Introduction
Juvenile individuals play a key role in the dynamics of animal populations, particularly in long-lived species such as seabirds, for which they can represent up to 50% of the total population (Schreiber and Burger Reference Schreiber, Burger, Schreiber and Burger2002). Juvenile birds show extensive foraging times, lower foraging efficiency and different feeding strategies than adults (Schreiber and Burger Reference Schreiber, Burger, Schreiber and Burger2002). These differences in feeding efficiency have been attributed to differences in individual experience (Wunderle Reference Wunderle1992). Moreover, it has been argued that juvenile birds can compensate the low feeding success by means of extending foraging bouts, selecting different size and type of prey, using different foraging tactics, or stealing food from other individuals (Wunderle Reference Wunderle1992, Bertellotti and Yorio Reference Bertellotti and Yorio2000a). How such less experienced individuals use their environment and interact with human activities has been identified as a priority for research (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012). Gulls stand out as an interesting model for considering this kind of ecological study, given their well-documented relationship with human activities (Schreiber and Burger Reference Schreiber, Burger, Schreiber and Burger2002). In general terms, gulls have generalist food habits, exploiting different habitats during the breeding and non-breeding seasons (Burger and Gochfeld Reference Burger, Gochfeld, del Hoyo, Elliot and Sargatal1996, del Hoyo et al. Reference del Hoyo, Elliott and Sargatal1996). Clearly, the habitat use of gulls outside their breeding period has an important and profound effect on the survival of individuals, recruitment, and quality of the forthcoming breeding season (Birkhead and Furness Reference Birkhead, Furness, Sibly and Smith1985).
Olrog’s Gull Larus atlanticus is endemic to the southern coast of South America (Harrison Reference Harrison1983). Its breeding range is restricted to only two nesting areas along 2,500 km of the central Argentinian coast. The number of breeding sites varied between seven and 12 per year, with locations differing among years and the global breeding population was estimated at 8,000 pairs (Yorio et al. Reference Yorio, Petracci and Borboroglu2013). The breeding season starts at the end of October and hatching usually occurs in early November–December. During the austral winter the species disperses chiefly to the north, reaching the coasts of Buenos Aires province (Silva Rodríguez et al. Reference Silva Rodríguez, Favero, Berón, Mariano-Jelicich and Mauco2005, Favero et al. Reference Favero, Copello, García, Mariano-Jelicich, Ravasi, Seco Pon and Athor2016), Uruguay (Azpiroz Reference Azpiroz2003), and occasionally, southern Brazil (Pacheco et al. Reference Pacheco, Olinto Branco and de Queiroz Piacentini2009). The species is listed as ‘Near Threatened’ on the IUCN Red List (BirdLife International 2018) and is listed in Appendix I of the Convention on Migratory Species (CMS Reference CMS2015) due to its confined distributional range, low population size, specialised food requirements, and conservation threats (Yorio et al. Reference Yorio, Bertellotti and García Borboroglu2005).
