Food preservation technology prior to canning and refrigeration involved salting, fermenting, drying or smoking foods, among other processes. Seamen during the age of sail (AD 1571–1862) relied on such techniques on lengthy voyages to lessen the likelihood of food degradation, but the most salient of these methods is salting. Salt is given special attention in historical shipboard accounts because the sailors’ diet was heavily treated with it.Footnote 1 Eugenio de Salazar expressed this sentiment when he wrote in The Landlubber's Lament (1573) that ‘Lady Sea will not tolerate or conserve meat or fish that is not dressed in her salt. Everything else that is eaten is rotten and stinking’.Footnote 2 John Collins, an English author, merchantman and accountant, wrote that it is a ‘great hazard of Mens[’] Lives … to salt the Provisions of a Ship or Garrison either with a bad salt, or ignorantly’.Footnote 3 During the Ship Biscuit and Salted Beef Research Project, a project that aims to determine the effects of the maritime diet on the nutrition and health of sailors during the seventeenth century, we noted that recipes repeatedly referenced ‘bay salt’ for brining meat brought on voyages. We used one such recipe to re-create the salted beef and pork for the project. Upon retrieving some of the aged meats for laboratory analysis, we noted that their interiors had a pleasant pink colour, much like meats cured with nitrate, and became curious as to what may have caused this colouration in the meat. Thus began our research into bay salt and other historical salts to understand more about why recipes specified bay salt's usage and whether the red colouration was typical of its use.
During the seventeenth century, various types of culinary salt were produced. Salts were described by colour (‘black’, ‘grey’ and ‘green’ salts), origin (similar to how Himalayan salt today indicates that it is salt from Pakistan) or, in the case of bay salt, its production method.Footnote 4 Originally, bay salt referred to the provenience of the salt, as it was most famously produced in the Bay of Bourgneuf, France, which dominated the market during the seventeenth and eighteenth centuries.Footnote 5 By at least the mid-seventeenth century, this term was applied to generic salt regardless of locale, made in salines (solar salt), shallow clay pans that were filled with salt water that was allowed to evaporate in the sun until the brine crystallized into salt.Footnote 6 Various sources document this salt production method, including De Re Metallica (1556) by Georgius Agricola, Salt and Fishery (1682) by John Collins, The Surgeon's Mate (1617) by John Woodall and Charlotte Mason's The Lady's Assistant (1775), among others (Figure 1).Footnote 7 Such sources, and the natural and practical knowledge they documented, also fed into the development of early modern scientific theories of salt, which played a crucial role in the evolution of matter theory from Aristotelian concepts of the elements to Newtonian chymistry.Footnote 8 In addition to bay salt, other types of salt made using other techniques were present at the time (Figure 2). Sea salt was made by boiling seawater over fire, rock salt was mined out of the ground, and basket salt was made from boiling brine from salt springs.Footnote 9

Figure 1. Agricola's illustration showing bay salt production. Georgius Agricola, De Re Metallica (tr. H.C. Hoover), New York: Dover Publications, 1950, p. 547.

