmacodynamics of subanaesthetic doses of ketamine that lead to its euphoric effect is a topic of research. In animal studies, subanaesthetic doses were associated with acute increases in extracellular glutamate and dopamine in the prefrontal cortex (Reference Moghaddam, Adams and VermaMoghaddam 1997) and anterior cingulate cortex (Reference Stone, Dietrich and EddenStone 2012), leading to excitatory neurotransmission. Other mechanisms of action include activation of amino methyl isoxazole propionic acid (AMPA) and motor-evoked potentials (Reference Di Lazzaro, Oliviero and ProficeDi Lazzaro 2003).
Critical appraisal of current trial evidence
The studies in question (Table DS1) have a number of flaws that caution against the widespread use of ketamine.
First, selection bias occurred in the Reference Ghasemi, Kazemi and YoosefiGhasemi et al (2014) trial, as patients randomised to the ketamine group had lower mean depression scores than those randomised to the ECT group (30.22 v. 35.88, P = 0.07, which is approaching statistical significance). Second, in all of the studies, the follow-up period was too short to rule out addiction and complications associated with regular ketamine infusion. Third, ketamine was used with other psychotropic medications in some studies (Reference DiazGranados, Ibrahim and BrutscheDiazGranados 2010b; Reference Zarate, Brutsche and IbrahimZarate 2012; Reference Diamond, Farmery and AtkinsonDiamond 2014; Reference Lapidus, Levitch and PerezLapidus 2014). Researchers were not able to distinguish whether the antidepressant effect was due to other medications or the sole effect of ketamine. Fourth, in one study (Reference Mathew, Murrough and aan het RotMathew 2010) the depression rating scale used as the indicator of response was administered twice within 3 days. This could lead to bias, because participants might remember their previous responses and give better ones, especially in an open-label trial. Fifth, Reference DiazGranados, Ibrahim and BrutscheDiazGranados et al (2010a) reported rapid resolution of suicidal ideation after a single infusion of ketamine. This study used the Beck Scale for Suicidal Ideation (SSI), on which higher scores indicate higher suicidal ideation. Participants were separated into two groups on the basis of scores (range 0–10) derived from analysis of their total SSI scores: those with significant suicidal ideation (scores >3) and those without significant suicidal ideation (scores <4). DiazGranados et al note that this cutoff threshold of 3/4 has been used before to indicate clinically significant suicidal ideation. However, the study to which they refer (Reference Holi, Pelkonen and KarlssonHoli 2005) was in an adolescent population. In adult populations some studies (e.g. Reference Sokero, Melartin and RytsalaSokero 2003) have used a cut-off of >6. Thus, the suicidal ideation scores were relatively low to begin with. There was a small reduction in the magnitude of suicidal ideation, but the results might not be clinically significant. Sixth, the studies were not able to ascertain the differences between the rapid antidepressant effect of ketamine and the ‘drug high’ of recreational drugs. Reference Mathew, Murrough and aan het RotMathew et al (2010) proposed head-to-head comparison of intravenous ketamine and intravenous amphetamine, but such a study might not be ethical. Seventh, having a placebo arm is crucial in antidepressant trials, because placebo achieves a response rate between 30% and 40% in people with MDD (Reference Sonawalla and RosenbaumSonawalla 2002). As a result, the results of the open-label studies (Reference Mathew, Murrough and aan het RotMathew 2010; Reference Murrough, Perez and PillemerMurrough 2013a; Reference Diamond, Farmery and AtkinsonDiamond 2014) are less reliable. Eighth, although some of the randomised controlled trials (RCTs) were claimed to be double-blind, the effects of ketamine (e.g. dissociation) would be obvious even to masked (‘blinded’) assessors. This might lead to bias in assessment, because the assessors would know which participants were randomised to the ketamine group. Such bias might affect the accuracy of findings. Last, none of the 12 trials was conducted in Asia, Africa or South America, and this limits the generalisation of findings to non-Western populations. Meta-analyses of the studies demonstrate significant acute mood elevation shortly after ketamine infusion (Reference Coyle and LawsCoyle 2015; Reference Lee, Della Selva and LiuLee 2015). This is not surprising, because all of the trials showed positive results, and Reference Lee, Della Selva and LiuLee et al's (2015) meta-analysis did not perform Egger's regression test or show P-values when calculating the publication bias.