Despite the fact that the majority of gull species are considered generalist feeders, Olrog’s Gull has been considered historically as one of the few gulls with relatively specialised feeding habits (Burger and Gochfeld Reference Burger, Gochfeld, del Hoyo, Elliot and Sargatal1996). Several authors reported that individuals breeding in coastal wetlands feed primarily on crabs (Neohelice granulata, Cyrtograpsus altimanus and Cyrtograpsus angulatus; Devillers Reference Devillers1977, Delhey et al. Reference Delhey, Carrete and Martínez2001, Herrera et al. Reference Herrera, Punta and Yorio2005, Suárez et al. Reference Suárez, Retana and Yorio2011). Likewise, studies conducted on wintering grounds located in southern Buenos Aires province also refer to the importance of crabs in the diet of the species outside the breeding season (Burger and Gochfeld Reference Burger, Gochfeld, del Hoyo, Elliot and Sargatal1996, Copello and Favero Reference Copello and Favero2001). However, non-breeding adults and immatures have also been described as more generalist foragers, preying on other invertebrates, fish and resources facilitated by agricultural and fishery activities (Escalante Reference Escalante1966, Olrog Reference Olrog1967, Spivak and Sánchez Reference Spivak and Sánchez1992, Martínez et al. Reference Martínez, Isacch and Rojas2000, Berón et al. Reference Berón, Favero and Gómez Laich2007, Petracci et al. Reference Petracci, Delhey and Sotelo2007, Seco Pon et al. Reference Seco Pon, García, Copello, Moretinni, Lértora, Pedrana, Mauco and Favero2012, 2013, Berón et al. Reference Berón, Seco Pon, García, Paterlini, Mariano-Jelicich and Favero2013). Although the provision of food facilitated by commercial fishing activities such as waste offal and discards (Bertellotti and Yorio Reference Bertellotti and Yorio2000b, Furness Reference Furness2003, Giaccardi and Yorio Reference Giaccardi and Yorio2004) could be expected to be beneficial for Olrog’s Gull (Martínez et al. Reference Martínez, Isacch and Rojas2000), there is evidence showing the risk of incidental mortality and injuries due to entanglement in, and ingestion of, sport fishery gear may lead to an unsustainable increase in mortality (Berón et al. Reference Berón, Favero and Gómez Laich2007, Berón and Favero Reference Berón and Favero2009).
The south-eastern sector of Buenos Aires province lies within an important refuelling and wintering area for several coastal seabirds, including Olrog’s Gull (Silva Rodríguez et al. Reference Silva Rodríguez, Favero, Berón, Mariano-Jelicich and Mauco2005, Favero et al. Reference Favero, Copello, García, Mariano-Jelicich, Ravasi, Seco Pon and Athor2016). In particular, Mar Chiquita Lagoon is one of the most important areas of Buenos Aires province in terms of biodiversity (Isacch et al. Reference Isacch, Bó, Vega, Favero, Baladrón, Pretelli, Stellatelli, Cardoni, Copello, Block, Cavalli, Comparatore, Mariano-Jelicich, Biondi, García and Seco Pon2016). Due to its unique environmental features Mar Chiquita Lagoon was declared a World Biosphere Reserve by MAB-UNESCO in 1996, and a Provincial Reserve in 1999 (Isacch Reference Isacch2008). This environment is regularly used by Olrog’s Gull juveniles (first-year birds), subadults and adults with varying abundances throughout the year. The greatest abundance of gulls occurs during austral autumn and winter (June to September; Azpiroz Reference Azpiroz2003, Berón et al. Reference Berón, Favero and Gómez Laich2007, Berón Reference Berón2009), with juvenile individuals dominant in numbers (Berón Reference Berón2009). A study using radio transmitters (VHF), showed a high site fidelity in this species with immature individuals using mostly the mouth of the lagoon associated with crab beds and sport fishing activities (Berón et al. Reference Berón, Favero and Gómez Laich2007). Adult Olrog’s Gulls show greater abilities to catch and handle crabs compared to subadults and juveniles, and adults also dominate juveniles in the selection of foraging areas (Berón et al. Reference Berón, García, Luppi and Favero2011). Feeding methods used by Olrog´s Gull include surface seizing, surface plunging and walking (Copello and Favero Reference Copello and Favero2001). Once the crab is captured, it is often rinsed and swallowed whole or after dismembering (Delhey et al. Reference Delhey, Carrete and Martínez2001). The foraging behaviour of adult birds could force juveniles to use alternative food resources such as discards from sport fishing activities which are easier to obtain in terms of foraging skills. Understanding habitat use, foraging behaviour and the impact of anthropogenic activities in non-breeding areas is important to develop effective management and conservation strategies. As mentioned above, there is a previous study on habitat use by the species, however the tracking devices used showed low temporal and spatial resolution and only four juveniles were tracked (Berón et al. Reference Berón, Favero and Gómez Laich2007).