Figure 2. Facsimile detailing different types of salt from Randall Cotgrave's dictionary. Randle Cotgrave, A Dictionarie of the French and English Tongues, London: Printed by Adam Islip, 1611.
Even given the wide selection of salts available, the use of bay salt (generic solar salt, not specifically from France) in the English Navy during the seventeenth and eighteenth centuries is well documented in primary sources, and the consensus in existing historical studies is that bay salt was used because it was inexpensive to produce.Footnote 10 However, when historical sources are studied in depth, bay salt's persistent use throughout history, and people's adherence to it, suggest that its popularity may not be solely explained by economics, and its cultural and historical significance is more complex than existing historiography insinuates. This study explores potential reasons apart from economics that may explain bay salt's continued popularity through time, combining analysis of historical sources and methods from experimental archaeology. Experimental archaeology is an approach for filling gaps in our knowledge about the past which cannot be filled through other archaeological and historical research methods. It does so typically through replicating a process that was believed to have been followed in the past to test an archaeological hypothesis. Historical archaeology is a subdiscipline of archaeology that studies material culture and supports its interpretation using written records or oral traditions.Footnote 11 As this article shows, experimental archaeology and historical archaeology can provide valuable new insights into early modern experiential knowledge.Footnote 12
A bitter aftertaste? Historical and contemporary sources on bittern in bay salt
Many historical sources indicate that bay salt had a pejorative reputation. An Irish resident in Nantes attempted to start a salt beef processing business but was unable to acquire appropriate salt, and as a result his salt beef ‘held up badly on the long transatlantic crossing and was rejected on the basis that it spoiled because of the inferior, local sel de Guerande’.Footnote 13 Collins rallied against bay salt in maritime provisions, writing that ‘Dutch Mariners returning from long Voyages, look fat, healthful, and fresh Coloured, because their Flesh and Fish is saved with refined Salt. Whereas on the contrary our Mariners feeding on Provision cured with Bay Salt, are scorbutick and incombred with acrimonious Diseases’.Footnote 14 Bay salt was outlawed in certain countries for its poor quality – during the mid-fifteenth century in Brielle, only purified salt was allowed to be sold in town.Footnote 15 In 1471 in Bergen, a merchant in a legal dispute stated that his ship was loaded with salt on salt that had been purified in Bergen ‘from good green Bay salt’ as proof of its quality.Footnote 16 Other sources concur that fish could not be cured with bay salt and that the Dutch, who controlled much of the fishing trade at the time, had to refine bay salt to create ‘salt on salt’ to cure their fish, which the English then copied.Footnote 17 Creating salt on salt (salt upon salt) involved dissolving bay salt in water and reheating it by fire to purify the brine of calcium, a constituent of bittern.Footnote 18 Bittern is the bitter remains from the crystallization of salt made of calcium and magnesium chlorides and sulphates, bromides, iodides and other chemicals originally present in seawater.Footnote 19 Historical sources give several reasons for bay salt's supposed inferiority: its inclusion of bittern, organic impurity and inconsistent quality.Footnote 20
Because it was simply sun-evaporated from unfiltered saltwater, bay salt contained ‘dirt, sand, and bittern’, making it unsuitable for curing meat and fish.Footnote 21 Bittern ‘renders the Meat dry, hard, dirty, rotten, and by reason of the Bittern in it, consumes the goodness or nutrimental part of the Meat’, which was said to cause ‘Scurvies, Consumptions, and other acrimonious Diseases, in the Bodies of Seamen, or Soldiers in a besieged Garrison, that are compelled to the frequent and long use of it’.Footnote 22
Modern studies on the chemical composition of salts support the idea that magnesium and calcium salts, the main components of bittern, are unsuitable for curing and that seawater generally contains higher amounts of these chemicals, making salts derived from unpurified seawater detrimental for food preservation.Footnote 23 In 1920, Dr. D.K. Tressler experimented with the effects of different types of curing salt on fish, and found that salt with proportions of magnesium and calcium seen in seawater inhibited salt from easily penetrating the fish, preventing the inner meat from being cured before it spoiled. The effects were evident less than a week after curing, and similar experiments agree with these results.Footnote 24 Blesa et al. noted that water activity (aw), an indicator of microbiological activity and spoilage, increases when meats are salted with potassium, calcium and magnesium salts, which meant that salts containing bittern had difficulty penetrating ham and would require a greater post-salting time compared to regular table salt, nearly pure sodium chloride without the minerals in bittern.Footnote 25 The effects of bittern on preservation were so evident that herring cured with ‘coarse French Bay salt should be limited to 17 lasts per hundredweight of salt, as opposed to 22 lasts with peat salt’, and ‘all curers should brand their barrels with the appropriate initial letter, either a B or S, so that buyers could immediately tell which salt had been used’.Footnote 26 Boiling, which was done for many types of salt, including peat salt, sea salt and basket salt, but not bay salt, was known to decrease bittern, as the boiling removed calcium salts.Footnote 27
In addition to including bittern, bay salt has long been known to have inconsistent quality and to contain other impurities. In 1467, German merchants complained about the quality of bay salt which was ‘mixed with earth and caused herring cured with it to go bad’.Footnote 28 Seawater that was entering the salines was not filtered or cleaned of debris, and, at least in the Bay of Bourgneuf, the water entered freely through bungholes in the seawall, meaning that its quality varied greatly depending on what detritus the tides brought in. French bay salt was impure and described as grey, black or sometimes green, but was said to still be used because it was ‘large-grained, inexpensive, and nearby’.Footnote 29
However, even with such drawbacks, bay salt was consistently used for curing meats both for regular land use and for use at sea, as various primary sources show. Martin Frobisher, the sixteenth-century English privateer and seaman, had a suggested provision list dated to 26 March 1588 that included five tons of bay salt for his second voyage to the New World. In the same list, in a separate row under the subheading of salted beef, was written, ‘baye sawlte to preserve the same 55 bushels [of beef] at ijs [2.5] per bushel [of salt]’.Footnote 30 The fleet of the Mary Rose's last campaign was victualled with at least 2,134 quarters of bay salt.Footnote 31 Collins, although he contradictorily wrote against the use of bay salt, included several recipes for curing with bay salt and noted ‘our [English] Mariners feeding on Provision cured with Bay Salt’.Footnote 32 Eliza Smith's The Compleat Housewife (1727) includes a recipe for salt hams that uses a peck (two gallons) of bay salt, four ounces of saltpetre and three pounds of brown sugar.Footnote 33
Yet the consensus among modern scholars is that bay salt was popular and frequently used because it was inexpensive. It was exported from France to England and other European nations from at least the medieval period, and exports reached a peak in the seventeenth century when it became such a large trade that it was a topic of concern in England as France was often an enemy.Footnote 34 Bay salt's popularity is attributed to its cost efficiency because it required little labour and equipment and no fuel to produce.Footnote 35 It is estimated that bay salt from France during the fifteenth century was two-thirds to half the price of Lüneburg and other superior white salts (white salt being nearly pure sodium chloride), while English sources indicate that French bay salt was brought to London for less than fourpence per bushel, but salt from England saltworks cost sixpence.Footnote 36 While economics appeared to play a major role in salt-purchasing decisions, primary sources also state that bay salt was good for curing because it was large-grained. Even as far back as the thirteenth century in The Account Book of Beaulieu, coarse-grained salt was used to cure fish, and fine-grained salt was kept for common use.Footnote 37 In the eighteenth century, Hannah Glasse (1747) wrote that York hams were cured with Maldon salt brought from Essex that ‘is a large clear Salt, and gives the Meat a fine Flavour’.Footnote 38 Some modern scholars claim that large, coarse grains of salt penetrate the flesh better and produce a better cure, whereas fine-grained salt allegedly sealed the surface, preventing the salt from entering further than the surface tissues.Footnote 39
Only one source, Collins's, in his argument that bay salt is unfit for use, attributed its inferiority to the salt's large size. He stated the exact opposite of other authors – that the coarse grains’ slow dissolution rate prevented it from preserving the meat, specifically noting that a third of the salt does not dissolve in time, therefore requiring more salt to be used overall.Footnote 40 However, nearly all sources disagree with Collins, and his discourse has a political agenda to preserve the English salt industry. For example, a section labelled ‘Arguments for the Encouragement of English Salt, and hindring the Expence of Foreign’ argues against the importation of French salt.Footnote 41 Even the name of today's salted beef (corned beef) is derived from the term ‘corns’, which was the seventeenth-century word for small bits of material, in this case, coarse salt crystals.Footnote 42
Salt grain size is determined by the speed of evaporation. A fast boil agitates the crystals of salt and prevents them from accumulating into large grains. Evaporation by the sun or other methods of slowly drying allows large crystals to form, as seen in bay salt.Footnote 43 As bay salt did not require fuel to evaporate the seawater, it was more economical, but there is evidence that coarse-grained salts were so much more preferable that salt producers were careful to produce the large grains even when expenses had already been made to clarify the salt. In 1678, Thomas Rastel, in a salt-clarifying recipe, specified not to stir the brine and egg white mixture too much in order to produce large-grained salt similar to that of bay salt.Footnote 44 While most historical sources indicated coarse salt was beneficial for curing, modern experiments by Arvill Bitting have indicated that the salt size did not affect the rate of penetration of salt into fish.Footnote 45 Furthermore, most shipboard meats were wet-cured and stored in brine, thus making grain size irrelevant.Footnote 46
In short, historical sources show that bay salt was inferior in quality but still utilized because it was inexpensive and served its purpose satisfactorily, but low costs cannot explain everything. Curiously, although Collins repeatedly criticized bay salt and wrote that it ruined food, he specifies its use in several salted meat and fish recipes (Figure 3).Footnote 47 In The Art of Making Common Salt (1748), William Brownrigg, a London physician, wrote, ‘For certain uses such as curing fish English white salt and rock salt are not as good as Bay salt which is imported from France.’Footnote 48 Historical studies have demonstrated that affluent households used bay salt for curing but more costly white salt for the table even when they had the means to use white salt for curing if desired, implying that regardless of financial means, different types of salt were still purchased for set purposes.Footnote 49 The English Navy relied heavily on bay salt and, interestingly, ‘only reluctantly gave up using French [bay] salt’ during the end of the seventeenth century, even though by then it had become more expensive than local salts produced from Portsea.Footnote 50 Furthermore, even after bay salt became less expensive than white salt in England during the mid-seventeenth and the eighteenth centuries, it became common to mix bay and white salt for curing.Footnote 51 Thomas Wilkins was said to have leased Hampshire saltworks in England in 1701 and experimented with the production of solar salt, paving some of the evaporation pans with brick, which produced large-grained white salt. He also made other salts on unpaved ground, which produced salt the colour of French bay salt. He wrote, ‘And [the gray salt] pleasing those best, who fancied the Bay salt to have some particular virtue in it; I gave myself no further trouble to pave the pans.’Footnote 52 While bay salt contained organic debris and bittern, sources suggest that it also had some quality to it that was extremely desirable so that it was used even when inexpensive and supposedly higher-quality white salts were available. Even today, unrefined solar salt, such as salt from Guérande and Salinas de Añana, is used by five-star chefs who boast of its quality in gourmet cuisine.