Further debate
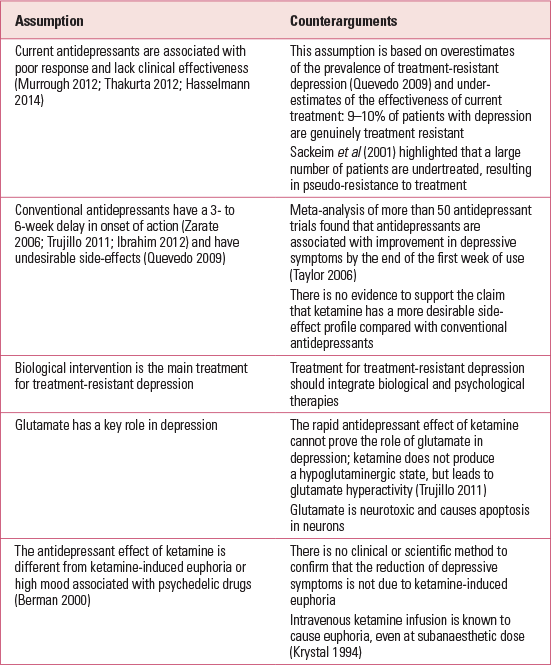
Some researchers assume that ketamine is a rapid antidepressant because depressive symptoms were lifted immediately after infusion. Following the same logic, if other psychedelic drugs of misuse, such as amphetamine or 3,4-methylenedioxymethamphetamine (MDMA), lift depression immediately, these drugs are also potential ‘rapid antidepressants’. Researchers are selectively focusing on the artificial mood elevation of ketamine, forgetting the long-term risk associated with its misuse and the fact that depression is a chronic illness. If ketamine causes addiction in patients with depression, the dual diagnosis will increase the healthcare burden. More points in the debate are outlined in Table 3.
TABLE 3 Assumptions held by ketamine researchers and counterarguments
Potential risk of repeated ketamine infusions
Exacerbated depression
As ketamine is usually used as an anaesthetic for single administration, the risks of repeated administration are unknown (Reference Salvadore and SinghSalvadore 2013). Psychiatrists need to be mindful that acute and chronic administration of ketamine have different effects on the brain. Acute ketamine use increases (Reference Moghaddam, Adams and VermaMoghaddam 1997) whereas chronic ketamine use decreases (Reference Narendran, Frankle and KeefeNarendran 2005) brain dopamine levels (Reference Tang, Morgan and LauTang 2015). If ketamine is an effective antidepressant for chronic use, why did Reference Morgan, Muetzelfeldt and CurranMorgan et al (2010) record increasing depression scores (i.e. increasing depressive symptoms) among frequent ketamine misusers over a 12-month period? Moreover, patients who demonstrate poor initial response will not respond to further ketamine infusions (Reference Murrough, Perez and PillemerMurrough 2013a). Thus, the rapid antidepressant effect after a single ketamine infusion does not imply that regular ketamine infusion will have a long-term antidepressant effect, let alone that it is safe for long-term use.