This study was designed with the objectives of: (1) identifying areas of importance for juvenile gulls during the winter period and (2) determining the use of habitat by juvenile individuals while preying on natural (crab beds) and anthropogenic resources (fishing discards and by-products from sport fishing activities) in one of the most important wintering grounds for the species in Argentina. We hypothesised that during winter, the distribution and habitat use of juvenile Olrog’s Gull in Mar Chiquita are affected by the sport fishing activity along the coast. Our predictions are: (1) that juvenile gulls are distributed in areas where sport fishing is concentrated, irrespective of the occurrence of crab beds. That is, a greater spatial overlap with fishing effort compared with crab densities; and (2) that on weekdays, due to reduced fishing effort, gulls will spend less time in areas where fishers are distributed, compared to weekends when the sport fishing activity is more intense. That is, a lower spatial overlap with fishing effort during weekdays than during weekends.
Methods
Study area
The study area was located at the Mar Chiquita Reserve (Buenos Aires province, Argentina, 37°46’S, 57°27’W; Figure S1 in the Online supplementary material). It encompasses a 46-km2 body of tidal brackish water along the coast, with mudflats surrounded by Spartina densiflora grassland and inhabited by large numbers of various groups of macro-invertebrates, including the intertidal crabs, C. angulatus and N. granulata as the most conspicuous organisms (Spivak et al. Reference Spivak, Luppi, Bas and Iribarne2001). Fish diversity is relatively high (including both benthic and pelagic fishes), many of which are frequently targeted by sport fishing activities in the area (Pellegrino and Cousseau Reference Pellegrino and Cousseau2005).
Habitat use by gulls
In order to identify habitat use and movements of juvenile individuals (first-year birds) during winter, 22 GPS devices (CatTraQ live 3 Mr. Lee, 17 g) were deployed on gulls during the following periods: 9–14 July 2013 (3 males, 4 females and 1 undetermined sex) and 26 May–12 July 2014 (eight males and six females). A total of 17 tags sent enough fixes in order to make the data analysis (Table S1). The devices weighed less than 3% of the total weight of the gulls (949 ± 112 g; n = 35 birds; unpubl. data), which is within the maximum recommended to avoid adverse effects on bird behaviour (Phillips et al. Reference Phillips, Xavier and Croxall2003). On average, gulls were followed for 2.5 days (range 1–5 days, both sexes combined). When necessary, the device was removed from birds when the battery was depleted. However, given that bird recapture was not always successful, and that the batteries had low capacity, the tags transmitted for only a short period of time. Individuals were captured using a Bal-chatri trap (Bloom Reference Bloom, Giron Pendleton, Millsap, Cline and Bird1987) with fish as bait (once the capture was completed the fish was removed from the area).
The devices were deployed on the back of the birds using Tesa® tape 4651 (Wilson et al. Reference Wilson, Puetz, Peters, Culik, Scolaro, Charrassin and Ropert-Coudert1997) and programmed to transmit one position every 30 minutes. Locations were received as a text message (SMS) on a mobile phone via a GSM system (Tomkiewicz et al. Reference Tomkiewicz, Fuller, Kie and Bates2010). Each individual bird was marked with a numbered plastic ring that allowed its remote identification (Table S1). A drop of blood from the wing vein of captured individuals was collected and settled on filter paper for further molecular sex determination following methods described in Quintana et al. (Reference Quintana, López and Somoza2008).
Availability of natural and anthropogenic food resources
To determine the overlap between the distribution of gulls and food resources, the focal area (kernel 75%) used by gulls was selected for analysis. The densities of C. angulatus and N. granulata (the two species providing the bulk of crab biomass in the estuary, and a large proportion of the diet of Olrog’s Gull) were assessed following different methodologies depending on their behaviour (burrowing vs. non-burrowing species). For N. granulata, an inter-tidal burrowing crab species, 39 stratified random plots measuring 50 x 50 cm were placed in different environments previously characterised in Isacch et al. (Reference Isacch, Costa, Escapa, Gagliardini, Iribarne, Rodríguez-Gallego and Conde2006) and sampled during 18–22 July 2014 (Figure S1). In each quadrat, the number of burrows was counted as a proxy indicator of crab abundance (Suárez et al. Reference Suárez, Retana and Yorio2012). For the sub-tidal C. angulatus, seven transects were performed during low tide along the edge of the estuary during 22–25 July 2014 (Figure S1). Each transect was 100 m long and 10 m wide, covering 5 m in the subtidal zone and 5 m in the intertidal zone. The number of crabs observed was recorded along each transect.
