Human immunodeficiency virus (HIV) infection is caused by the HIV virus, a blood-borne virus transmitted by three main routes: through sexual contact, via blood or blood products, and from mother to child during pregnancy or breastfeeding. The virus produces cellular immune deficiency characterised by a decrease in the number of CD4+ T-cells, a particular type of lymphocyte. This decrease results in inversion of the normal CD4/CD8 T-cell ratio and dysregulation of B-cell antibody production (Reference Frazer, Mackay and CrapperFrazer 1986; Reference Schechter, Boyko and CraibSchechter 1987). The infection advances in three stages: acute infection (or acute seroconversion), asymptomatic infection (or clinical latency) and acquired immunodeficiency syndrome (AIDS) (Reference LevyLevy 1993). The World Health Organization (2007) classifies HIV-related symptomatology into four stages, from asymptomatic to AIDS (Box 1). Average survival time after infection with HIV is estimated to be 9–11 years without treatment (UNAIDS 2007). However, highly active antiretroviral therapy (HAART) has led to a reduction in HIV-related morbidity and mortality, and the life expectancy of HIV-positive individuals has improved significantly. Indeed, the life expectancy for a newly diagnosed young adult has been raised to 20–50 years (Antiretroviral Therapy Cohort Collaboration 2008).
BOX 1 Stages of HIV-related symptomatology
-
Stage 1: asymptomatic or persistent generalised lymphadenopathy
-
Stage 2: moderate weight loss, recurrent respiratory tract infections, and minor mucocutaneous manifestations such as herpes zoster, angular cheilitis, recurrent oral ulcerations and fungal nail infections
-
Stage 3: severe weight loss, chronic diarrhoea, persistent fever, persistent oral candidiasis, oral hairy leukoplakia, severe bacterial infections, including pulmonary tuberculosis
-
Stage 4: AIDS (wasting syndrome, severe opportunistic infections such as recurrent bacterial pneumonia, pneumocystis pneumonia, extrapulmonary tuberculosis, cytomegalovirus infection, chronic herpes simplex infection, oesophageal candidiasis, central nervous system toxoplasmosis, cryptococcal meningitis, and neoplasms such as Kaposi's sarcoma and lymphoma)
The United Nations Report on the Global AIDS Epidemic estimated that 36.7 million people were living with HIV in 2015, with 2.4 million living in Central and Western Europe and North America (UNAIDS 2016). However, the number of new cases is declining: in 2015 there were 2.1 million new infections, a reduction of 39% since 2001. Although a cure is yet to be found, continued advances in the treatment of HIV with antiretroviral medicine and its greater availability (17 million people living with HIV were accessing treatment in 2015) has led to the significant decline in deaths worldwide, with 1.1 million dying in 2015, a 36% reduction since 2010 (UNAIDS 2016).
Relationship between HIV and psychiatric symptoms
The relationship between HIV and psychiatric symptoms and conditions is complex and the direction of effects between severe mental illness and HIV infection is unclear. In general, people with severe mental illness are at increased risk of contracting and transmitting HIV, and the prevalence of HIV infection among them is higher than in the general population (Reference Gottesman and GroomeGottesman 1997; Reference De Hert, Cohen and BobesDe Hert 2011). There may be several reasons for this. These include a higher incidence of risk behaviours than in the general population, such as substance misuse (including intravenous drug use) and sexual risk behaviours (e.g. unprotected sex, prostitution), and a reduced knowledge about HIV-related issues (Reference De Hert, Cohen and BobesDe Hert 2011). Also, people with psychiatric conditions are more likely to have been tested for HIV than those without mental illness, giving rise to a higher rate of detection than in the general population (Reference Yehia, Cui and ThompsonYehia 2014). At the same time, HIV infection may predispose to the development of psychiatric conditions. It is becoming ever more likely, therefore, that clinicians will encounter patients with HIV infection and psychiatric manifestations or comorbidities. However, there has been a relative lack of treatment guidelines for HIV-positive individuals with psychiatric conditions (Reference Blank, Himelhoch and WalkupBlank 2013).
Our literature review
The aim of our review was to summarise the current evidence on prevalence, manifestations and treatment of psychiatric conditions in HIV-positive adults (aged over 18), with particular emphasis on HIV-associated neurocognitive disorders (HAND). Very briefly, our study method was as follows (the online supplement to this article gives greater detail). We used the National Institute for Health and Care Excellence's Evidence Search (www.evidence.nhs.uk) to search in multiple healthcare databases. The years covered were 1990–2016 and we limited the search to the English language. We also checked reference lists of relevant articles.
In total, 129 journal articles met the eligibility criteria and were included in this review. Heterogeneity allowed only for narrative synthesis of data.
Prevalence of psychiatric conditions
The prevalence estimates for psychiatric conditions in HIV-positive individuals vary greatly. There may be several reasons for this, including sampling method (e.g. use of convenience samples, such as from certain institutions or geographical areas, use of administrative data, use of remnant blood specimens, true epidemiological samples) and other methodological considerations (e.g. adjustment for confounding factors) (Reference Blank, Himelhoch and WalkupBlank 2013). Depression is the most common psychiatric condition that we identified, followed by substance misuse, anxiety, psychosis, adjustment disorder and bipolar affective disorder. Online Table DS1 summarises the evidence on prevalence of various psychiatric conditions in HIV-positive and HIV-negative individuals, as derived from the studies in our review, and Fig. 1 shows the combined prevalence in HIV-positive individuals.