Figure 3. A folio from John Collins's Salt and Fishery specifying bay salt for use in salted meat and fish. John Collins, Salt and Fishery, London: A. Godbid and J. Playford, 1682, p. 121.
It is unclear whether adherence to bay salt for curing was simply due to tradition and economics, or whether there is a scientific reason, such as better preservation or taste, for its use in curing meats. Given that there are numerous variables that could lead to better preservation or taste, and historical documents do not provide a specific modern scientific hypothesis, we decided to approach this in a more open-ended way and analyse all probable variables we could think of. We hypothesized that if bay salt truly had a beneficial effect on meat preservation, either it contained nitrates or nitrites, or it had higher salt or other mineral content that could better preserve meats, or the salts influenced preservation via inhibiting detrimental microbes or encouraging beneficial microbes for preservation.
These hypotheses were borne out of observations made during the Ship Biscuit and Salted Beef Research Project and from general knowledge about food spoilage. The main processes through which meat spoilage occurs are the oxidation of fat and myoglobin and microbial decomposition.Footnote 53 The odour and rancidity of decayed fat are the results of the oxidation of unsaturated fat molecules, while oxidation of the myoglobin changes the meat from red, to brown, to dark brown or black.Footnote 54 The colour changes are correlated with bacterial growth, especially if there is water on the surface of the flesh.Footnote 55 Bacterial growth can be prevented using a high salt content, such as the saturated brine the meat is kept in, which then enhances the osmotic pressure of the meat and inhibits the growth of microorganisms.Footnote 56 The use of nitrate in meat also simultaneously inhibits certain undesirable bacteria and lipid oxidation, produces the characteristic red cured-meat colour, and improves the flavour.Footnote 57 As noted, the meats from the Ship Biscuit and Salted Beef Project exhibited a pleasant red and pink colouration, although no nitrates were added. As such, it was decided to base the project around the mineral (including nitrate, nitrite and ammonia) and microbiological content of the salts.
The experimental archaeological part of this project was split into two main portions that took place at different times. The first, re-creating the salted beef and pork using bay salt and gathering its microbiological and mineral data, was done earlier as a part of the Ship Biscuit and Salted Beef Research Project. At the time of re-creating the salted meat recipe, historical research on the other types of salt had not yet been done. The second, sourcing or creating the different types of historical salt and gathering their mineral and microbiological data, was done as a peripheral project after the salted beef and pork were observed to have an unexpected red colouration, and further research had been done on historical salts, prompting our curiosity. While it would have been ideal to further experiment by salting beef and pork using the different historical salts and comparing the results, this was not feasible as the salted beef and pork portion of the project had been completed, and obtaining new casks, meat and museum permission to store the casks on their premises was not possible. As such, conclusions on the effects of the historical salts, apart from bay salt, on meat can only be inferred from descriptive data, but not confirmed, until curing meat using the other salts is done to serve as comparison.
Choosing a recipe, salt sourcing and experimental production
Sea salt that was made by boiling seawater over fire was replicated, while rock salt that was mined and bay salt from salines were obtained from historically accurate sources. Basket salt, made from boiling brine from salt springs, was not acquired due to difficulties in locating local salt springs from which to produce it.Footnote 58
Bay salt, today commonly known as grey sea salt (sel gris), was purchased from the San Francisco Salt Company, a salt distribution company that purchases their French grey sea salt from the Guérande region of France. Although not in the famous Bay of Bourgneuf (which no longer produces salt due to salt marsh conversion to agricultural activity), Guérande has long been known for salt production using a tradition that dates as far back as before the ninth century AD.Footnote 59 The Guérande salt marshes were built as a system of channels that funnel ocean water into the Guérande basin for evaporation. At high tide, when water is needed, workers open a sluice to let in the seawater, which is allowed to settle and begin evaporation. From here, the minor changes in seawater levels allow the water to flow into even smaller evaporation ponds where the water continues to evaporate and the salt concentration increases. In the final ponds, called ‘eyelets’, the salt is concentrated enough to crystallize and is raked into piles to further dry before being collected.Footnote 60 The San Francisco Salt Company notes that the salt is imported directly from Guérande in its natural state and has not been further processed, meaning that this salt contains bittern, as the historical bay salt from the region did in the past. This method is comparable to that described in Collins's Salt and Fishery and Agricola's De Re Metallia.Footnote 61
Rock salt, or mined salt from the ground, was sourced from Grand Saline, Texas. The salt mines are located 228.6 meters (750 feet) below the surface and were mined from around 1886. The salt flats above the mine were used by Native Americans as early as AD 800 as a source of salt. The mine's temperature stays around 23.9 °C (75 °F) year-round, and salt is mined from the dome that is 98.5 per cent sodium chloride.Footnote 62 Several large pieces of rock salt were generously provided by the Salt Palace Museum in Grand Saline, Texas for use as a comparison to the other historical salts. As noted by Woodall, rock salt is not produced but mined from the ground: ‘The third is the salt that groweth concrete, hard and pure in the bowels of the earth, such is the Sal Gemm, and this last is held the best, both in meate and medicine, it is in colour like Christall transparent, and growth in great quantity in Polonia, neere the City of Cracovia.’Footnote 63
Sea salt, salt that is made by boiling seawater until crystals form, was made from seawater from the second sandbar of 11 Mile Beach in Galveston, Texas. The water was collected into two five-gallon buckets, then strained through a grade 50 mesh (twenty-eight by twenty-four threads per inch) cotton cheesecloth before being boiled in a sixteen-quart aluminium stock pot on a modern gas stove. As the brine boiled, it became cloudier from the increasing salt concentration, and after the second day of boiling, the first signs of crystallization occurred. The newly formed crystals were flaky and off-white in colour. On the third and final day of boiling, the pot containing the salt crystals and the remaining water was allowed to cool and then immersed in an ice bath to facilitate the crystallization process. After crystallization was complete, the salt was strained through cheesecloth to remove any excess brine (Figure 4). All salt crystals were stored in Whirlpak WhipLock™ bags. Historically, square or rectangular cauldrons made of copper, iron or lead were used, while fuel sources from straw to wood were used.Footnote 64 Period equipment was impossible to obtain, and the heat source used was a gas stove, which is different to what was used in the past, although the concept of how sea salt was produced was similar.

Figure 4. Newly formed sea salt crystals strained in cheesecloth for drying. Photograph by John McQuitty.
An issue of concern was whether the salts sourced and produced would be exactly like what was produced in the past. While there is no way to know the exact composition of different historical salts, salts used in the past would have also varied widely depending on locale and other external factors; for example, rock salts from different mines would still be considered rock salt even if they had different mineral compositions.Footnote 65 The categorization of salts that contemporaries used was based on production methodology, so this was used as the defining variable of how to produce the salt, and the geographic location was not prioritized (e.g. rock salt from Texas salt mines would still have been considered rock salt in the past even if it was not from European salt mines).
For the Ship Biscuit and Salted Beef Project, a grass-fed steer of approximately twelve months of age, having no known prior exposure to antibiotics, was sourced. The live body weight was roughly 363 kilograms (800 pounds), and the hanging weight was about 193 kilograms (425 pounds). The butchering and salting processes were based on the Salt and Fishery recipe in Collins's 1682 discourse, which can be found in Figure 3.Footnote 66 The primal cuts were butchered into four-pound slabs. Each slab was based on what was believed to be common cuts on board seventeenth-century ships. These data were obtained from the project's zooarchaeological analysis of remains from Warwick, the seventeenth-century English galleon which sank off the coast of Bermuda in 1619, other shipboard faunal remains from archaeological reports, and primary historical documents.Footnote 67 The beef slabs were then laid in a new oak cask (seasoned) with a thick blanket of French Bay of Guérande salt. The layers of beef had a thick blanket of salt between them to thoroughly dry-cure the meat. No ratio was specified in the recipe, but the project used a total of about 86 kilograms (190 pounds) of salt on 118.2 kilograms of bone-in beef during the dry-cure process. After twelve days of dry salting, the beef was removed, excess salt was shaken off, and the meat juices that had gathered at the bottom of the cask were removed and boiled to produce brine. Meanwhile, more brine was made using natural untreated aquifer water that was saturated with bay salt until an egg could float in the solution. The beef was put back into the cask, and the cooled meat juices and brine were poured into the cask until it was topped off so that all the pieces were submerged, completing the pickling process according to the recipe (Figure 5).