The number of coastal fishers was used as a proxy for the availability of anthropogenic food resources (i.e. discards and offal by-products of the sport fishery) (Smallwood et al. Reference Smallwood, Pollock, Wise, Hall and Gaughan2011). To analyse the distribution of this resource, sport fishing effort was estimated by conducting point counts where the number of fishers (including absences) was recorded following procedures described in Smallwood et al. (Reference Smallwood, Pollock, Wise, Hall and Gaughan2011). For this, 146 counts (88 and 58 for weekends and weekdays respectively) of fishers in different locations within the study area (at the mouth of the lagoon and subsidiary streams) were conducted between 26 May and 12 July 2014.
Data analysis
Given the varying number of locations obtained for each bird (mean = 60; range = 10–247; Table S1) due to the performance of the GPS devices, an interpolation of fixes every 15 minutes was carried out using the function ‘redisltraj’ of the package adehabitatLT in program R (Calenge Reference Calenge2006). Daily distance travelled per individual was determined by summing the distances obtained between consecutive points per day. Then to evaluate the habitat use of tracked gulls, a kernel analysis was fitted using utilization distribution (UD areas, Spatial Analysis Toolbox, ArcGis 10.1). This analysis calculates the magnitude of locations per unit area using a smoothing algorithm (Worton Reference Worton1989). The bandwidth (‘smoothing parameter’ h) used in this study was 50 km and the kernel contour levels were estimated for 50% (core area), 75% (focal area) and 95% (range) of the locations (Hyrenbach et al. Reference Hyrenbach, Keiper, Allen, Ainley and Anderson2006). This analysis has frequently been used to assess important areas for seabirds (Quintana et al. Reference Quintana, Dell’ Arciprete and Copello2010, Copello et al. Reference Copello, Seco Pon and Favero2014).
To analyse the differences in size of core areas and daily distances travelled in relation to years and sexes, Generalized Linear (GLM) and Mixed (GLMM) Models were used, respectively. A gamma error structure and loglink function were used in both models (Crawley Reference Crawley2007, Zuur et al. Reference Zuur, Ieno, Walker, Saveliev and Smith2009). The sex and year variables were used as fixed effects and the identity of individuals was modelled as a random effect. Model fit was evaluated by visual inspection of the residuals. Analysis of variance (ANOVA) was used for inference and model selection (McCullagh and Nelder Reference McCullagh and Nelder1989).
Comparison of average fishing effort between weekdays and weekends was analysed using the Wilcoxon signed-rank test (W) because distribution of data was not normal (Zar Reference Zar2010). To analyse the degree of overlap between the areas used by gulls and availability of food resources (i.e. crabs and discards) a cell size of 30 x 30 m was used. The density of the two crab species in the whole area was estimated using the inverse distance weighting univariate interpolation method (Fortin and Dale Reference Fortin and Dale2005) in the Spatial Analysis toolbox of ArcGIS using the samples mentioned above. Then the number of fixes of gulls in each cell was calculated using the Spatial Join tool in ArcGIS. Finally, a spatial overlap index (SOI) was calculated using the estimated maps. This index is widely used to assess the extent of spatial correlation between a predator and its prey (Williamson Reference Williamson1993), and is defined as:
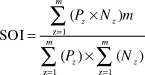 $${\rm{SOI}} = {{\sum\limits_{{\rm{z}} = 1}^m {\left( {{P_z} \times {N_z}} \right)m} } \over {\sum\limits_{{\rm{z}} = 1}^m {\left( {{P_z}} \right) \times } \sum\limits_{{\rm{z}} = 1}^m {\left( {{N_z}} \right)} }}$$
$${\rm{SOI}} = {{\sum\limits_{{\rm{z}} = 1}^m {\left( {{P_z} \times {N_z}} \right)m} } \over {\sum\limits_{{\rm{z}} = 1}^m {\left( {{P_z}} \right) \times } \sum\limits_{{\rm{z}} = 1}^m {\left( {{N_z}} \right)} }}$$where z is a sample location, m is the total number of samples, P z is the predator density (Olrog’s Gull) and N z is the density of prey (i.e. crab species or sport fishery discards and offal). A SOI value of one represents a uniform distribution of predator and prey populations, while an SOI value > 1 represents a greater overlap than expected and an SOI value < 1 represents a lesser overlap than expected (McClellan et al. Reference McClellan, Read, Price, Cluse and Godfrey2009, Harden and Williard Reference Harden and Williard2012).