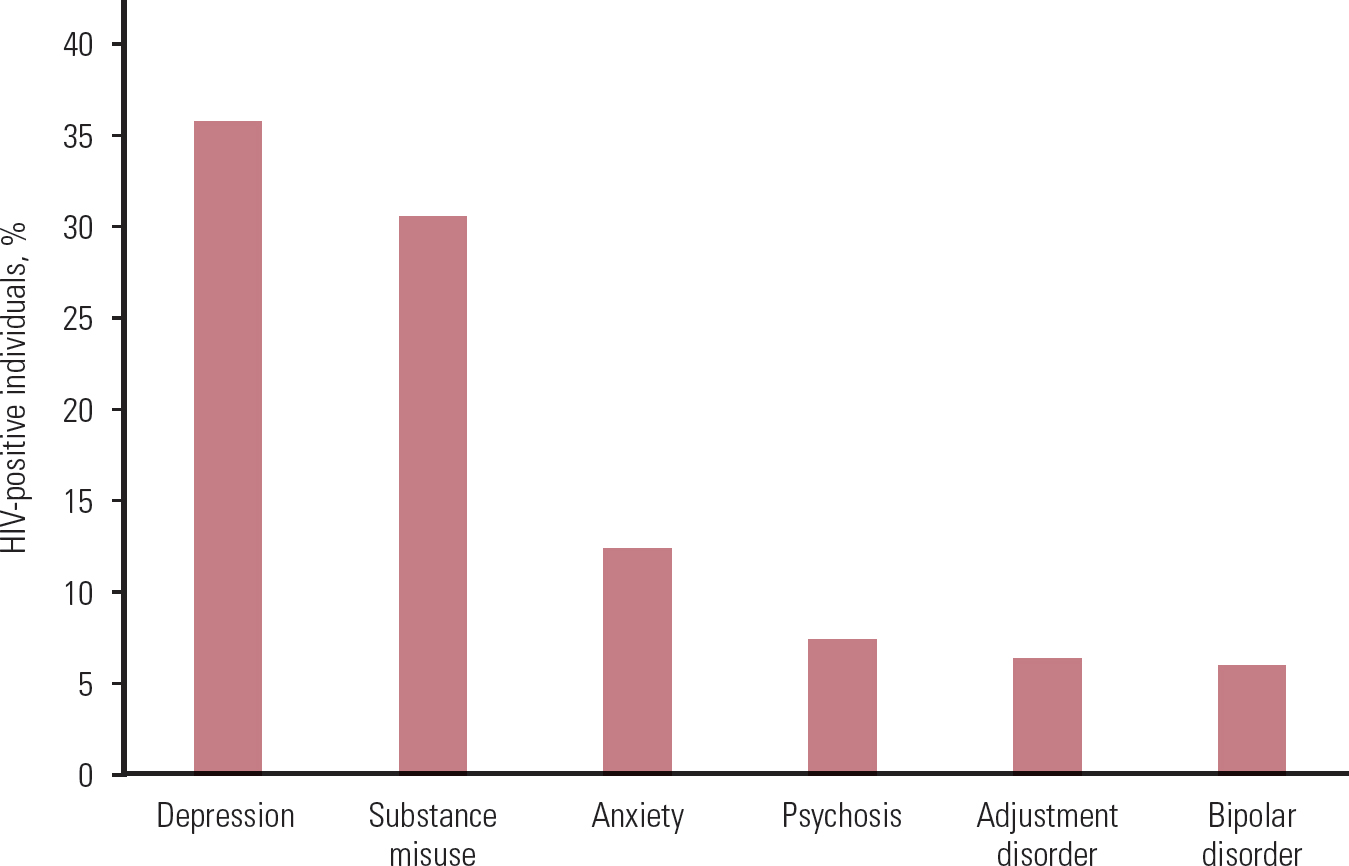
FIG 1 Combined prevalence of psychiatric conditions in HIV-positive individuals from the studies reviewed (for study details and references see online Table DS1).
Manifestation of psychiatric conditions
Depression
Neuropsychiatric sequelae of HIV have been a primary focus of clinical research since the beginning of the epidemic. Depression has received a considerable amount of attention, owing in part to its high prevalence in HIV-positive individuals, ranging between 5.8 and 36.0% (Table DS1). Typical features of depression are similar to those in HIV-negative people, although fatigue, loss of appetite and weight, impaired concentration, hopelessness and guilt are more common (Reference Puri, Hall and HoPuri 2014). Depression in anyone commonly has multiple causes, and in the context of being HIV-positive such causations only propagate and it becomes a greater challenge to the clinician to identify all the contributory factors.
The relationship between depression and HIV infection is complex. Depression can precede infection and may be associated with risk factors for infection in the first place (Reference Sherr, Clucas and HardingSherr 2011). For example, depression is more likely to be found among populations who are also at greater risk of contracting HIV (e.g. gay men, intravenous drug users) (Reference Clucas, Sibley and HardingClucas 2011). Depression can also emerge during the course of the infection. For example, it can be triggered by testing for HIV and receiving a positive result, by disclosure of illness (and/or sexual orientation) or by the prospect of taking lifelong treatment (Reference Sherr, Clucas and HardingSherr 2011). As a chronic and life-threatening illness, HIV infection can be stressful to manage and can therefore increase vulnerability to depression (Reference Blank, Himelhoch and WalkupBlank 2013). HIV infection is often associated with socially disadvantaged and marginalised populations (e.g. due to racial, ethnic or sexual minority status, poverty, substance use, prostitution or trauma), who are at increased risk for depression (Reference Blank, Himelhoch and WalkupBlank 2013). Stigma may also contribute to the development of depression, in particular by social isolation and lack of support (Reference Sherr, Clucas and HardingSherr 2011; Reference Blank, Himelhoch and WalkupBlank 2013). The medical sequelae of HIV infection, such as HAND or associated opportunistic infections, have also been associated with depression (Reference Blank, Himelhoch and WalkupBlank 2013). In particular, depression is more frequent in the initial stages of HIV dementia (Reference Puri, Hall and HoPuri 2014). Antiretroviral drugs and other drugs used in the treatment of the HIV infection, such as efavirenz, interferon, interleukin (IL)-2, steroids, zidovudine and vinblastine, are known to cause depression or depressive symptoms (Reference Puri, Hall and HoPuri 2014), making the treatment of the infection even more challenging. There is also evidence of an association between disease progression and depressive symptomatology (Reference Leserman, Pence and WhettenLeserman 2007).
Chronic immunodeficiency
Recent research has focused on the detrimental effects of chronic deficiency in immune function. A review of breast cancer survivors found that those who were fatigued had higher levels of serum markers associated with pro-inflammatory cytokines, including IL-1 and tumour necrosis factor (Reference Bower, Ganz and AzizBower 2002). Liu and colleagues found that IL-6 and IL-17 were elevated in major depressive disorders and in people with rheumatoid arthritis suffering from depression (Reference Liu, Ho and MakLiu 2012a,Reference Liu, Ho and Makb). Elevated plasma pro-inflammatory cytokine levels also contribute to the development of depression in HIV-positive individuals (Reference Rivera-Rivera, Vazquez-Santiago and AlbinoRivera-Rivera 2016). Importantly, those with very low CD4 T-cell counts (< 200 mm3) had significantly higher serum levels of pro-inflammatory cytokines, including IL-6 and IL-17, than those with CD4 T-cell counts between 200 and 300 mm3 (Reference Saing, Valdivia and HussainSaing 2016). These findings suggest that late-stage HIV infection with very low CD4 counts is associated with more severe depression than early-stage infection because of an increase in pro-inflammatory cytokines.