Figure 5. The beef cask after it was filled with brine. Photograph by Grace Tsai.
The pig was obtained from Chubby Dog Farm and was a Mangalitsa–Tamworth heritage boar with a live weight of approximately ninety-one kilograms (two hundred pounds) and a hanging weight of sixty-eight kilograms (150 pounds). The instructions for salting pork are the same as for the beef, as indicated by Collins,Footnote 68 with the exception that pork was normally butchered into 0.9-kilogram (two-pound) slabs according to several ration lists and primary documents.Footnote 69 Otherwise, the same procedure for dry salting and brining the pork was carried out. Due to the smaller size, the meat was placed in a smaller oak cask (also seasoned and new), and a total of sixty pounds of salt was used for dry salting.
While many other salted beef and pork recipes exist, Collins's recipe was chosen for the project because its minimalist ingredient list matched that seen in ship victualling lists and is specifically mentioned for ‘long keeping’, which was an important consideration that ship captains and victuallers had.Footnote 70 Various other recipes include spices and sugar that were not available to most common sailors.Footnote 71 A recipe for comparison that described how meat was prepared in the English Navy is recounted by Stephen Hales, writing in the eighteenth century that, at the naval victualling office,
they first rub it with white Salt only; then put it into Brine for five days to drain the bloody part out, for ’tis the Blood that is most apt to putrify: Then they pack it in Casks, strewing white and Bay Salt between each laying; then fill the Cask up with Pickle made of Water and Salt boiled so strong as to bear an Egg: They put three Pounds and half of Salt to a Gallon of Water. The proportion of Salt, Pickle included, is, to an hundred Weight of Flesh, four Gallons and a half of white, and one and a quarter of Bay Salt. The Pieces thus salted with dry Salt, after the infusion of the Brine or Pickle, must be laid to soak for some time in Water, before they be used; else they will be apt to be too salt: The dry Salt, as before observed, soaked very fast, into the thus brined Flesh; so that there is not the least danger, of its not keeping sweet there seeming rather, to be more danger of its being by this means too salt: Which may doubtless by further Experience be better regulated and proportioned, to the longer or shorter time it is intended to keep it.Footnote 72
On 19 August 2017, the beef in the cask was loaded onto the dock of Elissa, the nineteenth-century tall ship in Galveston, and the pork onto the deck of the ship (Figure 6). Samples for laboratory analysis were collected throughout. The specimen retrieval for the project was originally intended to increase exponentially in duration between collection days, as microbiological growth occurs exponentially, but due to Hurricane Harvey the scheduling was modified (Table 1).

Figure 6. Loading casks onto Elissa. Photograph by Grace Tsai.
Table 1. Schedule of shipboard food sample collection.

Taking samples and laboratory testing
On each sample collection day, two salted meat slabs from each beef and each pork cask were removed. One of the samples of the two taken from each cask was submerged in water from the freshwater cask on board the ship to mimic the desalting process that sailors would have peformed for a day prior to boiling the meat, while the other was left in its original state to test the meat's uncontaminated state directly from the cask. Each slab was separately placed into an individual Whirlpak WhipLock™ bag, one with fresh water and the other without. Both samples were then placed in a cooler for twenty-four hours. After this twenty-four-hour period, only the slabs that were submerged in water were separately boiled for ten minutes, as heating food at 100 °C (212 °F) for ten minutes greatly reduces the risk of foodborne illness.Footnote 73 However, for this portion of the experiment, only the raw slabs of meat were used for microbiological, nitrate, nitrite and ammonia analyses. Meanwhile, the mineral analyses were run on the cooked meats as a part of the Ship Biscuit and Salted Beef Research Project. The casks were refilled with pre-saturated brine after each sample was removed (except for the last sample collection) to ensure that the remaining meat was submerged, as sailors would have done in the past, and resealed.
Once transported to the lab, the samples were subjected to microbiological analysis and colorimetric analysis for nitrate, nitrite and ammonia, which are described in detail in the following paragraphs. Microbiological analysis involved plating the salt and meat samples on different growth media to understand the basic characteristics of the microbes and to measure increases and decreases in them over time. In addition, some colonies of bacteria had 16s rRNA sequencing for genotypic identification done to get a see more precisely whether they are genera typically beneficial or detrimental to preservation. Due to high costs and the time needed, only a few cultures have been sequenced thus far. As the interior of the meats showed the characteristic red and pink colouration indicative of nitrate, although no saltpetre was added, nitrate, nitrite and ammonia testing were done on the meats and the salts to identify whether any of the salts had these chemicals present, inferring that they would be superior to plain sodium chloride. Trace-mineral analysis was also done to determine whether there were minerals that may benefit or hinder preservation, such as a higher ratio of sodium for certain salts that lead to better preservation or greater concentrations of bittern. From this, it emerged that while bay salt and sea salt had greater bittern values, they also had higher values of nitrate, enough to cure meat. Furthermore, bay salt also carried a high number of microbes, compared to other salts, that may aid in nitrate reduction.
Microbiological testing procedure
An interior portion of one of the salted meat slabs at each sample collection was subjected to microbial analysis via excision of three inner pieces of meat (each weighing approximately between three and fifteen grams) from each slab. These interior pieces were washed with a ten-second submersion in 70 per cent alcohol to sterilize potential contamination that may have occurred on the surface of the inner piece of meat during the excision process. The sample was then rinsed to remove residual alcohol via submersion in a beaker of sterile deionized water, quickly withdrawn, and subsequently diluted with fresh sterile deionized water (one to one ratio of meat to water) and homogenized to draw out any solution containing bacteria from the centre of the piece. Resultant solutions of the salted meat interior were then serially diluted and plated, as described below. Brine samples, which are also representative of the surface of the slabs, collected from each flask were not diluted with deionized water like the meat, but were serially diluted as described above and plated to ascertain microbial characteristics representative of the exterior surface of the salted slabs. Historical salts were also diluted (one gram of salt to five millilitres of sterile deionized water) and then similarly serially diluted before being plated.
For the serial dilution and plating, one hundred microliters of 10-1 to 10-5 dilutions of each sample were spread onto the following three different types of media: mannitol salt agar for selective recovery of halophilic and halo-tolerant bacteria (such as Staphylococcus), trypticase soy agar with 5 per cent sheep's blood for non-selective determination of total culturable aerobes, and MacConkey agar for selective recovery and differentiation of enteric Gram-negative bacteria often representative of food and waterborne pathogens.
After inoculation, the plates were incubated for twenty-four hours at 37.5 °C before being counted, except for mannitol salt agar plates, which were counted after forty-eight hours of incubation due to slower growth. Unique, well-isolated colonies were then carefully picked, restreaked to their respective medium, and incubated as before to ensure purity, and resultant pure cultures were stored at –80 °C in 20 per cent glycerol. Select isolates were subjected to 16s rRNA sequencing for genotypic identification (Figure 7).