Risk of dependence
Researchers have claimed that ketamine does not cause dependence (Reference Diamond, Farmery and AtkinsonDiamond 2014), but this contradicts current evidence. There is biochemical and behavioural evidence to suggest that ketamine indeed causes dependence with chronic use, even at subanaesthetic doses. Anticholinergic or amphetamine-like antidepressant medications are misused by patients (Reference Nunes, Deliyannides and DonovanNunes 1996). A subanaesthetic dose of ketamine is associated with psychomotor activation, which, applying the psychomotor stimulant theory of drug reward (Reference WiseWise 1988), increases the addictive potential of the drug (Reference Trujillo, Smith and SullivanTrujillo 2011). Animal studies show that acute ketamine infusion produces immediate mood elevation by increasing dopamine levels in the prefrontal cortex (Reference Tan, Lam and WaiTan 2012). This reward effect enhances animals’ self-administration of ketamine (Reference Carroll and StotzCarroll 1983; Reference De Luca and BadianiDe Luca 2011).
Reference Sanacora and SchatzbergSanacora & Schatzberg (2015) have expressed concerns about the misuse potential of ketamine as an antidepressant owing to its effect on the mu opioid receptor. Reference Xu and LipskyXu & Lipsky (2015) believe that the effects of ketamine withdrawal are underestimated because there is no objective scale to assess withdrawal from the drug. In one study, more than half of ketamine misusers reported withdrawal symptoms, including exacerbation of depression, irritability, aggression and fatigue (Reference Chen, Lee and ChanChen 2005).
Ketamine is not suitable for oral administration because of its high first-pass rate (Reference Salvadore and SinghSalvadore 2013) and poor bioavailability (Reference Sinner, Graf, Schuttler and SchwildenSinner 2008). After oral ingestion, the ketamine's nitroso derivative, N-nitrosoketamine, causes genotoxic effects in cell culture (Reference Toyama, Shimizu and SuzukiToyama 2006). Oral – and intranasal – S(+) ketamine is reinforcing and addictive (Reference Shram, Sellers and RomachShram 2011) and makes the ‘slippery ketamine slope’ (Reference SchatzbergSchatzberg 2014) more slippery. Ketamine should be administered in tightly regulated hospital-based treatment under direct observation.
Systemic complications
The proponents of long-term ketamine treatment should be mindful of complications experienced by chronic users (Fig. 2). Their argument that low doses of ketamine would not lead to cognitive impairment is not supported by the evidence. In fact, frequent ketamine users demonstrate impairments in working memory and executive function (Reference Morgan, Muetzelfeldt and CurranMorgan 2010). The claims based on some short-term trials that ketamine does not cause cognitive impairment are premature, because impairments will become evident only after repeated infusions over the long term. Chronic ketamine use causes pathological changes, including reduction in grey matter in the bilateral frontal cortex that resembles the pattern in schizophrenia (Reference Liao, Tang and CorlettLiao 2011) and epithelial inflammation in the urinary tract that resembles chronic interstitial cystitis. Furthermore, animal studies have demonstrated neurotoxicity after repeated administration of ketamine (Reference Olney, Labruyere and WangOlney 1991).
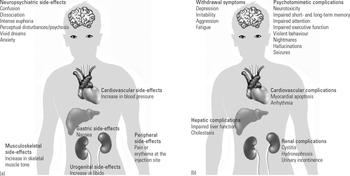
FIG 2 (a) Systemic side-effects of ketamine at subanaesthetic dose (Reference Salvadore and SinghSalvadore 2013); (b) systemic complications of chronic ketamine use (Reference Noppers, Niesters and AartsNoppers 2011; Reference Pappachan, Raj and ThomasPappachan 2014; Reference StahlStahl 2014).
Challenges to the glutamate theory
Some researchers suggest that ketamine is a potential antidepressant owing to its antagonism of NMDA receptors (Reference HasselmannHasselmann 2014). There is evidence that challenges this postulation. First, ketamine demonstrates a modest affinity for the NMDA receptor over other receptors, implying that the acute mood elevation effect is due to other mechanisms of action (Reference Hillhouse, Porter and NegusHillhouse 2014; Reference Sanacora and SchatzbergSanacora 2015). These mechanisms might include its effect on mammalian target of rapamycin (mTOR) receptors (Reference Li, Liu and DwyerLi 2011b), BDNF (Reference Autry, Adachi and NosyrevaAutry 2011), the dopamine system (Reference Tan, Lam and WaiTan 2012), sigma receptors (Reference Robson, Elliott and SeminerioRobson 2012) and nicotinic acetylcholine receptors (Reference Nishitani, Nagayasu and AsaokaNishitani 2014).
