Introduction
The Chinese Crested Tern Thalasseus bernsteini (CCT) was first described in 1863 and historical records include sightings in Indonesia, Malaysia, Philippines, Thailand, and China (Chen et al. Reference Chen, Fan, Chen and Lu2011, Collar et al. Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). In 1937, 21 specimens were collected at Muguan Islet in Shangdong, China; from 1978 to 2000, there were no confirmed sightings of CCTs (Collar et al. Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001). In June 2000, four nesting pairs of CCT were sighted amongst a colony of Greater Crested Terns T. bergii (GCT) in the Matsu Archipelago, to the excitement of ornithologists around the world (Collar et al. Reference Collar, Andreev, Chan, Crosby, Subramanya and Tobias2001).
In 2004, Chen et al. (Reference Chen, Chang, Liu, Chan, Fan, Chen, Yen and Guo2009) found 10–20 CCT breeding amongst c.4,000 GCT in the Jiushan Archipelago off the Zhejiang coast of China. In 2008, two pairs of CCT were discovered in a breeding colony of c.3,000 GCT in the Wuzhishan Archipelago in Hangzhou Bay, 100 km north-west of the Jiushan Archipelago (Chen et al. Reference Chen, Fan, Chen, Lu and Wang2010). Liu et al. (Reference Liu, Guo, Qiao, Zhang and Cai2009) suggested that the historical breeding colonies along the Qingdao and Shandong coastlines to the north were extirpated during the 1950s due to human development. Surprisingly, in 2016 the National Institute of Ecology in South Korea found two breeding pairs of CCTs inside a Black-tailed Gull Larus crassirostris colony in Yeonggwang County, Jeollanam-do, South Korea (Song et al. Reference Song, Lee, Lee, Lee, Kim, Choi, Shin, Park, Lee and Kim2017). This is the easternmost and northernmost record of breeding CCT.
The global population of CCT was recently estimated to be less than 100 individuals and it is listed as ‘Critically Endangered’ in the IUCN Red List (BirdLife International 2017). Most breeding CCT have been found nesting synchronously within large colonies of GCT, and the species has been known to shift its breeding site on an annual basis (Chen et al. Reference Chen, Fan, Chen and Lu2011).
Colony shifts between or within breeding seasons in terns (Sterninae) and gulls (Larinae) have been reported widely in many studies, and are associated with a variety of factors, including changes in food resources, predation, competition, human disturbance, and weather conditions (Erwin et al. Reference Erwin, Galli and Burger1981, Burger Reference Burger1984, Visser and Peterson Reference Visser and Peterson1994, Martinez-Abrain et al. Reference Martinez-Abrain, Oro, Forero and Conesa2003). Chen et al. (Reference Chen, Fan, Chen and Lu2011) reported that the mixed-species colony of CCT and GCT most likely shifted from the Jiushan Archipelago to the Wuzhishan Archipelago because of nesting failure after illegal egg collection by local fishermen; consequently, monitoring and safeguarding of the colony was considered paramount. Moreover, in-season shifts in colony location by GCT after nesting failure may limit nesting success, as delayed chronology of the nesting period means encountering poor weather conditions and/or low food availability, both associated with low nesting success in GCT (Langham and Hulsman Reference Langham and Hulsman1986, Crawford Reference Crawford2003, Tayefeh et al. Reference Tayefeh, Zakaria, Mohamadi, Amini and Ghasemi2015, McLeay et al. Reference McLeay, Page and Goldsworthy2017).