Treatment adherence
Depression is not only a mental health problem in itself, but is also a significant barrier to attendance at HIV clinics (Reference Schumacher, Mccullumsmith and MugaveroSchumacher 2013) and adherence to HAART. A number of studies have shown an inverse relationship between depression and adherence to HAART medication (Reference Ammassari, Antinori and AloisiAmmassari 2004; Reference Remien, Stirratt and DolezalRemien 2005; Reference Campos, Guimaraes and RemienCampos 2010; Reference Schumacher, Mccullumsmith and MugaveroSchumacher 2013). A recent systematic review and meta-analysis showed that the likelihood of achieving good adherence to antiretroviral therapy was lower among HIV-positive individuals with depressive symptoms compared with those without (Reference Uthman, Magidson and SafrenUthman 2014). It has also been found that the relationship between depression and non-adherence to antiretroviral therapy is consistent across samples and over time (Reference Gonzalez, Batchelder and PsarosGonzalez 2011). There are clearly some features of depression which, if present, are of greater detriment to HIV-positive individuals as they reduce medication adherence; these include low motivation, poor concentration and feelings of worthlessness.
Suicide risk
Depressed HIV-positive individuals are at high suicide risk. In a study undertaken between 2000 and 2002 of 2909 HIV-positive individuals from four US cities (San Francisco, Los Angeles, Milwaukee and New York City), it was found that 19% of participants reported thoughts of suicide in the past week. Factors that correlated positively with suicidal ideation included being homosexual, having more severe HIV-related symptoms and medication side-effects, marijuana use and elevated affective symptoms of depression (Reference Carrico, Johnson and MorinCarrico 2007). A systematic review covering 66 research papers published between 1989 and 2009 found that, on average, 9.4% of HIV-positive individuals had died by suicide and approximately 20% had self-harmed (Reference Catalan, Harding and SibleyCatalan 2011).
Apathy
Apathy has also been more commonly reported among HIV patients than in the general population (Reference Castellon, Hinkin and MyersCastellon 2000). Although symptoms of depression and apathy overlap, apathy specifically refers to the reduction in self-initiated cognitive, emotional and behavioural activity. This mood disorder has more commonly been associated with AIDS dementia complex (HIV-associated dementia), along with other symptoms of subcortical dysfunction, including memory impairment, poor concentration and reduced psychomotor rate. However, elevated levels of apathy have also been reported in patients without dementia (Reference Castellon, Hinkin and MyersCastellon 2000) and also in patients with less severe disease progression; for example, one study reported that 26% of HIV patients with an average CD4 cell count of greater than 300 exhibited clinically significant apathy (Reference Tate, Paul and FlaniganTate 2003).
Substance misuse
Studies of HIV-positive individuals have found high prevalence levels of substance misuse (ranging between 7.0 and 58.3%) (Table DS1). A systematic review of adherence to antiretroviral therapy among drug users found that active substance use was associated with poor adherence, as well as depression and low social support (Reference Malta, Strathdee and MagnaniniMalta 2008). Clearly, not only can a diagnosis of HIV drive substance misuse, but also intravenous use of social drugs (heroin, cocaine and methamphetamine) puts an individual at greater risk of contracting HIV. From 2008 to 2011, injection drug use was responsible for approximately 10% of HIV cases in the USA (Centers for Disease Control and Prevention 2015). The most obvious risk is direct-route transmission during intravenous drug use, but alcohol and other drugs can also lower an individual's inhibitions, leading to reduced risk-averse behaviour.
Prescription opioid injection
Although opioids are commonly prescribed to patients living with HIV/AIDS, their illicit use is also common; Lake and colleagues report that, between 2005 and 2013, the median prevalence of prescription opioid injection among HIV-positive injection drug users in Vancouver, Canada, was 24.2% (Reference Lake, Kerr and BuxtonLake 2016). Buprenorphine, a partial opioid agonist, is commonly prescribed by addiction specialists in the UK and intravenous buprenorphine misuse is on the rise (Reference Sarkar, Subramaniam and KonthoujamSarkar 2016). Intravenous buprenorphine misuse can transmit HIV and lead to public health problems. Infectious disease physicians need to monitor for other complications in HIV-positive individuals who misuse intravenous buprenorphine, including infective endocarditis and cutaneous complications (Reference Ho, Ho and TanHo 2009a,Reference Ho, Ho and Makb). Training is required to raise the awareness among infectious disease physicians about the risks and benefits of buprenorphine in HIV-positive individuals (Reference Sullivan, Tetrault and BangaloreSullivan 2006).
Substance misuse and mortality
Substance misuse can also exacerbate or induce mental illness in individuals who are more vulnerable, such as those living in poverty or those with a history of abuse. DeLorenze and colleagues studied whether treatment v. non-treatment of comorbid psychiatric or substance use disorders in HIV-positive individuals affected their risk of dying. Not being treated for depression resulted in a statistically significant increased risk of dying, but, interestingly, there was almost no statistically significant difference in the risk of dying between those not being treated for substance misuse and those who received treatment (1.88 v. 1.75) (Reference DeLorenze, Satre and QuesenberryDeLorenze 2010). It is noted that treatment was taken to mean only what was offered in the Kaiser Permanente Northern California health plan, and other treatment options, such as Alcoholics Anonymous or self-pay services, were not included in the analysis. Despite this, there is strong evidence that substance misuse is associated with poor adherence to HAART (Reference Hinkin, Hardy and MasonHinkin 2004; Reference Samet, Horton and MeliSamet 2004) and that it can limit medication effectiveness (Reference Samet, Horton and TraphagenSamet 2003; Reference Palepu, Horton and TibbettsPalepu 2004). In particular, among HIV-positive homosexual men, the use of stimulants (e.g. methamphetamine) has been associated with decreased odds of antiretroviral therapy use and difficulties with adherence (Reference Horvath, Alemu and DanhHorvath 2016).