All spatial and statistical analysis were performed using ArcGIS 10.1 (ESRI) and the free program R i386 3.1.2 (http://www.r-project.org). In all cases, differences were considered significant at P < 0.05.
Results
A total of 1,088 positions of tracked individuals was obtained throughout the study period (299 locations in 2013 and 789 in 2014). Juvenile gulls were distributed in a relatively small area lying between 37°26’–37°44’S, and 57°27’– 57°30’W (Figure 1A). The distribution range of tracked individuals (kernel 95%) was chiefly restricted to the vicinity of the mouth of the lagoon (Figure 1B), in an area of 4.5 km2. However, the core area (kernel 50%) was restricted to a smaller section of 0.3 km2 around the convergence of a number of subsidiary streams. Core areas from 82% of individuals overlapped with each other, averaging 31.4 ± 27.9% overlap. The remaining individuals were distributed in core areas away from the village of Mar Chiquita, especially in the north and within subsidiary streams. The focal area (kernel 75%) included the mouth of the lagoon covering an area of 1.2 km2 (Figure 2A). No offshore locations were observed (both years combined).
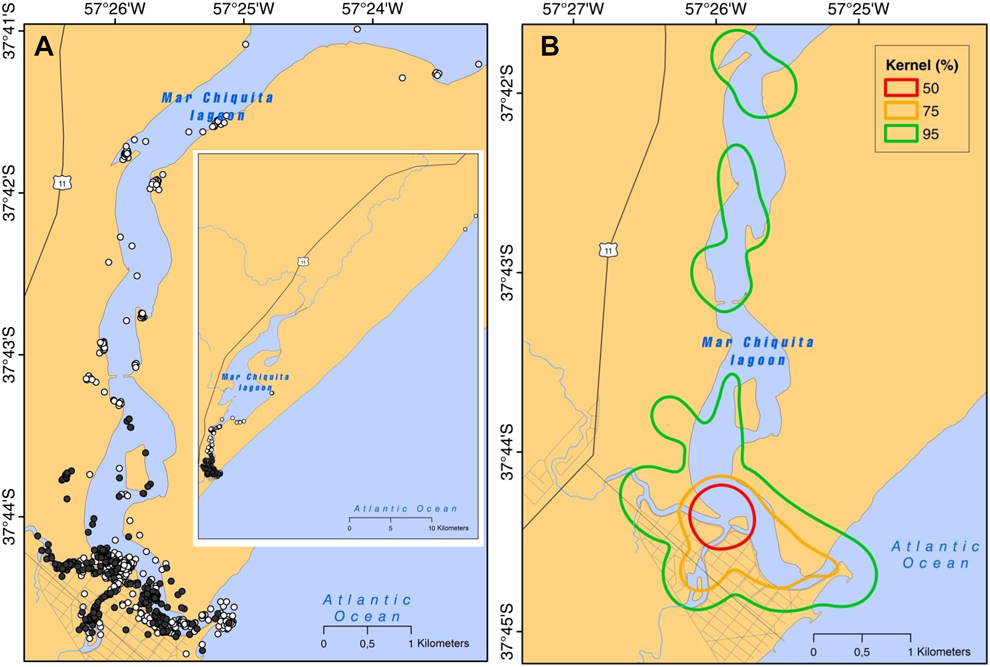
Figure 1. (A) Locations obtained from juvenile Olrog’s Gull followed by GPS during the 2013 (black dots) and 2014 (white dots) winter seasons (inset: detail of locations in 2014 including one individual that ranged 42 km to the north of the estuary); (B) areas of use (Kernel plot) pooled for winters 2013 and 2014.