GCT and CCT mainly prey on anchovy (Engraulidae) near their breeding colonies (Walter Reference Walter1984, Chen et al. Reference Chen, Fan, Chen and Lu2011) and Crawford (Reference Crawford2003) showed that GCT colony size in South Africa was significantly related to the combined biomass of available anchovies and sardines Sardinops sagax. McLeay et al. (Reference McLeay, Page, Goldsworthy, Paton, Teixeira, Burch and Ward2010) indicated that GCTs spent more time foraging in zones with higher chlorophyll-a concentrations, while in the Taiwan Strait and the East China Sea, the larval anchovy assemblage is closely correlated with chlorophyll-a concentration (Kim et al. Reference Kim, Kang, Oh, Suh and Hwang2005, Hsieh et al. Reference Hsieh, Lo, Chen and Meng2016). In several cases, satellite imagery of chlorophyll-a concentrations has been used in studies of trophic factors as they influence seabird distribution and population dynamics (Adams et al. Reference Adams, Takekawa, Carter and Yee2010, Le Corre et al. Reference Le Corre, Jaeger, Pinet, Kappes, Weimerskirch, Catry, Ramos, Russell, Shah and Jaquemet2012, Suryan et al. Reference Suryan, Santora and Sydeman2012, Velarde et al. Reference Velarde, Ezcurra, Horn and Patton2015). Chlorophyll-a concentrations within foraging range of seabird colonies may be a good indicator of primary productivity and food availability (Grémillet et al. Reference Grémillet, Lewis, Drapeau, van Der Lingen, Huggett, Coetzee and Ryan2008, Votier et al. Reference Votier, Bearhop, Witt, Inger, Thompson and Newton2010).
Typhoons, also called tropical cyclones or hurricanes, can cause massive breeding failures of nesting seabirds or disruption of their breeding cycles (King et al. Reference King, Hicks and Cornelius1992, Wiley and Wunderle, Jr. 1993). Chen et al. (Reference Chen, Fan, Roby, Lu, Chen, Huang, Cheng and Zhu2015) found that 28% of CCT breeding failures at colonies along the coast of Zhejiang, China were attributed to the synergistic effects of illegal egg harvest and typhoons. There are no other published studies, however, investigating the effects of colony size, in-season colony shifts, weather conditions, and food availability on nesting success of CCTs.
In this study, we report the results of censuses of CCT and sympatric GCT breeding at the Matsu Islands Tern Refuge, Taiwan, from 2008 to 2017. We combined this with historical records (2004–2007) to assess the impact of typhoons, in-season colony shifts, and variability in chlorophyll-a concentrations on annual peak colony size and breeding productivity of GCTs and CCTs at the Matsu Archipelago. We also make recommendations for future conservation actions at the MITR to enhance restoration of CCTs and GCTs.
Methods and materials
Matsu Islands Tern Refuge
The Matsu Archipelago, which is 20 km east of the Ming River estuary (Fujian Province, China) consists of five main islands (Nangan, Beigan, Dongyin, Xiju and Dongju) and numerous small islands and islets (Figure 1). In 2000, the Lienchiang County Government of Taiwan designated eight islands in the archipelago as the Matsu Islands Tern Refuge (MITR) to protect breeding colonies of GCT, CCT, Black-tailed Gull, Bridled Tern Onychoprion anaethetus, Roseate Tern Sterna dougallii, and Black-naped Tern S. sumatrana. These eight uninhabited islands are all isolated from the five main islands in the archipelago, except for Sheshan, which is connected to Xiju at low tide. Further, they designated the protected islands as core areas of the MITR, where any unapproved entry or human activity during the seabird breeding season (May to August) is strictly prohibited.

Figure 1. Locations of the seven protected islands in the Matsu Islands Tern Refuge where Chinese Crested Terns have nested in the Matsu Archipelago: 1. Baimao (1.88 ha), 2. Tiejien (1.29 ha), 3. Zhongdao (2.02 ha), 4. Sanlianyu (1.39 ha), 5. Jinyu (3.12 ha), 6. Liuquanjiao (1.09 ha), 7. Sheshan (3.16 ha). The relative positions of Taiwan, China, the Wuzhishan Archipelago (a), the Jiushan Archipelago (b), and the Matsu Archipelago (c) are shown at the bottom right corner. (Dongyin Township in the eastern part of the Matsu Archipelago is not included in this figure).
From 2000 to 2007, evidence of nesting by CCT and GCT was found on seven of the eight MITR islands, and was not found on any island outside the MITR (Chang Reference Chang2008). The mixed-species tern breeding colonies were usually located on relatively higher and flatter ground, and characterised by low-lying vegetation. Chenopodiaceae, Tetragonia, and Polygonaceae species such as round-leaved goosefoot Chenopodium acuminatum subsp. virgatum, garden sorrel Rumex acetosa, Japanese dock Rumex crispus var. japonicus, and New Zealand spinach Tetragonia tetragonoides were dominant on those islands.