Anxiety
The prevalence of anxiety among HIV-positive individuals ranges from 4.3 to 44.4% (Table DS1). As with depression, the relationship between anxiety and HIV infection is complex. Anxiety can precede HIV infection, but it can also be triggered by an HIV diagnosis (Reference Clucas, Sibley and HardingClucas 2011). Infection with a virus that has no cure and can be unpredictably progressive understandably creates stress and anxiety. This often begins before HIV test results are known and continues even when results are negative; it is exacerbated by social and economic stressors (symptoms of a urinary tract infection, poor education, food insecurity, violence) (Reference Mayston, Patel and AbasMayston 2015). Stigma, social isolation and fear of disclosure may add to the stressors, making HIV-positive individuals more vulnerable to anxiety (Reference Clucas, Sibley and HardingClucas 2011).
Generalised anxiety disorder tends to be the most common reported type of anxiety. It is free floating and persistent, with feelings of apprehension about everyday events, muscle tension and functional impairment. As with depression, anxiety can impair concentration, making individuals less likely to adhere to their medication; it is also associated with avoidant coping behaviour and substance misuse. Anxiety regarding death due to the illness (death anxiety) is also commonly reported among HIV-positive individuals (Reference Miller, Lee and HendersonMiller 2012).
Post-traumatic stress disorder (PTSD) is more prevalent among HIV-positive individuals than in the general population. The relationship between post-traumatic stress symptoms and HIV infection is complex. Post-traumatic stress symptoms may be due to factors associated with HIV infection, such as the notification process (Reference Clucas, Sibley and HardingClucas 2011). Trauma such as sexual abuse can precipitate post-traumatic stress symptoms and also sexual risk behaviours, such as unprotected sex, that precede HIV infection (Reference McLean and FitzgeraldMcLean 2016).
Adjustment disorder
The prevalence of adjustment disorder among HIV-positive individuals ranges from 3.8 to 67.6% (Table DS1). Its presentation is variable, as it can often present with depressive or anxiety symptoms, or even with obsessive–compulsive or somatic symptoms (Reference Puri, Hall and HoPuri 2014).
Psychosis
Psychosis is an uncommon psychiatric symptom of HIV, with a prevalence of 6 to 17.1% (Table DS1). Harris and colleagues note that persecutory, grandiose and somatic delusions were the most common symptoms found in new-onset psychosis in HIV disease, followed by hallucinations and affective disturbance (Reference Harris, Jeste and GleghornHarris 1991). It has been suggested that paranoid delusions in the absence of the usual affective symptoms may present an ‘elementary model’ of acute psychosis in HIV patients (Reference De Ronchi, Bellini and CremanteDe Ronchi 2006). There is also evidence that HIV patients with psychosis have a higher mortality rate than those without; in one study the mean survival time was 23 v. 51 months for patients with and without psychosis (Reference Sewell, Jeste and AtkinsonSewell 1994). Dolder and colleagues carried out a review of 41 papers that had examined new-onset psychosis in HIV disease and HIV infection in adults with psychotic disorder. They report that HIV tends to lead to greater morbidity and mortality in patients with schizophrenia than in the general public because patients are more likely to find it difficult to comply with medical care, have more trouble explaining symptoms to medical personnel and are less likely to receive attention from medical personnel for physical complaints (Reference Dolder, Patterson and JesteDolder 2004).
Bipolar affective disorder
A study conducted in Brazil (Reference De Sousa Gurgel, Da Silva Carneiro and Barreto Reboucasde Sousa Gurgel 2013) found that the prevalence of bipolar affective disorder among its sample of HIV-positive individuals was four times higher than that in the general population in the USA. Puri et al report the prevalence of mania in HIV-positive individuals as 1.5% (Reference Puri, Hall and HoPuri 2014). Mania can be a predisposing factor for HIV infection because of the related impulsivity, high sexual drive and non-adherence to safe sex practices. Mania in HIV-positive individuals may also be associated with social problems, especially if it presents early in the course of HIV infection; if it presents later it may be associated with HIV dementia (Reference Puri, Hall and HoPuri 2014). Other causes of HIV-induced mania include side-effects of antiretroviral medications such as zidovudine and lamivudine, direct effects of HIV infection on the central nervous system (CNS), metabolic disturbance and CNS opportunistic infection (e.g. toxoplasmosis cerebritis, cryptococcal meningitis) and CNS opportunistic tumours such as non-Hodgkin's lymphoma (Reference Puri, Hall and HoPuri 2014). Managing manic episodes in HIV-positive individuals can be challenging because the patients are often non-adherent to antiretroviral therapy, and there is the risk that they will spread HIV infection to other people owing to their high sexual drive and impaired insight.
HIV-associated neurocognitive disorder
HIV-associated neurocognitive disorder (HAND) is the umbrella term for the spectrum of neurocognitive impairment induced by HIV. This ranges from asymptomatic impairment to severe impairment with pronounced deficits in self-efficacy and interference with day-to-day activities, including financial management, driving and medication adherence (Reference Dufour, Marquine and FazeliDufour 2013), a significant factor that has been reported to be directly related to suicidal ideation (Reference Carrico, Johnson and MorinCarrico 2007).
In 2007 the National Institute of Mental Health (NIHM) and the National Institute of Neurological Disorders and Stroke (NINDS) updated the diagnosis of neurological manifestations of HIV infection originally defined by the AIDS Task Force of the American Academy of Neurology. In what have become known as the Frascati criteria, the spectrum of neurocognitive deficiency was delineated into three categories of severity (Reference Antinori, Arendt and BeckerAntinori 2007):
-
1 HIV-associated asymptomatic neurocognitive impairment (ANI)
-
HIV-associated mild neurocognitive disorder (MND)
-
HIV-associated dementia (HAD).
These categories are defined in Box 2.
BOX 2 HIV-associated neurocognitive deficiency: the Frascati criteria
-
1 Asymptomatic neurocognitive impairment: does not interfere with everyday functioning, does not meet the criteria for delirium or dementia and there is no evidence of another pre-existing cause for it
-
Mild neurocognitive disorder: mild interference with everyday functioning (e.g. reduced mental acuity, inefficiency at work or homemaking, poor social functioning), does not meet the criteria for delirium or dementia and there is no evidence of another preexisting cause for it
-
Dementia: marked interference with day-to-day functioning (work, home life, social activities), does not meet the criteria for delirium and there is no evidence of another pre-existing cause for it.