Figure 7. Mannitol salt agar plate of bay salt at 10-1 dilution (plate 146-1) showing the red and orange colours of the bacterial colonies grown from the salt used in this project. Photograph by Grace Tsai.
Mineral testing procedure
Mineral testing was outsourced to Texas A&M AgriLife Extension Service Soil, Water, and Forage Testing Laboratory in triplicate. Phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), zinc (Zn), iron (Fe), copper (Cu), manganese (Mn), sulphur (S) and boron (B) were measured in parts per million (milligrams per kilogram).
Nitrate, nitrite and ammonia analyses
The cured meat's outer surface turned a greyish brown colour soon after the twelve-day dry-curing period, indicative of oxidation. However, throughout the experiment, the interior of the meat had a bright pink or red colour and texture like that of meat preserved with nitrates or nitrites when it was cut open to remove inner samples for microbiological plating, although no nitrate or nitrite salts were added (Figure 8). Nitrates and nitrites are meat preservatives well known for their antimicrobial properties. They are particularly noted for inhibiting Clostridium botulinum, producing the characteristic cured-meat colour, inhibiting lipid oxidation and improving flavour.Footnote 74 Due to this observation, it was decided also to determine whether nitrate, nitrite and ammonia values changed in the meat and brine throughout the experiment, and if any were present in the salts.
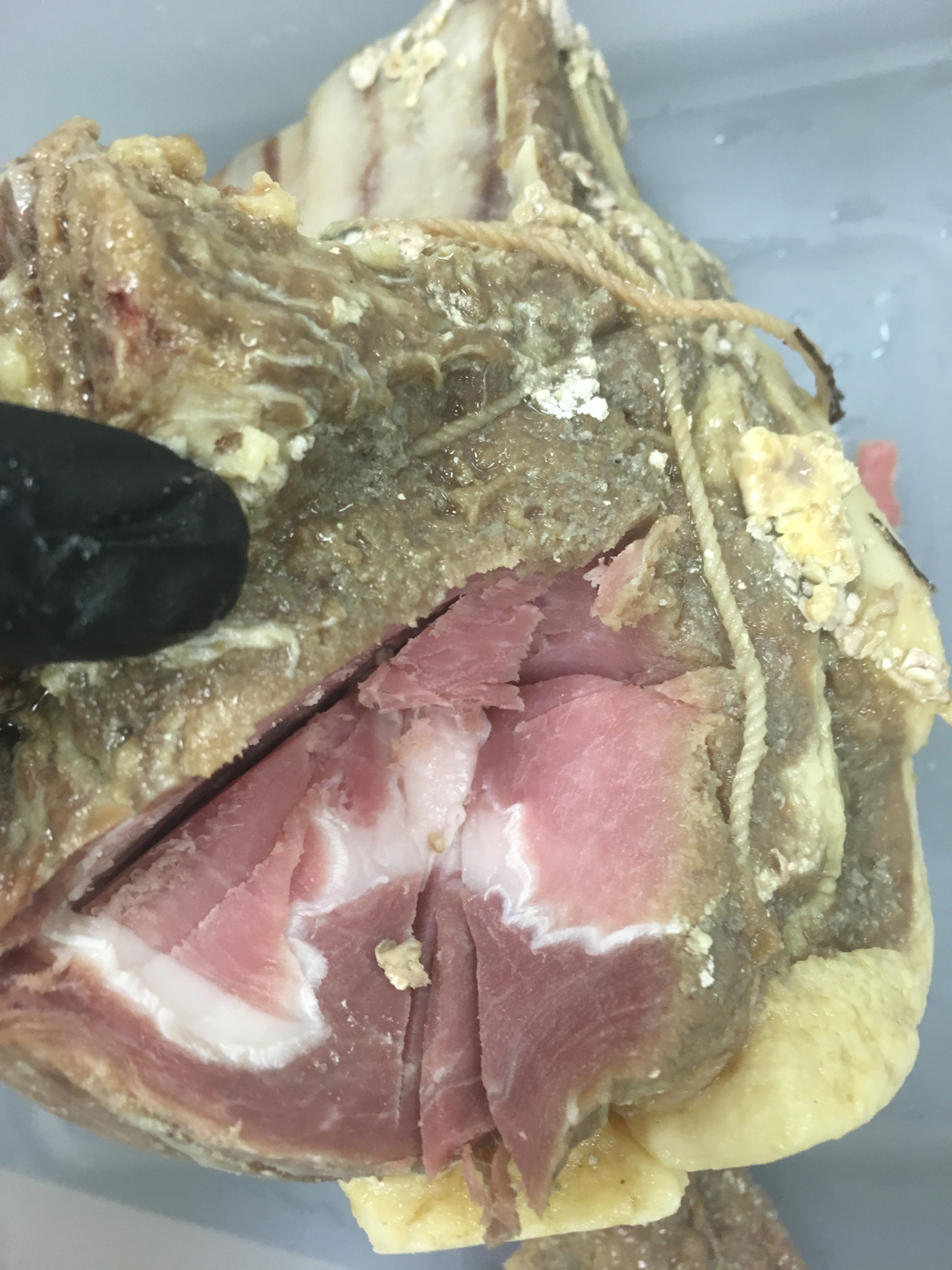
Figure 8. A slab of salted beef cut open to extract an internal sample for microbiological analysis. Notice the red and pink colouration. Photograph by Grace Tsai.
Each sample type was prepared in triplicate. Salt samples were diluted using one gram of salt dissolved in five millilitres of deionized water. Meat samples were prepared by grinding three grams of meat to fifteen millilitres of deionized water and the resultant liquid extracted.
Nitrate assay procedure
A total of twenty microlitres of sample was added to 13 × 100 millimetre tubes and analysed to determine nitrate content using the colorimetric method of Cataldo and colleagues.Footnote 75 A total of twenty microlitres of sample or standard (0, 0.75, 1.50, 3.0, 6, 12, 24 millimolar (mM) nitrate) was reacted with eighty microlitres for twenty minutes, then neutralized with 1.9 millilitres L 2 M sodium hydroxide. After ten minutes at room temperature, nitrate concentrations were calculated from the absorbance readings of standards and samples measured at 410 nanometres.
Nitrite assay procedure
Samples for the nitrite assay were further diluted (300 microlitres of sample to 200 microlitres of water) before undergoing nitrite analysis according to the protocol laid out by Schneider and Yeary.Footnote 76 Nitrite concentrations were measured in standards and samples after diazo-coupling to sulphanilamide and N-1-Naphthyl ethylendiamine dihydrochloride. Using the stock, 0.6 mM sodium nitrite made up to ten millilitres with water was used to prepare the standard curve (standard curve 0, 5, 10, 20, 30, 40, 50, 60 micromolar (μM) concentration). Then 0.5 millilitre of sulphanilamide reagent was added to each tube, and three minutes were allowed to pass before adding 0.05 millilitre N-1-Naphthyl ethylendiamine dihydrochloride reagent. The solution was mixed, and twenty minutes were allowed to pass before they were read at 540 nanometres.
Ammonia assay procedure
The ammonia assay was a catalysed indophenol reaction done according to the protocol set out by Chaney and Marbach.Footnote 77 The sample was added to each sample tube while the standard curve was prepared (standard curve ammonia mM 0, 0.75, 1.5, 3, 6, 12, 24). Then three millilitres of phenol reagent were added to each tube and mixed. Afterwards, 3 millilitres of hypochlorite reagent was added to each tube and mixed. Tubes were incubated at 39 °C for 20 minutes and mixed again before reading them at 630 nanometres.
Mineral analysis results
The results of the mineral analysis can be seen in Table 2. Sea salt exhibited the highest mineral concentrations in potassium, calcium, magnesium, sulphur and boron. Out of the three salts, the sea salt also had the lowest sodium content, whereas bay salt differed from the other salts by containing much higher amounts of iron. The rock salt had comparatively low mineral values except for sodium, of which it had the highest. Overall, no major trends or differences that would imply superior curing were found in the bay salt's mineral content. This was somewhat expected, given that bay salt refers to a method of salt production rather than to a specific locale of production, which is what would produce mineral composition changes.
Table 2. The mineral values in the various salts reported in milligrams per litre (parts per million)