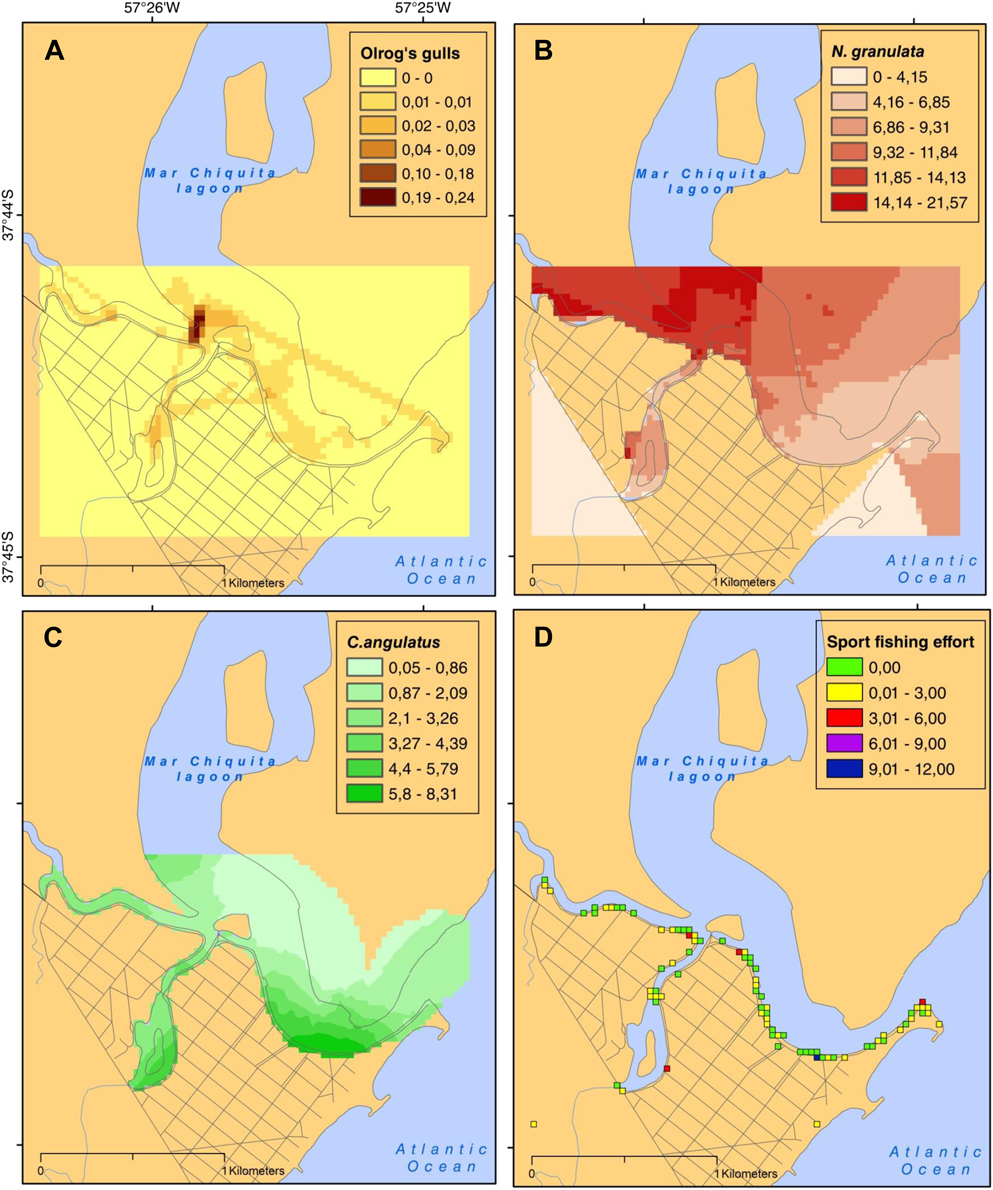
Figure 2. Distribution of Olrog’s gulls in the focal area (A) (time spent/900 m2), and density of natural and anthropogenic resources: N. granulata (B), C. angulatus (C) (crabs/900 m2), and sport and recreational fishing (D; number of fishermen /900 m2).
