Catatonia is a complex and heterogeneous psychomotor syndrome. Symptoms can be categorised into four groups: disturbances of motor function, disturbances of volition, disinhibition and autonomic instability (Walther Reference Takamiya, Kishimoto and Watanabe2012, Reference Tuerlings, Van Waarde and Verwey2016) (Table 1). There are numerous organic (Box 1) and functional causes of catatonia, including affective disorders, schizophrenia spectrum disorders, neurodevelopmental disorders, dementia and drug intoxication (Walther Reference Tuerlings, Van Waarde and Verwey2016). Without treatment, mortality from catatonia is high (Tuerlings Reference Sienaert, Dhossche and Vancampfort2010; Healy Reference Gibson and Walcott2012). The subtype of malignant catatonia is characterised by pyrexia, behavioural agitation, delirium and autonomic instability, alongside the motor symptoms typical of the disorder. This is a life-threatening condition where early diagnosis and treatment are crucial (Park Reference Neerukonda, Bliss and Jaroodifar2017). Patients with catatonia are also at an increased risk of malnutrition, dehydration, pneumonia and venous thromboembolism (Regestein Reference Petrides, Divadeenam and Bush1977; Penland Reference Northoff, Koch and Wenke2006). The underlying mechanism remains poorly understood. N-methyl-d-aspartate (NMDA) receptor hypofunction and dysfunction of the cortical and basal ganglia motor circuits have been implicated. For comprehensive reviews of this topic I suggest articles by Walther et al (Reference Tuerlings, Van Waarde and Verwey2016, Reference Walther and Strik2019) and Rogers et al (Reference Raveendranathan, Narayanaswamy and Reddi2019).
TABLE 1 Four categories of signs and symptoms of catatonia
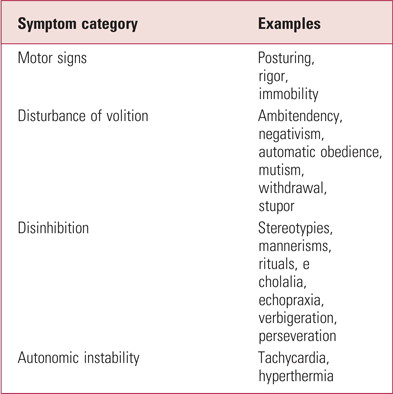
Source: Walther & Strik (Reference Tuerlings, Van Waarde and Verwey2016).
BOX 1 Organic causes of catatonia
Like most neuropsychiatric symptoms, catatonia can occur secondary to organic pathology, including infections (e.g. meningitis and encephalitis), autoimmune encephalitis, metabolic disorders (e.g. homocystinuria and hepatic encephalopathy), benzodiazepine withdrawal and structural disorders (e.g. stroke). It is therefore crucial that clinicians consider a wide differential diagnosis in the aetiology of acute-onset catatonia.
In clinical practice, benzodiazepines are the most commonly used treatment (Fink Reference Bush, Fink and Petrides2013; Sienaert Reference Schmider, Standhart and Deuschle2014). High doses may be required for effective treatment (Fink Reference Fink and Taylor2009; Lin Reference Healy, Le Noury and Harris2017). In life-threatening or treatment-resistant cases, electroconvulsive therapy (ECT) is the treatment of choice. However, there are no widely accepted clinical guidelines for the treatment of catatonia. Although numerous case reports and small studies are available, there is a paucity of higher-quality research into both the pathophysiology and the treatment of catatonia. The Cochrane review discussed here was conducted with the aim of summarising higher-quality evidence from clinical trials to help guide clinical decision-making in treating catatonia. However, the findings also raise the question of what constitutes feasible high-level evidence for the treatment of acute emergency presentations such as catatonia.
This month's Cochrane Review
Method
Zaman et al (Reference Walther and Strik2019) aimed to assess the efficacy of benzodiazepine treatment compared with other drugs, placebo or ECT for catatonia in people with schizophrenia or severe mental illness (SMI), which they specified as bipolar affective disorder or depression. Inclusion criteria were double-blind randomised controlled trials (RCTs) with participants diagnosed with catatonia alongside schizophrenia or other SMI. Quasi-randomised studies were excluded. Interventions included benzodiazepines of any type, compared with any other pharmacological agent, placebo or ECT. This was not limited by dose, frequency or route of administration.