Second, if ketamine is an effective antidepressant, we would expect other NMDA receptor antagonists to exhibit antidepressant effects. This is not the case. Memantine is a non-competitive NMDA receptor antagonist, but it does not have a rapid antidepressant effect (Reference Monteggia and ZarateMonteggia 2015). Riluzole exerts multiple actions on the ionotropic glutamate receptor system and inhibits NMDA receptors (Reference Debono, Le Guern and CantonDebono 1993). In trials, combined riluzole and ketamine treatment did not prevent relapse of depression (Reference Mathew, Murrough and aan het RotMathew 2010) or alter the course of antidepressant response to ketamine (Reference Ibrahim, Diazgranados and Franco-ChavesIbrahim 2012). Ketamine's primary site of action is the PCP site of the NMDA receptor (Reference Mathew, Murrough and aan het RotMathew 2010). NMDA antagonists do not have a rapid antidepressant effect, because these drugs do not act on the PCP site. Other researchers suggest that the acute mood elevation effect of ketamine occurs principally through AMPA receptor activation (Reference Sanacora, Zarate and KrystalSanacora 2008).
Ethical concerns
Ethical questions relating to the use of ketamine to treat depression have not been fully addressed. Reference Sisti, Segal and ThaseSisti et al (2014) expressed concerns about the mental capacity of severely depressed patients when consenting to ketamine treatment. If ketamine is approved by a health authority such as the Medicines Healthcare products Regulatory Agency (MHRA) or the Food and Drug Administration (FDA) as a licensed antidepressant, psychiatrists will feel pressurised to prescribe, especially by patients who are addicted to ketamine. The issues of beneficence and non-maleficence must be considered. Off-label use of ketamine is not without harm. Reference Kellner, Greenberg and AhleKellner et al (2014) reported that private anaesthesiologists started to use ketamine to treat depression, but with minimal antidepressant benefits. The authors were worried that such use of ketamine in severely ill people delays commencement of evidence-based treatments such as antidepressants or ECT. Reference Sisti, Segal and ThaseSisti et al (2014) reported that some clinics in the USA offer financial incentives to promote repeated ketamine infusion.
Authors’ declarations also reveal potential financial conflicts of interest in the research. The Icahn School of Medicine at Mount Sinai and some of its research employees own the patent for use of intranasal ketamine for the treatment of depression (US 8785500 B2), and they will receive financial benefits if the FDA approves the formulation. Teams at Icahn have conducted various studies demonstrating the positive effects of ketamine in treatment of depression (Reference Berman, Cappiello and AnandBerman 2000; Reference Mathew, Murrough and aan het RotMathew 2010; Reference Murrough, Perez and PillemerMurrough 2013a,Reference Murrough, Iosifescu and Changb; Reference Lapidus, Levitch and PerezLapidus 2014). A principal investigator at NIMH is listed as co-inventor of another patent application for the use of ketamine in depression (WO 2013056229 A1), although he has assigned his rights on the patent to the US government (noted in Reference Zarate, Brutsche and IbrahimZarate 2012). It is undeniable that ketamine has significantly reduced research costs in new drug development because it is readily available. The medical profession needs to consider the ethical implications if researchers and institutions will benefit financially by using a psychedelic drug that has misuse potential to treat vulnerable patients. It is crucial to have more clinical trials conducted by independent researchers who have no financial conflicts of interest related to ketamine. This will allow more objective and accurate evaluation of ketamine as a rapid antidepressant.
Safety monitoring during ketamine research
Not all psychiatric departments and hospitals have issued guidelines for researchers on conducting safe and ethical research into the use of ketamine to treat patients with depression. Table 4 summarises a safety framework that we have compiled from the literature.