The size of core areas (2.2 ± 0.9 km2) was not significantly different between years or sexes (F 1,14 = 2.28, P = 0.09; F 1,14 = 1.33 P = 0.27, respectively). Similarly, the daily distance travelled (5.7 ± 7.9 km) was similar between years and sexes (X 12 = 3.76, P = 0.05; X 12 = 2.73, P = 0.1, respectively).
The mean density for N. granulata and C. angulatus were estimated as 0.3 ± 0.1 and 0.1 ± 0.1 individuals per m2, respectively. While the highest densities of N. granulata were found in the northwest section of the study area, for C. angulatus the highest densities were concentrated in the south-east of the mouth of the lagoon and subsidiary streams (Figures 2B and C).
The average effort of sport fishing was significantly higher at weekends (we) than weekdays (wd) (1.8 ± 3.3 and 0.8 ± 1.9 fishers/900 m2, respectively; W 1,147 = 3051.50, P < 0.05). A higher concentration of fishing effort was observed at the south-east coast of the mouth, although some isolated points of high effort were observed in subsidiary streams (Figure 2D).
The overlapping analysis revealed a patchy distribution of gulls and prey (crabs and discards) (SOI ≠ 1) which deviated from an expected uniform spatial co-distribution. The overlap between crab densities and gulls was lower than expected by a uniform distribution (SOIwd = 0.714 and SOIwe = 0.686 for N. granulata and SOIwd = 0.897 and SOIwe = 0.972 for C. angulatus). However, the overlap index obtained between gulls and sport fishing effort was higher than expected by a uniform distribution during weekdays (SOI = 3.499) and lower during weekends (SOI = 0.401).
Discussion
Our results showed that tracked juveniles use a small area, concentrating their activities in the vicinity of the mouth of the lagoon. This pattern is consistent with a previous study using radio telemetry (Berón et al. Reference Berón, Favero and Gómez Laich2007). This limited space use may be due to large food abundances in the area. Olrog’s Gulls have easy access not only to their main natural food (Copello and Favero Reference Copello and Favero2001, Berón Reference Berón2003) but also to sport fishery discards as a supplementary source (Berón et al. Reference Berón, Seco Pon, García, Paterlini, Mariano-Jelicich and Favero2013). This observation is supported by the predator-prey overlap index, indicating that, at least during weekdays, the overlap between gulls and fishing effort distribution was higher than expected by a uniform distribution (SOI > 1). During weekends, the overlap with fishing effort was lower than expected by a uniform distribution (SOI < 1). These results are partially in line with the predictions formulated for this study and also with the results reported by Berón et al. (Reference Berón, Favero and Gómez Laich2007) that showed a strong fidelity of the species to foraging patches with crab-bed areas and beaches where sport fishing activities occurred. We predicted a greater overlap with fishing effort during the weekends and the opposite was found. The difference in the overlap values between weekdays and weekends could be due to the spatial spread of fishing effort along the lagoon during weekdays. Juvenile gulls were more closely associated with fishermen during weekdays, while at weekends when the fishers’ distribution was more restricted, the overlap was less marked because gulls may compete intraspecifically for access to the discards. On the other hand, the greater degree of recreational and tourist activities (for example kite-surfing, kayaking, etc.) during weekends compared with weekdays in the area (Friedman, pers. comm. 2017) could cause greater disturbance to juveniles, potentially diminishing the time in association with the fishing activities.