The first of the primary outcome measures was a 50% improvement in symptoms as measured using a visual analogue scale (VAS; Box 2). Additional primary outcome measures included the duration of hospital admission, change in satisfaction with care and the incidence of clinically important adverse effects.
BOX 2 Visual analogue scales
A visual analogue scale (VAS) is an external and objective assessment of variables that cannot be otherwise directly measured. It predominantly measures the intensity of symptoms that lie on a continuum, as opposed to a categorical scale, which records, for example, whether a symptom is present or absent. It is widely used in the assessment of pain but can also be applied to the symptoms of catatonia that are described in Table 1.
Electronic searches were performed using the Cochrane Schizophrenia Group's study-based register of trials using the terms ‘catatonia’ (under Health Care Condition) and ‘benzodiazepine’ (under Intervention) (Box 3). The references of the included studies were inspected for further relevant studies. Citations were independently inspected by three review authors to identify relevant abstracts. The search results were appropriately displayed as a flow diagram. The risk of bias was independently assessed by two study authors using standardised criteria from the Cochrane Handbook for Systematic Reviews of Interventions. Overall, this review used an appropriate search method and included discussions of how they intended to further analyse their data for heterogeneity if multiple studies were included. The consistency of the methodology used allows for direct comparison with a previous search in 2007, which resulted in an ‘empty review’ (Gibson Reference Gibson and Walcott2008).
BOX 3 The Cochrane Schizophrenia Group's study-based register of trials
This register was developed in 1994 and is regularly updated through systematic searches of online databases, hand-searching of literature, searching grey literature, email alerts, checking references of relevant papers and their citations and by contacting relevant researchers and organisations. The register is compiled using multiple electronic databases without limitations on language, date, document type or publication status.
In May 2018, the register contained 25 328 reports for 18 079 coded studies. It includes studies where randomisation is either described or implied and includes quasi-randomised and non-clinical studies. The register can only be accessed by the Cochrane Schizophrenia Group's Information Specialist on behalf of the Group's authors.
Results
There were no new records identified compared with the previous empty review. However, data were analysed from one of the studies that was previously awaiting assessment to resolve disagreements between investigators (Schmider Reference Regestein, Alpert and Reich1999). This was a direct comparison between two benzodiazepines, lorazepam and oxazepam – two short-acting agents with similar pharmacokinetics but differing pharmacodynamics. Twenty-one participants were originally recruited for the study, of whom 17 were included in the final analysis. The mean age of participants was 50.8 years (range 21–77 years) and they had a range of mental illness diagnoses. There was no difference in catatonia symptoms between treatment groups as defined by at least a 50% improvement on the VAS (RR = 0.95, 95% CI 0.42–2.16; n = 17) or average total score on the VAS (mean difference 1.18; 95% CI −1.99 to 4.35; n = 17).
The quality of evidence was very low, owing to the small number of participants and risk of bias. The trial was of short duration (3 days): baseline observations were undertaken on day 1, the intervention received on day 2 and participants crossed over on day 3. Participants received lorazepam 2 mg sublingually (n = 7) or oxazepam 60 mg sublingually (n = 10) before being crossed over to the alternative treatment on day 3. Intramuscular and sublingual routes of benzodiazepine administration may be preferred to oral in catatonia because of concerns over oral intake and the safety of swallowing, the sublingual route being a less restrictive intervention than intramuscular. Of note, sublingual benzodiazepines are not licensed for use in the UK. The included study used 2 mg lorazepam, the equivalent of 20–40 mg oxazepam and a dose much lower than is used in clinical practice in the treatment of catatonia. However, the other arm received 60 mg of oxazepam – approximately twice the equivalent dose of benzodiazepine compared with the lorazepam arm. The choice of oxazepam dose was documented as being as in accordance with the manufacturer's advice. The degree of catatonic symptoms was the only outcome measure that was reported in this study.
There was no discussion of whether and how participants were randomised. However, the paper did state that the study was of a ‘double-blind’ design. The method by which allocation was concealed was not discussed. The study was therefore rated high for selection bias. It was also rated as having an unclear risk of attrition bias, with four participants (19%) not included in the final analysis. Adverse reactions were not reported and the study was therefore deemed to have an unclear risk of reporting bias.