Nitrate, nitrite and ammonia assay results
None of the salts had detectable ammonia (minimum observable level being 0.0469 mM at the lowest standard concentration), but nitrate was present in all salt samples, particularly in the bay salt and sea salt. Nitrite was only detected in the sea salt (Table 3).
Table 3. The ammonia, nitrate and nitrite values in the salt and salted meats are reported in parts per million (ppm) (milligrams per kilogram). Note that the start dates for the meats are samples that were taken after the twelve days of dry salting, so they are not indicative of levels in the fresh meat (shown in Table 4). The minimum observable level was 0.047 mM for ammonia, 0.056 mM for nitrate, and 0.039 μM for nitrite.

Table 4. Nitrate and nitrite levels in fresh beef and pork, limit of quantification (LoQ) for meats were 4.5 milligrams per kilogram for the nitrite ion and 9.6 milligrams per kilogram for the nitrate ion.Footnote 79

Ammonia in the beef slightly decreased from beginning to end. The pork increased slightly in ammonia in the final experimental sample. Nitrate for both meats was undetectable at the beginning but increased greatly in the experimental end samples. Nitrite decreased in the end samples compared to the start samples (Table 3).
The brines showed an increase in ammonia, nitrate and nitrite concentrations, except for the nitrate value in the pork brine, which decreased minimally (Table 3).
Other studies note that the values of nitrate and nitrite are low in fresh beef and pork and standard sea salt (Table 4).Footnote 78 The values of nitrate found in the experimental meats and brine are greater than expected in fresh meat or sea salt alone. The values in the bay salt also cannot account for nitrate and nitrite in the experimental meat and brine.
Today's maximum allowable limit of nitrate and nitrite in commercial foods depends on the method of curing used and the type of product, but the levels found in the experimental meat are below the regulatory limit and within safe levels for consumption.Footnote 80 While large changes in nitrite were not observed in the samples, the nitrogen cycle is highly complex, and many compounds are highly unstable; nitrites normally decrease rapidly, being converted to nitrous acid, nitric oxide, ammonia and nitrates, and are therefore difficult to determine.Footnote 81 Nitrite can change into these different components depending on temperature, pH and various reducing agents, including bacteria, and it is possible that some of the increase in ammonia seen in the meat and brine samples is from nitrate and nitrite reduction after it had already cured the meat.Footnote 82 Even with degradation, nitrite levels were likely enough to cure the meat and produce the red colouration, because as little as two to fourteen milligrams per kilogram of sodium nitrite is sufficient for colour development.Footnote 83
Microbiological analysis results
After each microbiological plate was counted, its number was recorded and converted to log10 based on their dilution to detect statistical outliers. These values were averaged, while those that were marked ‘TNTC’ (too numerous to count) or below detection (count of 0) were excluded from the average. The averaged log10 values were then reconverted into arithmetic values, which are presented in Table 5.
Table 5. Average microbial counts (colony-forming units (CFU) per millilitre for liquids or CFU per gram for solids) for beginning, middle and end samples averaged. The limit of detection (LoD) is 20 CFU per millilitres or 20 CFU per gram, depending on the sample.

Colony counts determined on blood and MacConkey agars for the meats and brines decreased from beginning samples to end samples. These two media select for populations of total culturable aerobes and Gram-negative aerobes respectively. Meanwhile, the microbe counts on mannitol salt, which selects for salt-tolerant aerobes, increased greatly in the meats and brines. This suggests that the warm salty environment that the meats were stored in inhibited the growth of common bacteria, including many pathogens, but selected for halophilic and halotolerant bacteria.
The microbiological plating results of historical salts can be seen in Table 6 and show that bay salt exhibits much higher microbiological activity compared to the other salts, and is the only one to grow exponentially in the mannitol salt agar.
Table 6. Average microbial counts in salts (CFUs per gram). The limit of detection (LoD) is 20 CFU per gram.

The select microbes that were selected from 16s rRNA analysis were obtained from the salted beef and can be seen in Table 7. Most are commonly found in the soil and water environments, but three are uncharacterized species, or species that have not yet been classified and are largely ‘new’ or ‘unknown’. Of the previously characterized species, all are able to assimilate nitrogen via the nitrogen fixation process through the conversion of atmospheric or free nitrogen in the environment into nitrogen salts such as nitrate. The uncharacterized species discovered here also belong to genera known to be capable of nitrogen fixation, but are also able to reduce oxidized nitrogen compounds such as nitrate to nitrite through the removal of an oxygen molecule. Among the most efficient nitrate-reducing organisms are micrococci, and at least one of the isolated species falls under the genus Micrococcus.Footnote 84
Table 7. Characteristics of identified microbes from the beef or brine. The unknown species (marked *) were characterized based on genus, but it is possible for their features to differ.

Nitrate, nitrite and ammonia results
The lab results suggest that bay salt's advantage over other salts is not solely economic or due to adherence to tradition, but because bay salt contains nitrate levels higher than other salts, and possibly because it contains nitrogen-fixing and denitrifying bacteria that produce nitrate and nitrite which help to preserve meat better. A comparison of the beginning and end values of pork and beef nitrate levels shows that they increased over the course of the experiment. These increases correlate with an increase in halophilic, or at least halotolerant, bacteria, as seen in the mannitol salt agar counts (Tables 8 and 9). However, the possible microbiological benefits of bay salt denitrification cannot be shown without further experimentation via re-creation of salted meats with the other historical salts for comparison.
Table 8. Beef changes in nitrate, nitrite and ammonia, and microbes growing in mannitol salt from beginning to end.

Table 9. Pork changes in nitrate, nitrite and ammonia, and microbes growing in mannitol salt from beginning to end.