The association between gulls and sport fishing could be considered beneficial for the species given the supplementary supply of food, as has been shown in other gull species (Furness Reference Furness2003). Besides, assuming more limited skills in juveniles to manipulate crabs, the alternative food obtained from sport fishing activities could be beneficial for this particular age-class. However, more information is needed in order to determine the effect of this alternative food on the Olrog´s Gull population. In addition, this species has been reported to regularly attend semi-industrial trawl and purse seine fishing operations in northern Patagonia outside the breeding season (Seco Pon et al. Reference Seco Pon, García, Copello, Moretinni, Lértora, Pedrana, Mauco and Favero2012, 2013), so at this stage it has become apparent that the use of anthropogenic sources of food by Olrog’s Gull could be more important than originally thought. It could also be that the species might have expanded its dietary spectrum, chiefly driven by the expansion of commercial fisheries and changes in coastal areas during recent decades, similarly to other pelagic seabirds such as albatrosses (Mariano-Jelicich et al. Reference Mariano-Jelicich, Copello, Seco Pon and Favero2017). It is important to note that the species has been recorded interacting with sport fishing in the study area, with negative effect for the birds in terms of direct mortality due to fishing gear ingestion and severe injuries due to entanglement with fishing lines (Berón and Favero Reference Berón and Favero2009).
In relation to spatial segregation in seabirds, there is abundant evidence indicating that different sexes show variation in distances travelled, at least in adults during the breeding season (Guilford et al. Reference Guilford, Meade, Willis, Phillips and Boyle2009, Rayner et al. Reference Rayner, Taylor, Gummer, Phillips and Sagar2012, Hedd et al. Reference Hedd, Montevecchi, Phillips and Fifield2014, Camphuysen et al. Reference Camphuysen, Shamoun-Baranes, Loon and Bouten2015). However, there is little information about spatial segregation in juvenile seabirds. In this study, we found no differences between the daily distances travelled and the size of the core area between males and females, nor between years. Further studies are needed to evaluate the degree of competition between the sexes in this species, as well as to evaluate the relative contribution of natural and anthropogenic food resources in the diet of female and male gulls.
Given the evidence of undesired impacts resulting from the interaction between coastal gulls (Larus atlanticus and Larus dominicanus) with sport fishing activities in Argentina (Berón and Favero Reference Berón and Favero2009, Yorio et al. Reference Yorio, Marinao and Suárez2014) and the ‘Near Threatened’ conservation status of the study species, our results are relevant to the development of conservation strategies in Mar Chiquita Lagoon, considered the main wintering ground of this endemic species. However, some shortcomings in the study must be acknowledged: 1) the low battery lifetime of the tags and as a consequence the short tracking period; 2) the use of GPS devices without storage of the positions recorded in case of failure of GSM coverage; and 3) the fact that samples of natural and anthropogenic food resources were not taken concomitantly with tracked birds. It will be crucial to consider in further studies the tracking of individuals for a longer period of time considering that many juvenile gulls ringed at Mar Chiquita lagoon were sighted in surrounding areas such as Mar del Plata harbour (the most important commercial harbour in Argentina) (authors’ unpubl. data). Besides, it would be also relevant to track adult and sub-adult birds.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270919000029
Acknowledgements
This study was financially supported by grants from the National Agency for the Promotion of Science and Technology (Agencia Nacional de Promoción Científica y Tecnológica, PICT 2012-295, 2013-711), the National Research Council (PIP CONICET 00070), and the National University of Mar del Plata (Argentina). The authors thank Javier Rojo, Giselle Magalí Fuentes and Joaquín Hernán Pinciroli for field assistance; Amalia Soroeta for logistical support and Rupert Pilkington for language editing. We also thank Organismo Provincial para el Desarrollo Sostenible (Buenos Aires) for issuing permits. We thank Dr. Pablo Yorio (Associate Editor) and two anonymous reviewers for providing comments that helped to improve the manuscript.