Relatively early indicators of HAND include impairments in psychomotor function (e.g. slowed movements, impaired coordination and gait), in simple and divided attention and concentration (including impaired performance on working memory tasks), in mental agility (e.g. impaired performance on reaction time tests) and in mental flexibility (Reference Richardson, Morgan and VielhauerRichardson 2005). Language, by contrast, is often well preserved. HAND relates not only to cognitive and memory impairment, but also to personality changes, including apathy, irritability and inertia (Reference Owe-Larsson, Sall and SalamonOwe-Larsson 2009), as well as changes in behaviour, particularly social withdrawal and impaired activities of daily living. Neurological signs include poor coordination, loss of balance and tremor; later in the illness, hyperreflexia, dysdiadochokinesia, ocular pursuit abnormalities, myoclonus and frontal lobe signs are observable (Reference Jefferies and AgrawalJefferies 2009).
Episodic memory
Poor episodic memory is the most frequently reported cognitive difficulty in HIV-positive individuals. Episodic memory is being considered in two components: retrospective memory (the recollection of past experiences, encounters, people and words) and prospective memory (the recollection of needing to do something after a delay, i.e. remembering to remember). Although retrospective memory testing is commonly used in both objective and subjective memory research in HIV, prospective memory testing is thought to be a stronger performance indicator for instrumental activities of daily living such as medication adherence (Reference Woods, Carey and MoranWoods 2007), even after controlling for potential confounders, including demographics (e.g. education), traditional cognitive measures or retrospective memory and executive functions and psychiatric factors (e.g. depression) (Reference Zogg, Woods and WeberZogg 2010). Specifically, HIV-associated prospective memory impairment correlated with worse performance on measures of strategic verbal encoding, working memory, executive functions and complex information processing speed (Reference Woods, Carey and MoranWoods 2007). These findings overlap with results in other research, which showed that the most common neuropsychological test abnormalities in those with HIV dementia were in the domains of verbal memory, construction and motor speed (Reference Sevigny, Albert and McDermottSevigny 2007; Reference Owe-Larsson, Sall and SalamonOwe-Larsson 2009). Also, those with HAND were disproportionately vulnerable to prospective memory deficits at longer ongoing task delay intervals. These deficits appear to be driven by strategic dyscontrol of prospective memory, i.e. by a delay between encoding a future intention and detection of the retrieval cue, which is consistent with the preferential disruption of prefrontal systems (Reference Morgan, Weber and RooneyMorgan 2012). Interestingly, it has also been noted that informal caregivers are often the first people to recognise cognitive impairment such as poor concentration and communication difficulties in HIV-positive individuals and therefore may be uniquely placed to identify cognitive decline otherwise missed by physicians (Reference Murray, Cummins and BatterhamMurray 2016).
Neurocognitive impairment
There is considerable variability among individuals in both presentation and severity of HAND and this can be problematic when trying to distinguish between true neurocognitive impairment and depression. For example, one study found that high levels of depressive symptoms were significantly associated with reduced cognitive functioning in HIV infection, particularly in the domains of attention, psychomotor speed and construction, although the study included only female participants (Reference Fialho, Pereira and MendoncaFialho 2013). Further complexities arise in that, from at least three separate cohort studies, a sizable proportion of patients have a fluctuating course of neurocognitive impairment, and normalisation of symptoms is possible (Reference Antinori, Arendt and BeckerAntinori 2007; Reference Robertson, Smurzynski and ParsonsRobertson 2007). It is also of interest to note that in one study neuropsychological tests were positive for cognitive impairment in up to 78% of HIV-positive individuals, but only 9% of these were clinically found to have cognitive impairment, in which psychomotor slowing was the most common deficit (Reference Muniyandi, Venkatesan and ArutselviMuniyandi 2012).
Only a few studies have compared neurocognitive performance between men and women with HIV and these were often of very small sample size. One study reported that HIV-positive men and women had cognitive impairment rates of 52% and 55% respectively. The greatest differences in cognitive functioning were in the domains of verbal memory for texts, where women were far more likely to be impaired (42% v. <10%) (Reference Maki and Martin-ThormeyerMaki 2009). Another found no significant difference between the genders and levels of cognitive impairment, but rather that older age, lower cerebral reserve scores and not being on zidovudine treatment were all associated with lower global neuropsychological scores and with the presence of cognitive impairment (Reference Pereda, Ayuso-Mateos and Gomez Del BarrioPereda 2000).
Risk factors for HAND
Evidence of which individuals are more at risk of developing neurocognitive impairment and the timescale of development is a continually progressing area of research. There is growing evidence that one of the most important risk factors for HAND is a low nadir CD4 cell count: the lower the cell count the more likely the individual is to develop neurocognitive impairment (Reference Robertson, Smurzynski and ParsonsRobertson 2007; Reference Tozzi, Balestra and BellagambaTozzi 2007; Reference Muñoz-Moreno, Fumaz and FerrerMuñoz-Moreno 2008; Reference Cysique, Vaida and LetendreCysique 2009; Reference Heaton, Franklin and EllisHeaton 2011). A study that followed 396 individuals with advanced HIV infection for a median of 25.2 months found that those who died during that period were more likely at baseline to have an abnormal neurocognitive status, as well as a lower CD4 count, an AIDS-defining illness, a lower haemoglobin level, a higher plasma HIV RNA level and higher plasma and cerebrospinal fluid (CSF) macrophage chemoattractant protein 1 levels (Reference Sevigny, Albert and McDermottSevigny 2007). Furthermore, HIV-associated dementia was significantly associated with time to death after adjusting for immune markers (including CD4 count and plasma MCP-1 level) and other demographic and clinical covariates. Interestingly, it has also been reported that baseline CFS MCP-1 levels are associated with time to HIV-associated dementia (Reference Sevigny, Albert and McDermottSevigny 2004).
Higher prevalence of HAND in US studies
There is an interesting discrepancy between the findings of US researchers and those of other countries with regard to the prevalence of HAND (Reference Barber, Bansi and PozniakBarber 2017). For many years, starting with the description of the AIDS dementia complex in the 1980s, US researchers have reported much higher prevalence of neurocognitive problems compared with other researchers, including those in the UK. The reasons for such discrepancy are unclear, but it may that those reporting higher rates may apply lower thresholds for abnormality, or that other factors contributing to cognitive impairment, such as mood disorder or substance misuse, have not been excluded.