Bay salt may have been chosen out of the other available historical salts for its superior curing properties, specifically because it provided nitrate and the starter culture needed for nitrate reduction compared to the other salts.
While the Galveston sea salt also exhibited higher values of nitrate compared to rock salt, it had low bacterial levels as its production required boiling to evaporate the seawater the salt is in. Nitrate is the prerequisite to nitrite and then nitric oxide, which is the highly active curing compound in the meat matrix.Footnote 85 Nitrate by itself is a generally inert compound, and so nitrate reduction through denitrifying microorganisms is a key event in meat preservation. Interestingly, the addition of this compound is effective only in raw meat products that are cured at ambient temperatures, which are the conditions in which the salted meats in this study were cured.Footnote 86 Today's meat curing involves starter cultures that contain staphylococci, micrococci and/or some lactobacilli with efficient nitrate reductase activity.Footnote 87 Existing research on waste-water purification and saline environments has also found various extreme halophiles that reduce nitrate.Footnote 88 Nitrogen-fixing bacteria likely obtained nitrogen from the environment, converting it into nitrate (NO3-). Denitrifying bacteria then converted the nitrate into nitrite (NO2-), then to an intermediate compound, nitric oxide (NO), before converting into ammonia (NH3) and/or nitrogen gas (N2). These compounds and reactions are constantly changing in bioactive environments and can serve as oxidizing, reducing or nitrosylating agents.Footnote 89 As mentioned previously, the reason nitrite levels may be low in the results may not be due to their absence but possibly due to nitrite's unstable nature, making it difficult to determine in assays, especially under varying circumstances (changing temperatures, brine concentration, meat-to-brine ratio, moisture and so on). However, the levels of ammonia rose in the samples, perhaps indicating that nitrite was produced and then reduced into ammonia, although other processes such as the degradation of protein from the meat could also lead to higher ammonia concentrations. Not only did ammonia increase, but so did the nitrate in the experimental meats and brines. Nitrates are known to act as reservoirs of nitrites; they are gradually reduced into other stages of the nitrogen cycle that produce compounds that play a role in cured meat's red colouration, antioxidant effects, flavour improvement and antimicrobial properties.Footnote 90
While colour may seem of secondary importance, this is typically an important attribute in meats, and even today meat colouration is a critical factor in consumer acceptance.Footnote 91 Nitric oxide, a product of reduced nitrite, can bind to the iron ion of myoglobin, abundant in muscle tissue of red meat, and form nitrosomyoglobin, which, when heated or in low acidic environments, produces the characteristic pink colour of cured meats (nitroso-myochromogen).Footnote 92
Lipid oxidation in meats corresponds to rancidity and spoilage. Nitrites at low levels tend to inhibit warmed off-flavour development because they are effective antioxidants.Footnote 93 Morrissey and Techivangana showed that just fifty milligrams per kilogram of nitrite reduced thiobarbituric acid values, a common indicator of lipid oxidation, by 50–64 per cent for beef and pork and 35 per cent for fish.Footnote 94 As such, greater nitrite values lead to general flavour improvement.
While nitrites lead to flavour improvement, the cured meat flavour appears to be unique to nitrite and cannot be reproduced with other antioxidants, which can also help prevent lipid oxidation. Furthermore, only a small amount is needed; approximately forty to fifty milligrams per kilogram of ingoing nitrite is enough to produce a significant flavour contribution to cured meat.Footnote 95 This is because nitrites bind with several volatile and non-volatile compounds to produce the overall flavour of cured meats.Footnote 96 Pegg and Shahidi identified 135 volatile compounds in nitrite-cured ham, showing that the flavour is unique, but the complex reactions involved in the flavour development of nitrite in meats are still not fully understood.Footnote 97
Lastly, nitrate, and in particular nitrite, have bacteriostatic and bactericidal properties for various microbes that cause spoilage and illness.Footnote 98 Many studies have focused on Clostridium botulinum and found that 150 milligrams per kilogram of nitrite are required for efficacy against it and that there is a positive correlation between C. botulinum inhibition and nitrite levels.Footnote 99 Nitrite is also effective against Gram-negative pathogens, such as Salmonella and E. coli (including shiga-toxin-producing E. coli strains in fermented sausages). It also has a significant effect on Gram-positive bacteria.Footnote 100 Furthermore, ammonia can be used to produce ammonia hydroxide (NH4OH), which is formed when total saturation of salt in the brine solution occurs, much like in the brines of the salted meats sailors ate. Ammonia hydroxide is a natural meat preservative commonly sprayed on meats as an antimicrobial agent today.Footnote 101
It is also a possibility that the other bacteria in the bay salt had other benefits on the meat quality. Bay salt is known to be abundant in bacteria; in a study by Henriet et al., the solar salts tested had some of the highest microbiological counts when plated (Guérande salt had 6.5 log10 CFU/g on their chemically defined medium) and had wider diversity compared to non-solar salts.Footnote 102 Furthermore, salted and fermented foods today contain halophilic archaea, although their roles in preservation or spoilage are not yet fully explored.Footnote 103
Nitrate, salt and historical ideas on salt
The data point to a few key conclusions that suggest that the bacteria in the bay salt increased nitrate and nitrite.
• The meat and brine, with no addition of saltpetre (i.e. nitrate), increased in nitrate and ammonia in most samples.
• Common knowledge today indicates that curing meat with brine made from modern purified salt does not spontaneously generate nitrate or nitrite.
• Fresh meat by itself and the aquifer water have below detectable or trace amounts of nitrate and nitrite. The bay salt and sea salt (both of which come from the sea) have about twice as much nitrate as other forms of salt and several times more than in fresh meat.
• The increase in nitrate in the experimental meats and brines positively correlates to halophilic and/or halotolerant bacterial counts in the same experimental meats and brines.
• Bacterial counts of the experimental meats grown in other media that did not select for halophilic and/or halotolerant bacteria decreased over time.
• The bay salt, compared to the other salts, grows halophilic and/or halotolerant bacteria when plated. All other historical salts had low levels of bacterial counts.
• Although only a fraction of the bacteria have been isolated and sequenced, the few that have been identified appear to be nitrogen fixers or reducers.
The results make a strong case that bacteria in the bay salt are the primary responsible agent in producing the nitrate and nitrite in the meats. The other salts harboured few viable bacteria, and microbiological counts in the mannitol salt media correspond to nitrate levels. As such, it is possible that past victuallers chose bay salt for its nitrate content and ability to fix and reduce nitrate and nitrite. Two clues are present in historical sources that suggest this is valid: the fact that coarse salt was preferred, as was salt that made meat red.
We suggest that while past provisioners and sailors attributed the bay salt's superior curing properties to its coarse grain size, it was likely due to the nitrogen fixation and denitrification activities of the salt's indigenous microbes. Some secondary sources in this article noted that coarse salt is preferred for curing due to its ability to penetrate meat better. However, as noted earlier, grain size is not relevant for curing, especially not in meats that are submerged in brines. We suggest that the reason why coarse salt (of which bay salt was the primary sort) was historically preferred to fine salt was because salt grain size is a result of the rate of its evaporation during production – most other methods of salt production other than mined and bay salt require boiling, such as in the cases of the sea salt, basket salt and refined salt (salt on salt). Boiling, while removing calcium salts that made up the bittern, purifying the salt of organic debris and expediting the saltmaking process, also kills most of the microbes in the salt (the exception perhaps being spore formers that are able to survive the boiling process), and produces fine-grained salt. Bay salt grains remain large due to slow evaporation by the sun; this method does not destroy the microbiome of salt. Interestingly, a study on the formation of salt spheroids by Perthuisot and Castanier noted that extremely halophilic bacteria play a large role in inducing precipitation in solar salines, adding another potential reason why not boiling the brine would produce larger salt grains and in the act also preserve microbes in the salt that could produce and reduce nitrate and nitrite.Footnote 104 Metagenomic research is under way to confirm the hypothesis that the nitrogen-fixing and/or reducing microbes are present in the bay salt and how the microbial composition changed in the salted meats.