Rat eradication was conducted in the MITR beginning in 2010. Each year, 15 medium-sized Sherman live traps baited with sweet potato chunks were deployed on Sheshan, Tiejien, and Zongdao for 3–5 days in April and November. Brown country rats Rattus losea were frequently captured (1–25 individuals/year) on Zongdao, but there was only one capture event on Tiejien and none on Sheshan.
Census of Greater Crested Terns and Chinese Crested Terns
From 2000 to 2007, local birdwatchers conducted censuses of breeding terns on an irregular basis on each island in the MITR from a boat during the tern breeding season (May to August). Collected data included the numbers of observed adult terns of each species, numbers of observed juvenile CCT, and the location of the breeding colonies. Beginning in 2008, researchers from the Wild Bird Society of Taipei and National Taiwan University conducted censuses every other week from May to September. Using binoculars, 3–5 five researchers counted the total numbers of adult CCT and GCT, juvenile CCT and, beginning in 2011, juvenile GCT on each island. To minimise disturbance, surveys were conducted from fishing boats that circled each island from 50 m offshore for 10–15 minutes during daylight. All flying terns were counted using the snapshot method, and terns resting on the island were counted at the same time to minimise double counting (Tasker et al. Reference Tasker, Jones, Dixon and Blake1984). At the end of each breeding season, we also estimated the numbers of tern adults and fledged chicks present, but fledged GCT chicks were not counted until 2011.
Due to restrictions imposed by local authorities, researchers were prohibited from doing ground counts on protected islands. The actual number of individuals participating in breeding and the number of active nests were not determined. Beginning in June 2011, to improve census methods despite this difficulty in enumerating the number of active breeding pairs and monitoring tern breeding success, we deployed 4–6 surveillance cameras (KeepGuard SD-1039 and Reconyx HC600) on several islands before June each year.
During 2011–2014, 2016, and 2017, we found that the number of nesting terns declined dramatically during the incubation stage; over half of breeding terns left the breeding colony and large numbers of tern eggs were abandoned. We defined nest abandonment events as when at least 50% of breeding adult terns left the colony. We also observed another type of breeding phenomenon: terns would initiate nesting colonially on one island, but then later in the same nesting season shift to another island within the MITR and resume nesting. When evidence of breeding (e.g. eggs or chicks) was found on both an initial colony on one island, and then a subsequent colony on a second island within a single nesting season, we recorded this phenomenon as a colony shift.
Frequency of typhoons and chlorophyll-a concentration
We collected daily wind speed and precipitation data from the local weather station in Matsu since 2004. The intensity, radius, paths, warning period, and impact reports of typhoons were downloaded from the Typhoon DataBase of the Taiwan Central Weather Bureau (http://rdc28.cwb.gov.tw/). Our definition of typhoon was based on that of the Regional Specialized Meteorological Center (RSMC Tokyo-Typhoon Center), except in this study we additionally considered precipitation. When peak wind speed exceeded 32.7 m/s and rainfall exceeded 100 mm during a typhoon warning period, we classified it as a typhoon event. We included a criterion of precipitation because heavy rain can cause hypothermia in young tern chicks directly, and flooding or runoff can induce loss of tern eggs from nest scrapes (Wiley and Wunderle Jr Reference Wiley and Wunderle1993, Bugoni et al. Reference Bugoni, Sander and Costa2007).
Chlorophyll-a concentrations were obtained from the MODIS-Aqua satellite, and we downloaded monthly data with 4-km resolution from NASA’s OceanColor Web (https://oceancolor.gsfc.nasa.gov/). Foraging ranges of GCTs during chick-rearing were previously found to be about 40 km from the breeding colony (McLeay et al. Reference McLeay, Page, Goldsworthy, Paton, Teixeira, Burch and Ward2010). To analyse the linkage between chlorophyll-a concentrations, colony sizes, and breeding productivity, we used chlorophyll-a data obtained within 40 km of the colony. We used the zonal raster statistic tool in QGIS 2.18.15 (QGIS Development Team 2017) to determine the average chlorophyll-a concentration within 40 km of the MITR from July through August during 2004–2017.