Effect on caregivers
It should be noted that caregivers of patients with cognitive impairment or dementia often suffer from depression and anxiety as well (Reference Sallim, Sayampanathan and CuttilanSallim 2015). Those who appear to be more at risk are female caregivers, caregivers with male care recipients and caregivers who have a spousal relationship with the care recipients. Not surprisingly, the greater the care burden the more likely the carer was to be depressed, including having to provide more care for the HIV patient, having to help others besides that person and the duration of care required (Reference Pirraglia, Bishop and HermanPirraglia 2005).
Treatment
Highly active antiretroviral therapy
Highly active antiretroviral therapy (HAART) was introduced in 1996. HAART uses several classes of antiretroviral agent which act on different stages of the HIV life cycle, decreasing the patient's total burden of the disease while aiming to maintain the function of the immune system and prevent opportunistic infections that could otherwise lead to death (Reference Moore and ChaissonMoore 1999). These newer antiretroviral medications have led to a reduction in HIV-related morbidity and mortality (Reference Detels, Muñoz and McfarlaneDetels 1998). In the pre-HAART era, plasma HIV RNA levels (the plasma viral load) very early in infection were associated with the development of HIV dementia (Reference Childs, Lyles and SelnesChilds 1999), and HIV RNA levels in the CSF (the CSF viral load) correlated with the severity of disease (Reference Brew, Pemberton and CunninghamBrew 1997). However, HAART-era studies have not found a relationship between plasma or CSF viral loads and neurological dysfunction in individuals taking HAART, possibly as a result of the new treatment restricting the range of plasma HIV RNA levels (Reference Robertson, Smurzynski and ParsonsRobertson 2007). It is clear that neurocognitive impairment remains a significant problem even in the HAART era.
A multicentre cohort study undertaken between 1990 and 1998 that included 2734 HIV-positive men looked at the effects of HAART medication on rates of infection, malignancy and dementia (Reference Sacktor, Lyles and SkolaskySacktor 2001). The study found that the incidence rates for cryptococcal meningitis, CNS lymphoma and dementia had all decreased dramatically since the introduction of HAART. In particular, the incidence of dementia decreased by 50% between 1990 (21.1/1000 person-years) and 1998 (10.5/1000 person-years), indicating that HAART at the very least slowed the onset of HIV-associated dementia.
As yet it is not known why, despite effective HAART, neurocognitive impairment remains frequent. It has been argued that it may represent ongoing active disease, or past pre-HAART injury which has remained permanent, or a combination of the two (Reference Robertson, Smurzynski and ParsonsRobertson 2007; Reference Tozzi, Balestra and BellagambaTozzi 2007). However, more recent research has demonstrated that HIV DNA isolated from monocytes correlates with cognitive performance irrespective of plasma HIV RNA and CD4 lymphocyte counts in both pre- and post-HAART settings, indicating that CNS injury may be related to migrating infected monocytes (Reference Shiramizu, Gartner and WilliamsShiramizu 2005; Reference Valcour, Shiramizu and SithinamsuwanValcour 2009). Further complexities arise from studies which appear to show conflicting evidence, in that greatest neurocognitive improvement is seen in patients treated with better CNS-penetrating HAART regimes, or that viral suppression in the CSF corresponds to better neurocognitive outcomes (Reference Letendre, Mccutchan and ChildersLetendre 2004; Reference Cysique, Vaida and LetendreCysique 2009; Reference Heaton, Clifford and FranklinHeaton 2010).
Antidepressants
A study that followed 765 HIV-positive women for 7 years found in multivariate analyses (controlling for clinical treatment, viral load, CD4 cell count, sociodemographic characteristics and substance misuse) that participants with chronic depressive symptoms were twice as likely to die as those with limited or no depressive symptoms (Reference Ickovics, Hamburger and VlahovIckovics 2001). There is also evidence that depressed HIV-positive individuals who were being treated with antidepressants were more likely both to receive treatment for their HIV and to have significantly lower monthly healthcare costs (Reference Sambamoorthi, Walkup and OlfsonSambamoorthi 2000). More recently, in the era of HAART, evidence still shows that depressive symptoms are related to disease progression and mortality and are more severe in the terminal phase of illness (Reference Cook, Grey and BurkeCook 2004; Reference Leserman, Pence and WhettenLeserman 2007); this effect has been found even when controlling for HAART adherence (Reference Lima, Geller and BangsbergLima 2007). The importance of treating depression is highlighted in a meta-analysis of 29 studies of 12 243 HIV-positive individuals, which showed that treatment of depression and psychological distress improved antiretroviral therapy adherence and that the odds of a person adhering were 83% higher if their depression was treated (Reference Sin and DimatteoSin 2014). A systematic review and meta-analysis concluded that antidepressant medication was efficacious in treating depression among HIV-positive individuals (Reference Himelhoch and MedoffHimelhoch 2005). A review of interventions for comorbid depression and HIV infection showed that psychotropic drugs were generally effective in the treatment of depression, but the combination of psychological therapies with drugs was more effective than either intervention alone (Reference Sherr, Clucas and HardingSherr 2011).
It is important to note that some antiretroviral medications are associated with depression; these include efavirenz, stavudine, zidovudine, atazanavir, darunavir, raltegravir and lopinavir with ritonavir. Reference Brogan and LuxBrogan & Lux (2009) give a summary of treatment recommendations for various psychiatric conditions in HIV-positive individuals, with cautionary comments. For depression, selective serotonin reuptake inhibitors (SSRIs), selective noradrenaline reuptake inhibitors (SNRIs), mirtazapine and bupropion are recommended as first line, although levels of bupropion may be increased by efavirenz and some protease inhibitors, including ritonavir and nelfinavir, but decreased by lopinavir with ritonavir.
In 2010, Freudenreich and colleagues completed an internet-based consensus survey of current practices in the psychiatric treatment of people with HIV/AIDS in which 62 members of the Organization of AIDS Psychiatry participated. It was found that several medications could be considered first-line treatments by consensus: escitalopram or citalopram for depression, quetiapine for psychosis and secondary mania, and clonazepam for anxiety (Reference Freudenreich, Goforth and CozzaFreudenreich 2010). For the treatment of depression, the first-line choice of antidepressant was not influenced by HIV treatment (i.e. combined antiretroviral therapy-naive, protease inhibitor-based regimen or efavirenz-based regimen) and in case of no response within 8 weeks, a switch to an SNRI was considered most appropriate, with bupropion or another SSRI also considered appropriate choices.