TABLE 4 Safety framework for conducting ketamine research in people with depression
Research implications of ketamine
The past 50 years have seen significant advances in psychopharmacology that have reduced the suffering of people with mental illnesses. However, in our opinion the field has now reached a steady state and improvements brought about by new antidepressants are expected to be less dramatic than those achieved 50 years ago. The sudden revisiting of an old drug with misuse potential and claims of therapeutic triumph in depression, a condition closely linked with psychosocial aetiologies, require further thought. Treatment-resistant depression is a chronic and complex clinical problem associated with multiple risk factors and to be targeted by integrated therapeutic strategies combining antidepressants with psychosocial interventions. Owing largely to underpowered RCTs and lack of comparison with other antidepressants or stimulants, the evidence base for the therapeutic benefits of ketamine is controversial and not particularly encouraging with regard to maintenance of antidepressant effect. However, the research discussed here has focused on S(+) ketamine, and researchers have started to investigate the antidepressant properties of R(−) ketamine. Recent animal studies have demonstrated that the R(−) isomer of ketamine has a sustained antidepressant effect with addictive potential (Reference Zhang, Li and HashimotoZhang JC 2014; Reference Yang, Shirayama and ZhangYang 2015). This finding requires replication in human studies.
Researchers from several countries are competing to replicate the rapid antidepressant effects of ketamine by conducting ‘me too’ studies and presenting their findings as a breakthrough. One of the reasons could be lack of research funding for the development of new psychotropic drugs. This debate will continue. On one hand, the research findings outlined in Table DS1 support the sensible use of ketamine in a controlled medical setting to treat depression. Some researchers advocate that ketamine, a class B drug in the UK, should not be excluded from further study. On the other hand, medical professionals need to be mindful of the potentially addictive properties of ketamine, resulting in misuse, possible pressure from patients demanding more frequent ketamine infusions and concerns from the public and legislators, especially in communities seriously hit by ketamine misuse. Such controversies will lead to cognitive dissonance and are best resolved by applying current knowledge to develop safer NMDA receptor-modulating antidepressants.
Implications for psychiatrists and professional bodies
Using the psychedelic properties of a potentially addictive drug to treat psychiatric disorders presents significant ethical questions. Researchers mainly focus on effect sizes in ketamine trials and underestimate the potential harm of ketamine. Severely depressed patients are desperate for dramatic cures, but psychiatrists should not allow desperation to cloud their clinical judgement (Reference Kellner, Greenberg and AhleKellner 2014). Psychiatrists should not overuse ketamine for non-treatment-resistant depression and should not overpromise its therapeutic benefits. Psychiatrists must present the risks and admit that much is unknown about ketamine. It would be unethical and dangerous to society if ketamine were to be widely (mis)used under the label of a ‘rapid antidepressant’. Psychiatrists need to be mindful of the possibility of triggering another anti-psychiatry backlash. Although an American Psychiatric Association task force has warned of the misuse potential and risk of neurotoxicity with regular administration of ketamine in clinical settings (Reference Newport, Carpenter and McDonaldNewport 2015), most other professional and regulatory bodies, including the Royal College of Psychiatrists, the Commission on Human Medicines and the Medicines and Healthcare products Regulatory Agency, have issued no guidance on this matter. There is an urgent need for policy discussion to monitor the use of psychedelic drugs in the treatment of psychiatric disorders. The conflicting views on ketamine presented by researchers and by the media (Reference McKieMcKie 2014; Reference SiddiqueSiddique 2014) are confusing for the public. We urge postgraduate training committees to help trainees to understand the ethical issues behind the use of ketamine as an antidepressant treatment. Trainees need to apply their knowledge and critical appraisal skills to evaluate published studies on the subject.
Box 2 summarises the key messages of this article.
BOX 2 Key messages about ketamine use in treating major depressive disorder or treatment-resistant depression
-
The current evidence is not sufficient to recommend ketamine as an antidepressant. Oral and intranasal ketamine carry risk of diversion and misuse.