The cross-over interval was short. The authors explained that this was for ethical reasons, to prevent a prolonged unmedicated period between interventions. However, even with short-acting benzodiazepines such as lorazepam and oxazepam, the pharmacokinetics of these drugs means that a carry-over effect cannot be excluded. The Cochrane review accounted for this by only using data from the first arm of the trial. Moreover, the cited ethical reasons also bring the appropriateness of the cross-over design into question. Such a design may have the advantage of reducing the sample size needed to adequately power the study, but would be more suited to a disease that is both chronic and stable, unlike catatonia, which can change rapidly (Box 4).
BOX 4 Cross-over trials
Cross-over studies are longitudinal studies that can be observational or interventional in nature. They have a repeated-measures design where each participant receives a sequence of two interventions (which may include a placebo). All participants therefore receive the same number of treatments for the same duration.
The advantage is that fewer participants may be needed to adequately power the study. However, this design may not be feasible for acute and rapidly changing clinical conditions such as catatonia. It is more suitable for chronic and stable conditions, the aim of which is improved quality of life as opposed to cure. After all, if the first treatment cures the patient then the second will not have a chance to demonstrate its efficacy. It is also a commonly used trial design for bioequivalence studies.
Discussion
Zaman et al's review included a single study that compared two benzodiazepines in the treatment of catatonia. This was very low-quality evidence, because of the small sample, short duration and numerous sources of bias. The reason for the review's limited findings might be the genuine lack of randomised trials, but one cannot exclude bias in the search methodology that failed to identify relevant studies. The review authors quite rightly recognise that some randomised trials may not have been included because of lack of clarity about their study design or inappropriate use of statistics and outcome measures. This outcome most likely reflects the scarcity of research into catatonia, due to difficulties in study design and/or lack of interest into the field. One could suppose that, with improvements in early intervention services and a wide choice of psychotropic agents for affective and psychotic disorders, catatonia may be less common (although without reliable epidemiological data this is conjecture). In psychiatric settings, catatonia is only seen in extremis, as a life-threatening presentation that requires immediate treatment, often in patients that are not capacitous to consent. It may also be that catatonia continues to be underrecognised and underdiagnosed. This is further compounded by the differing diagnostic criteria in the most commonly used classifications, the DSM-5 and ICD-10 (Box 5).
BOX 5 Classification of catatonia
Catatonia is classified differently in the DSM-5 (American Psychiatric Association 2013) and the ICD-10 (World Health Organization 1992).
In the DSM-5, catatonia is coded under 293.89 as a specifier associated with another mental disorder (293.119), catatonic disorder due to another medical condition (293.120) or unspecified catatonia (293.121).
In the ICD-10, catatonia is coded either as an organic catatonic disorder (F06.1) or as catatonic schizophrenia (F20.2). Catatonia is therefore a recognised specifier across a wider spectrum of primary psychiatric disorders in the DSM-5 than in the ICD-10. In the upcoming ICD-11, catatonia is coded as unspecified catatonia (6A4Z), schizophreniform catatonia (6A20.Z), secondary catatonia syndrome (6E69), catatonia induced by substances or medications (6A41) or catatonia associated with another mental disorder (6A40). This is therefore more aligned with that of the DSM-5.
This review highlights the paucity of RCTs on the use of benzodiazepines in catatonia, but this does not necessarily equate to a lack of an evidence base (Box 6). With case series (Petrides Reference Park, Tan and Krzeminski1997; Mekala Reference Kate, Raju and Vishwanathan2020; Neerukonda Reference Lin, Hung and Tsai2020) and observational studies (Raveendranathan Reference Penland, Weder and Tampi2012) demonstrating their efficacy, the use of placebo-controlled trials for an acute, life-threatening state could arguably be both dangerous and unethical. Although RCTs are held to be high-quality evidence (Fig. 1), this raises the question of whether they are either necessary or appropriate to understand the role of benzodiazepines in treating catatonia. It is important that epidemiological studies are undertaken to establish the burden of catatonia in psychiatric settings. Also, an improved understanding of the pathophysiology and neural circuitry may elucidate new targets for treatment.