Antipsychotics
There are relatively few guidelines developed for clinicians who provide treatment for patients with both HIV and psychotic illness. Published reports suggest that new-onset psychosis in HIV-positive individuals generally responds well to antipsychotics. However, this group of individuals are more susceptible to antipsychotic side-effects, especially extrapyramidal symptoms and tardive dyskinesia (Reference Hriso, Kuhn and MasdeuHriso 1991; Reference Shedlack, Soldato-Couture and SwansonShedlack 1994; Reference Hinkin, Castellon and AtkinsonHinkin 2001). In a case series of 20 HIV-positive patients with psychotic symptoms, risperidone was found to be efficacious and to have fewer side-effects than first-generation (typical) antipsychotics (Reference Chandra, Desai and RanjanChandra 2005). It is also important to consider the type of HIV medication being used. A national survey of a random sample of 347 HIV experts, drawn from the American Academy of HIV Medicine, who were asked about HAART prescribing for AIDS patients with schizophrenia found that they would tend to avoid prescribing efavirenz, a medication with known neuropsychiatric side-effects (17.7% v. 45.5%, P <0.01) (Reference Himelhoch, Powe and BreakeyHimelhoch 2007a). Furthermore, many HAART medications and antipsychotics are metabolised by cytochrome P450 isoenzymes and thus there is competition between the two (Reference Singh and GoodkinSingh 2007).
Mood stabilisers
Mood stabilisers are not uncommonly used to treat HIV-positive individuals, although drug interaction is a particular problem in this subset. Lithium, for example, is not recommended because it is not well tolerated in HIV-positive individuals as they are at risk of developing lithium toxicity due to HIV-induced nephropathy (Reference Dube, Benton and CruessDube 2005). Sodium valproate is effective in treating mania in HIV-positive individuals, but requires monitoring of liver function. Carbamazepine, however, is not recommended owing to the risk of pancytopenia in HIV-infected patients and induction of metabolism of antiretroviral treatment.
Anti-dementia drugs
Currently, there are only four anti-dementia drugs licensed in the UK for patients of any age. These are the cholinesterase inhibitors donepezil, rivastigmine and galantamine, and the NMDA-receptor blocker memantine; the first three are used in mild to moderate dementia and the last for moderate to severe dementia. However, despite positive results of trials in different types of dementia, including Lewy body dementias (both Parkinson's disease dementia and dementia with Lewy bodies) and vascular dementia, cholinesterase inhibitors and memantine are licensed in the UK only for treatment of Alzheimer's disease, with rivastigmine also being licensed for dementia in Parkinson's disease.
Psychological therapies
One study randomly assigned HIV-positive individuals who had less than 80% adherence to their medication to four couples-focused adherence intervention sessions. The intervention consisted of education regarding the treatment and adherence to medication, identifying barriers to adherence, developing communication and problem-solving strategies and building confidence for optimal adherence. Compared with controls, post-intervention participants were significantly more likely to take their medication and to take it at the correct time (16% and 23% increase respectively). However, the improved adherence was measured only 2 weeks after the intervention and the effects diminished significantly with time at 3- and 6-month follow-up (Reference Remien, Stirratt and DolezalRemien 2005).
A recent systematic review and meta-analysis showed that mindfulness-based stress reduction and mindfulness-based cognitive therapies have psychological benefits in HIV-infected patients (Reference Yang, Liu and ZhangYang 2015). Another systematic review showed that cognitive–behavioural therapy (CBT) was efficacious in improving depression and quality of life in the short term, and improving medication adherence in the long term, compared with standard care for HIV-positive individuals (Reference Fu, Wu and HuFu 2014). Cognitive–behavioural interventions may also have a positive effect in the treatment of depression and anxiety in HIV-positive individuals (Reference Crepaz, Passin and HerbstCrepaz 2008; Reference Spies, Asmal and SeedatSpies 2013). A review of interventions for comorbid depression and HIV infection showed that psychological interventions, especially those that incorporated a cognitive–behavioural component, were particularly effective in the treatment of depression (Reference Sherr, Clucas and HardingSherr 2011). Group psychological interventions for depression in HIV-positive individuals have been shown to have a significant effect on depressive symptomatology, and group CBT appears to be an acceptable intervention for comorbid depression (Reference Himelhoch, Medoff and OyeniyiHimelhoch 2007b; Reference Honagodu, Krishna and SundaracharHonagodu 2013). For the treatment of anxiety in HIV-positive individuals, psychological interventions (especially cognitive–behavioural stress management and CBT) were generally more effective than pharmacological interventions (Reference Clucas, Sibley and HardingClucas 2011). Stress-management interventions reduce mental health difficulties such as anxiety and depression, distress and fatigue, and improve quality of life (Reference Scott-Sheldon, Kalichman and CareyScott-Sheldon 2008).
Exercise
A growing body of evidence indicates that physical activity may be of particular relevance in reducing HAND. Extensive research has shown that increased exercise consistently improved cognitive performance. A meta-analysis of 30 trials (a total of 2020 participants) from 1970 to 2003 concluded that exercise training increases cognitive function in people with dementia and related cognitive impairment (Reference Heyn, Abreu and OttenbacherHeyn 2004). A number of studies have recently extended this concept to HIV-positive individuals (Reference Honn, Para and WhitacreHonn 1999; Reference Fillipas, Oldmeadow and BaileyFillipas 2006; Reference Dufour, Marquine and FazeliDufour 2013). The largest of these (Reference Dufour, Marquine and FazeliDufour 2013), sampling 335 individuals, found that those who reported any amount of time exercising in the past 72 hours were half as likely to have neurocognitive impairment than those who reported no exercise (15.66% v. 30.95%). It is important to note that, on average, those who reported no exercise had had HIV for longer, had lower nadir CD4 counts and were more likely to have an AIDS diagnosis. Interestingly, 12.5% of individuals who did not exercise had a current major depressive disorder, compared with only 2.5% of those who did exercise, although there was almost no difference between the two groups when comparing major depressive disorders over their lifetime (55.0% for those who exercised v. 58.2% for those who did not). A recent Cochrane Collaboration systematic review reported that aerobic exercise can be safe and beneficial for HIV-positive individuals who are medically stable. Performing aerobic exercise or a combination of aerobic and resistive exercise on a regular basis is safe and can lead to improvements in cardiopulmonary fitness, body composition, strength and quality of life for adults with HIV (Reference O'Brien, Tynan and NixonO'Brien 2016).