Second, the desire for red colouration in preserved meats and the importance of nitrate and nitrite for historical food preservation are well documented. As far back as the late Roman period, nitrite was sought out for its ability to produce red colouration in meat, and during the fourteenth century AD there were already records of saltpetre being used to reinforce bay salt, keeping the meat pink and more palatable.Footnote 105 Prior to industrialization, saltpetre was secured from soil with decayed organic material (nitrous earth) that, when mixed with potassium and calcium carbonate (lye) and magnesium, would cause the carbonates to precipitate out and leave the saltpetre. The undesirable debris was then burnt from the solution.Footnote 106 This produced pure saltpetre, a nitrate of potassium or sodium. In some cases, sal prunella, or nitrite, was made by melting saltpetre in a crucible, then repeatedly throwing on charcoal dust which converted some of the potassium nitrate into nitrite, decreasing the time needed for bacteria to convert nitrate to nitrite and therefore allowing meat to be cured faster. Just a quarter to a half ounce of sal prunella was enough to cure forty-five kilograms (100 pounds) of meat, and many historical recipes actually stipulate dangerous amounts of nitrate and nitrite.Footnote 107 Today it is known that as little as two to fourteen milligrams per kilogram of nitrite can be enough to produce the red colouration in meats.Footnote 108 Indeed, the desire for the red colour in meats has been noted throughout history. For example, ‘in Virginia they cure their hams with bay salt; and it is there a common practice to rub them with the ashes of hick[o]ry wood, instead of salt-petre, in order to give them a red colour’.Footnote 109
This meat-reddening effect could also be achieved by some salts. In one instance, Portsea Island salt ‘granulates or kerns to any fitting desirable size, small or great, and of it are made … And on one part of the Ground, is made a reddish Salt, that serves to salt gammons of Bacon and Neats-Tongs, and renders them Red’.Footnote 110 This quote notes not only the desire for the reddish colour in meat, but also that the salt forms ‘kerns’ or coarse kernels. Furthermore, it notes that the salt is red; most halobacteria are pink or red due to the presence of bacteriorhodopsin and other retinol-based pigments.Footnote 111 These bacteria are responsible for the colour of the brines in saltworks (and, in some cases, the resulting salt), for the salt's coarse grain and possibly for the production of nitrate and nitrite, as noted above.
Early modern scientists, including Thomas Tymme, Robert Boyle and Nehemiah Grew, believed that salts were behind the colours and tastes of substances.Footnote 112 For example, Tymme believed that ‘earthy fixed salt had a simple salt taste, nitrous or sulphureous salt a sweet and oily one, and mercurial salt was sour’.Footnote 113 Grew noted that the tastes of plants were also based upon saline chemistry; he postulated that plants that had a pungent taste did so because their biting or nitrous salts remained on the tongue.Footnote 114 Based on the idea that salts were responsible for colour, Glauber ‘produced a comprehensive list of how nitre could be used to create artist's paints and differently hued glazes. Copper or lead dissolved in nitric acid produced a green colour. Salt peter could also whiten yellow wax, and could color glass.’Footnote 115 Tymme noted that nitre was white, but, after heating, dyed the alembic a variety of colours.Footnote 116 Similarly, Boyle subscribed to these ideas and noted the red colouration change in meat that had saltpetre added to it, writing,
And as Metalline, so likewise Mineral Solutions may produce Colours differing enough from those of the Liquors themselves. I shall not fetch an Example of this, from what we daily see happen in the powdring of Beef, which by the Brine imploy'd about it (especially if the flesh be over salted) do's oftentimes appear at our Tables of a Green, and sometimes of a Reddish Colour, (deep enough) nor shall I insist on the practise of some that deal in Salt Petre, who, (as I suspected, and as themselves acknowledg'd to me) do, with the mixture of a certain proportion of that; and common Salt, give a fine Redness, not only to Neats Tongues, but which is more pretty as well as difficult, to such flesh, as would otherwise be purely White.Footnote 117
It is unclear what kind of common salt was used in Boyle's brine, but he noted that meat treated with saltpetre produces a ‘fine redness’ and that powdered (salted) beef sometimes had a ‘reddish colour’, linking their colour to the salts used on them, and perhaps inferring taste based on the beliefs of the time. As Roos notes, salt was also intimately connected to ideas on medicine and physiology, factors that were critically important for sailors and navies.Footnote 118 As such, scientific and everyday knowledge of the early modern period were interconnected, and the continued popularity of bay salt for curing may have been influenced by such ideas on taste, colour and possibly health.
Bay salt not only was an inexpensive salt purchased for its sodium chloride preservative properties, but also may have been used because it contains some nitrate which changed its colour, that in the salt theory of the time, was linked to other properties of taste and health. This could explain why, even when white salt could be procured more cheaply than bay salt and was preferred for regular table use, bay salt was preferable for curing. Further historical research is needed to see if the standardized addition of saltpetre in curing, which occurred around the early seventeenth century, corresponded to the less frequent usage of bay salt, or whether recipes that included bay salt reduced or eliminated the amount of saltpetre added.Footnote 119 In addition, it is unclear whether the reddish colouration and its inferred taste and health characteristics were regarded as more important than the ideas on bittern and its detrimental effects, which will require more study into philosophical ideas on bittern. Roos briefly discusses Stephen Hales's ideas from Philosophical Experiments Containing Useful, and Necessary Institutions for such as undertake long voyages at Sea (1739), which shows that he believed bittern caused putrefaction, but he also noted that seawater produced nitrous salts, although nitrous salts’ use for food preservation was not mentioned.Footnote 120
If this theory on the reasons why bay salt was preferred in historical curing is true, people were harnessing the power of microbes to produce nitrate prior to the advent of the germ theory. This provides an interesting case of ethnomicrobiology – the intersection of culture and people using microbes.Footnote 121 While early modern people did not understand bay salt as a more effective meat preservative than other salts in microbiological terms, through experience they had obtained knowledge of its qualities as a food preservative, having determined that it was superior for curing in terms of producing red colouration, preventing rancidity in meat, improving its flavour and reducing food-borne illness. The superiority of coarse bay salt for curing was attributed to grain size in the early modern period, and bay salt was understood to have a ‘particular virtue’. Therefore, even when other ‘better’ salts and nitrate became more economical and more easily obtained, people still insisted on using bay salt instead.Footnote 122 The experimental archaeological methods used for this article thus provide us not only with a contemporary understanding of the potential superiority of bay salt for meat preservation, but also with a new historical understanding of the experiential knowledge that early modern actors had of bay salt as a meat preservative that is impossible to obtain from analysing historical written sources alone.
Acknowledgements
We would like to thank Andrew Fielding of Ecosal-UK for discussing and sharing his knowledge on historical saltmaking with us during the inception of the SBSB Project. We would also like to extend our thanks to the reviewers for their insightful comments and suggestions, and to the editors, Marieke Hendriksen, Alexander Wragge-Morley, Rohan Deb Roy and Trish Hatton, for their assistance in publishing this article in this volume. Calvin and Karyn Medders and the various Aggie Research Scholars who participated in the SBSB Project deserve recognition for their help in the salted beef and pork preparation and for transporting the casks. We owe our gratitude to The Salt Palace for generously providing the rock salt for the project. Finally, we would like to thank the Institute of Nautical Archaeology, Texas A&M University, and Karbach Brewing Co. for funding the SBSB project from which this article originated.




