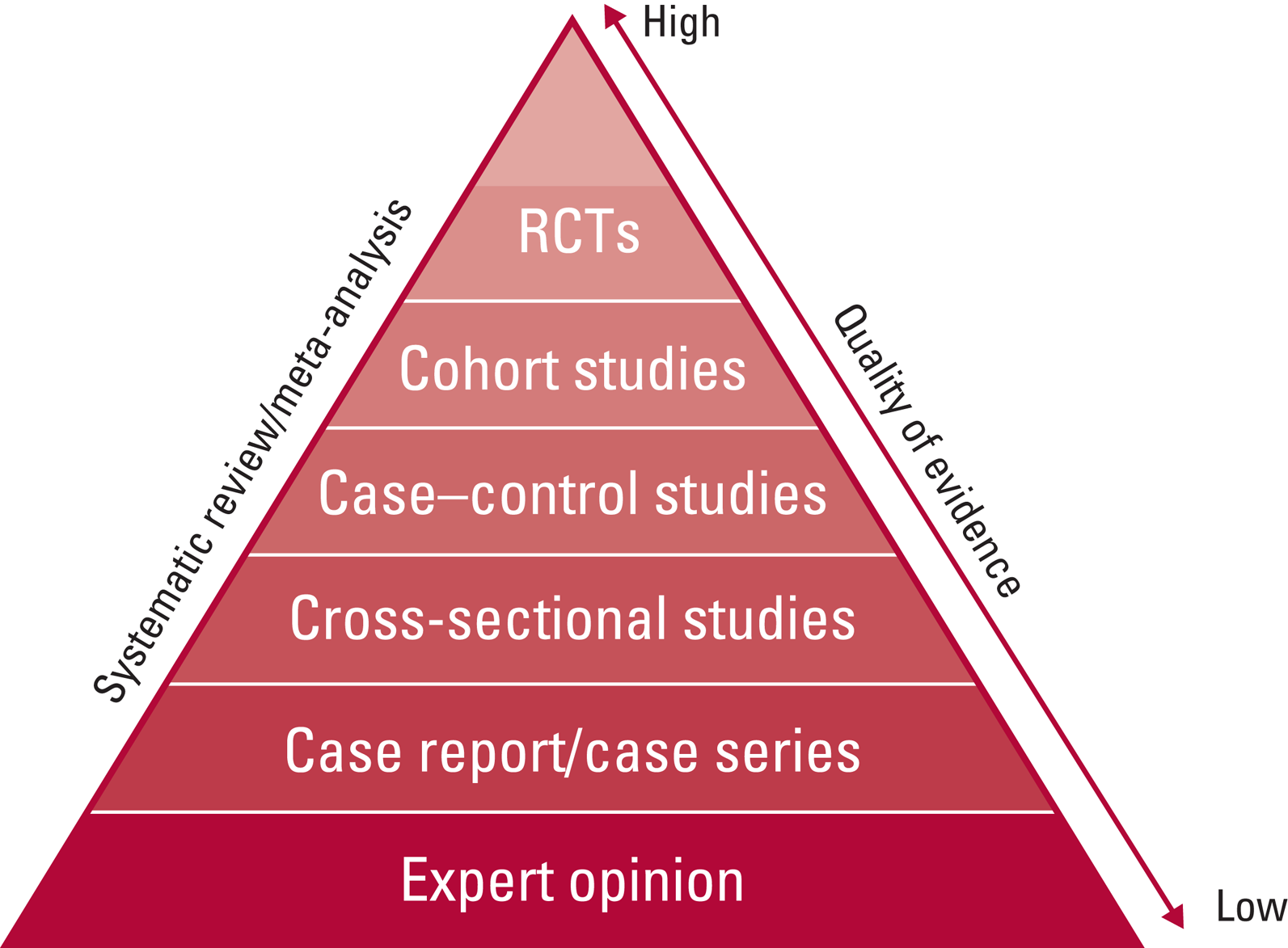
FIG 1 The hierarchy of evidence shows the study designs with the highest- to the lowest-quality evidence, which may then be used to inform clinical decision-making and guideline formation. RCTs, randomised controlled trials.
BOX 6 Evidence-based medicine (EBM)
Evidence-based medicine describes the use of clinical research in resolving clinical questions, including diagnosis, treatment, risk of adverse effects, prognosis and causation. As well as incorporating the hierarchy of evidence described in Fig. 1, EBM stresses the importance of using the evidence in the context of the individual patient, their wishes and values, as well as the risks and benefits of each intervention for that person.