Psychosocial interventions and social care support
Alongside the pharmacological treatment of HIV and its psychiatric complications, the role of psychosocial interventions cannot be emphasised enough. The multidisciplinary teams providing HIV care are expected to communicate effectively and collaborate with the professional leads of other services providing psychosocial support for people living with HIV (e.g. mental health, social care and voluntary sector services). A randomised controlled trial of an advanced practice nurse (APN) model of care management showed that the intervention was effective in reducing depression and improving quality of life in HIV-positive individuals with serious mental illness. This intervention consisted of assignment of the services of an APN, who provided in-home consultations, coordinated medical and mental health services and collaborated with prescribing providers, pharmacists and case managers to organise medication regimens, help participants cope with barriers to medication adherence and promote self-care (Reference Hanrahan, Wu and KellyHanrahan 2011).
Specific psychosocial interventions have been reported as effective in reducing psychological distress (e.g. depression, anxiety, anger) and improving quality of life. These include spiritual mantra repetition, life review, art psychotherapy, art therapy, case management community care, peer support counselling and relaxation techniques (Reference Clucas, Sibley and HardingClucas 2011; Reference Sherr, Clucas and HardingSherr 2011).
Psychosocial interventions are of great value in the care of people with HIV, and clear pathways should be defined between services providing clinical treatment and those offering psychosocial support. Many people with HIV are likely to require social care support at some point during their illness. As HAART has made a substantial impact on suppressing HIV, the social care needs of these people have changed. Social care is no longer about supporting people with a terminal illness. Some people with HIV may indeed have physical care needs; others, however, may experience psychological distress, mental illness or neurocognitive impairment and they may have different types of care needs. They can also be disproportionately affected by poverty, and may therefore be in need of social support to address poverty-related needs such as housing, benefits advice and other financial problems.
Future research directions and service development
Genetics play a role in predisposing people with medical disorders such as stroke or systemic lupus erythematosus to develop depression (Reference Mak, Kong and MakMak 2013) or other neuropsychiatric complications (Reference Ho, Ong and ThiaghuHo 2016). This area is, however, underresearched in HIV infection. So far, research has mainly focused on apolipoprotein genotypes and HIV-induced dementia and it has been found that there is no association between the ε4 allele of the apolipoprotein E (APOE) gene and cognitive outcomes in HIV disease (Reference Becker, Martinson and PenugondaBecker 2015).Footnote a Research is therefore required to study the relationship between serotonin genotypes, genes related to the immune system and risk of developing neuropsychiatric complications in HIV-positive individuals.
A significant proportion of HIV studies involve gay or bisexual men, or mostly or only men: women have received much less attention. Future studies will therefore need to focus more on heterosexual men and on women. There is also a paucity of studies from low- and middle-income countries, despite the higher rates of HIV infection.
Recent research suggests that internet access among non-urban and rural patients with HIV in the USA is adequate to support trials testing internet-delivered interventions. It is therefore time to develop and deliver internet interventions tailored for this population (Reference Modipane, Waldman and RitterbandModipane 2016). The development of mobile phone apps would also be useful to increase medication adherence and overall adherence to treatment among HIV-positive individuals (Reference Horvath, Alemu and DanhHorvath 2016). Text messaging has already been found to be promising in improving medication adherence in HIV-positive individuals (Reference Nigam, Mitra and LahueNigam 2014). Internet and smartphone apps can therefore increase medication adherence and deliver psychological therapy to HIV-positive individuals, and psychiatrists can play a key role in advocating and developing new modalities of psychotherapy (Reference Zhang, Ho and CheokZhang 2015). Owing to the stigma of the HIV infection, patients may not want to come forward to see a therapist for psychotherapy. Online psychotherapy can reach a larger population, especially those who live in rural areas (Reference Modipane, Waldman and RitterbandModipane 2016). Individualised treatment such as the APN model of care management (Reference Hanrahan, Wu and KellyHanrahan 2011; Reference Blank, Himelhoch and WalkupBlank 2013) is important for HIV-positive individuals with psychiatric symptoms.
Conclusions
The life expectancy of individuals who are HIV-positive has improved significantly over the years. Consequently, research into HIV and its comorbidities continues to grow and become ever more relevant. HAART has made a substantial impact on suppressing HIV, but neuropsychiatric complications persist and are likely to impose a significant burden on individuals with the disease. The degree of direct impact the virus has on these complications remains uncertain, although it is clear that effective treatment of both HIV and its psychiatric complications is critically important in order to maximise the quality and quantity of life-years.
MCQs
Select the single best option for each question stem
-
1 Which is the most common psychiatric condition in HIV-positive individuals?
-
a Substance misuse
-
b Anxiety
-
c Depression
-
d Psychosis
-
e Bipolar affective disorder.
-
-
2 Which of the following factors does not correlate positively with suicidal ideation in HIV-positive individuals?
-
a Having greater self-efficacy for coping
-
b Having more severe HIV-related symptoms
-
c Marijuana use
-
d Being homosexual
-
e Having more medication side-effects.
-
-
3 Which antidepressants are not recommended as first line for the treatment of depression in HIV-positive individuals?
-
a Selective serotonin reuptake inhibitors
-
b Tricyclic antidepressants
-
c Selective noradrenaline reuptake inhibitors
-
d Mirtazapine
-
e Bupropion.
-
-
4 Which of the following is not a relatively early feature in HIV-associated neurocognitive disorders?
-
a Impaired coordination
-
b Impaired performance
-
c Impaired performance on tests of reaction time
-
d Language impairment
-
e Slowed movements.
-
-
5 Which of the following statements about HIV-associated dementia is incorrect?
-
a It is the least severe of the HIV-associated neurocognitive disorders
-
b The pattern of cognitive impairment does not meet the criteria for delirium
-
c There is no evidence of another pre-existing cause for the dementia
-
d It is more severe than HIV-associated mild neurocognitive disorder
-
e Cognitive impairment produces marked interference with day-to-day functioning.
-
MCQ answers

| 1 | c | 2 | a | 3 | b | 4 | d | 5 | a |




eLetters
No eLetters have been published for this article.