-
Ketamine increases presynaptic glutamate, which is known to cause neurotoxicity. The acute mood elevation effect of ketamine does not explain the role of glutamate and NMDA receptors in depression.
-
The differentiation between subanaesthetic and recreational doses of ketamine is not supported by evidence. Repeated use of subanaesthetic doses may lead to addiction and neurotoxicity. Similarly, there is no scientific method to differentiate acute mood elevation, euphoria, a ‘drug high’ and an antidepressant effect of ketamine. The acute mood elevation effect of ketamine is a common intrinsic property of psychedelic drugs.
-
The findings from ketamine trials apply to a special group of patients in an experimental environment. These findings should not be generalised to antidepressant-naive patients, mild depression and out-patient settings.
-
Before conducting ketamine research or promoting ketamine as a rapid antidepressant, psychiatrists need to consider the legal status of ketamine in their own countries, the community prevalence of ketamine misuse and the risk of diversion.
-
Although ketamine is an old drug, some researchers and institutes will benefit financially if ketamine is approved to treat depression. The financial conflict of interest will become a major ethical problem if patients become addicted to ketamine after repeated use for depression. More clinical trials conducted by investigators without a financial conflict of interest are required.
Conclusions
The presentation of ketamine as a novel antidepressant is reminiscent of the ketamine-related controversy in schizophrenia research in the 1990s. There are contradictory reports about its risks and benefits, and both sides present supporting evidence. Psychiatrists should consider other matters, including the psychedelic properties and misuse potential of ketamine, the course of MDD and motives behind ketamine trials. The safety profile of prolonged ketamine use at subanaesthetic doses is not established and chronic use should therefore be restricted. We are concerned that both oral and nasal formulations of ketamine are at risk of diversion, and recommend that the drug only be administered in a hospital setting with tight control. In addition to being of proven efficacy, a novel antidepressant treatment needs to be safe, ethical and based on convincing pharmacodynamic mechanisms.
MCQs
Select the single best option for each question stem
-
1 In the 1990s, US researchers conducted a study that used ketamine to induce psychosis. Which of the following statements is false?
-
a The researchers recruited patients with schizophrenia
-
b The study was stopped owing to ethical concerns
-
c This was a single-centre study
-
d This study yielded important findings that changed antipsychotic prescription practice when treating schizophrenia
-
e This study aimed to understand the efficacy of drugs for schizophrenia.
-
-
2 Which of the following binding sites is the site of action for ketamine?
-
a Cannabinoid binding site of the NMDA receptors
-
b Dopamine binding site of the NMDA receptors
-
c Gamma-aminobutyric acid (GABA) binding site of the NMDA receptors
-
d Glutamate binding site of the NMDA receptors
-
e Phencyclidine (PCP) binding site of the NMDA receptors.
-
-
3 Which of the following side-effects is not common after a single ketamine infusion?
-
a Dissociation
-
b Incontinence
-
c Increase in skeletal muscle tone
-
d Increase in blood pressure
-
e Psychosis.
-
-
4 Which of the following has not been shown by trials of ketamine involving patients with depression?
-
a Patients with a small hippocampus are more likely to respond to ketamine infusion
-
b Patients with homozygous met/met alleles of the BDNF gene are more likely to respond to ketamine infusion
-
c There is a wide variation in response rate
-
d A single infusion of ketamine at 0.5 mg/kg can reduce severity of depression
-
e A single infusion of ketamine at 0.5 mg/kg often leads to permanent cure of depression.
-
-
5 Which of the following countries does not have legislation to control and restrict the use of ketamine?
-
a Canada
-
b Denmark
-
c The Netherlands
-
d The UK
-
e The USA.
-
MCQ answers
| 1 | d | 2 | e | 3 | b | 4 | e | 5 | c |









eLetters
No eLetters have been published for this article.