This Cochrane review highlights a clinical scenario in which study design is difficult, and therefore the objective evidence base is scant. Clinical practice then becomes informed more by experience than by evidence. Nonetheless, it is very important not to misinterpret the absence of evidence as evidence of null effect and for clinicians to be supported in the use of EBM not only in forming clinical guidelines, but also in their implementation, and how to approach situations of uncertainty.
The sublingual route of administration, as well as low dose of lorazepam and non-equivalence of the dose of oxazepam used, means that applicability of this study is also poor. Oxazepam is an agent that is rarely used in clinical practice. There were no studies that used benzodiazepines with differing pharmacokinetics, such as diazepam and clonazepam, or trials comparing this group of medications with alternative psychotropic agents, placebo or ECT.
The single study included in this Cochrane review used the outcome measure of a 50% improvement in symptoms, according to the VAS. This is traditionally used in the assessment of pain and has not be validated for use in catatonia. The sensitivity and specificity are therefore unclear. Alternative tools to screen for and monitor the progression of catatonic signs and symptoms include the Bush–Francis Catatonia Rating Scale and the Northoff Catatonia Scale, both of which have been shown to be reliable and sensitive to change (Bush 1996a, Reference Bush, Fink and Petrides1996b; Northoff Reference Mekala, Malik and Lone1999). These tools were available at the time of the study and it remains unclear why these rating scales were not used. This was not discussed by the authors in the original article (Schmider Reference Regestein, Alpert and Reich1999). A lack of recognition of these validated tools may be another contributory factor to the underdiagnosis of catatonia.
Immunomodulatory therapy and rTMS
Catatonia is a core presentation of NMDA-receptor encephalitis, indicating that the immune system may be important in its pathophysiology and that immunomodulatory therapy may be an effective treatment (Rogers Reference Raveendranathan, Narayanaswamy and Reddi2019). This includes the use of plasmapheresis, intravenous immunoglobulin, corticosteroids or rituximab (Rogers Reference Raveendranathan, Narayanaswamy and Reddi2019).
It remains to be seen whether novel interventional techniques may assist in more targeted treatments for catatonia such as repetitive transcranial magnetic stimulation (rTMS). This has already been published in several case reports (Kate Reference Hansbauer, Wagner and Strube2011; Shiozawa Reference Rogers, Pollak and Blackman2013; Takamiya Reference Shiozawa, Da Silva and Cordeiro2015) and represents an exciting prospect. The slow onset of efficacy means that its clinical use will likely be limited to patients unresponsive to benzodiazepines, or where ECT is either contraindicated or not available or where long-term maintenance treatment is required (Hansbauer Reference Fink2020). It may also enable a more in-depth understanding of the cortical and subcortical motor circuits that are implicated in the pathophysiology of this complex and potentially life-threatening phenomenon. TMS has already shown promise in specifically targeting the dorsolateral prefrontal cortex to treat depression, one of the most common causes of catatonia. With enhanced understanding of the neural circuits of interest, rTMS could provide an adjunctive or alternative treatment for catatonia without the risk of dependency or the need for a general anaesthetic.
Conclusions
The findings of this Cochrane review are not able to influence clinical practice. However, they do highlight the paucity of high-quality evidence for the management of this potentially life-threatening syndrome that can manifest as a feature of numerous mental disorders. It raises an interesting discussion about why this may be the case, including difficulties with recognition and diagnosis and with study design. Specifically, one is left wondering what constitutes high-level evidence for the acute treatment of catatonia and whether RCTs are both feasible and appropriate. Improved awareness of validated diagnostic tools and better concordance between classification systems may improve rates of diagnosis and subsequent epidemiological data about the prevalence of catatonia. Future randomised or observational studies should ensure that any pharmacotherapy used is clinically relevant (including formulation, dose, frequency and route of administration) to improve generalisability. It remains unclear whether benzodiazepines are superior to alternative agents, placebo or ECT and, if so, which formulation, dose and regimen is optimal.
Acknowledgements
I thank Dr Riccardo De Giorgi (Department of Psychiatry, University of Oxford) for his support on this project.
Author contribution
E.C. was the sole contributor to the writing of this manuscript.
Declaration of interest
None.
An ICMJE form is in the supplementary material, available online at https://doi.org/10.1192/bja.2020.79.






eLetters
No eLetters have been published for this article.