Data analyses
We used the ratio of the number of fledged chicks to the annual maximum number of breeding adult terns (chick:adult ratios) as a measure of breeding productivity for GCT and CCT, and used linear regression to investigate trends over time in annual productivity from 2004 to 2017. We tested for possible correlations between the numbers of GCT and CCT observed during bi-weekly colony counts in 2008–2017 and annual productivity (chick:adult ratios) observed from 2011 to 2017 using Pearson correlation coefficients.
We used generalized linear models to examine the effects of chlorophyll-a concentration (mg/m3), typhoon frequency (events per breeding season), and colony shifts (0 = remained on a single island, 1 = shifted between islands) on variation in chick:adult ratios (binomial distribution) and annual counts of CCT and GCT (normal distribution) at the MITR during 2004–2017. We used the R package ’MuMIn’ to find the best-fit model based on Akaike’s Information Criterion for small sample sizes (AICc) (Akaike Reference Akaike1974, Burnham and Anderson Reference Burnham and Anderson2003). In addition, the relationship between colony shifts and nest abandonment events was examined using Spearman rank correlation to investigate potential associations between these two phenomena. All analyses were carried out with R 1.0.136 (R Core Team 2017).
Results
Colony censuses
CCTs arrived at the MITR about one week later than GCTs during late May to early June in each year of the study period except 2012 and 2013 (Figure 2), and then CCTs built nests within the GCT colony upon arrival to form a dense mixed-species colony. Fluctuations in the annual census numbers of the two species were positively correlated during the study period (Pearson’s r = 0.59, P < 0.001, Figure 2). There was also a positive correlation between the chick:adult ratios for CCTs and those for GCT during 2011–2017 (Pearson’s r = 0.84, P = 0.017).

Figure 2. The colony sizes of Greater Crested Terns (GCT, black lines) and Chinese Crested Terns (CCT, grey areas) in the Matsu Islands Tern Refuge during the study period (2008–2017). Arrows indicate the name and date of typhoons that impacted the Matsu Archipelago during the tern nesting season (May–August).
Based on previous data and our observations of the annual maximum numbers of breeding CCT and GCT at the MITR, there were an average of 10 ± 4 (SD, min = 2, max = 16) adult CCT and 3,235 ± 1,072 (SD, min = 1,130, max = 5,500) adult GCT at the MITR during 2004–2017 (Table 1).
Table 1. Observed annual maximum numbers of individual Chinese Crested Terns (CCT) and Greater Crested Terns (GCT) in the Matsu Islands Tern Refuge during 2004–2017. Fledged chicks of GCTs were not counted separately from adults before 2011. The timing of nest abandonment was recorded as the range of dates between census dates before and after abandonment or based on surveillance camera images (underlined dates); dashes under “Nest abandonment” indicated insufficient data.
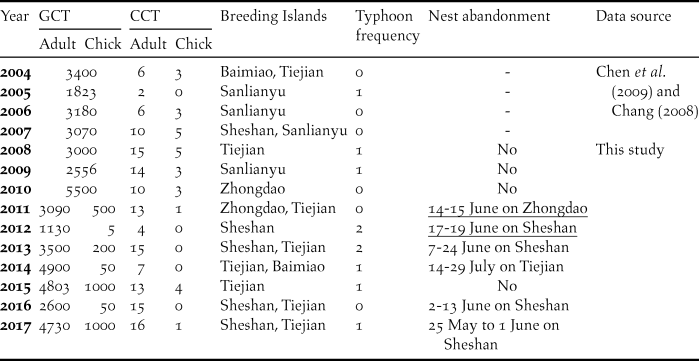
Most terns spent the beginning of the breeding season during 2008–2017 at Sheshan, but this incipient nesting attempt usually resulted in subsequent nest abandonment. During 2013–2017, three nest abandonment events occurred on Sheshan in June, and the tern colony shifted to Tiejien afterward. In 2012, typhoon “Talim” hit the Matsu Archipelago on 20 June, soon after a nest abandonment event occurred in Sheshan, and most of the terns left the MITR and no nesting colony formed again that season (Figure 2).
According to our records and classification criterion for typhoons, there were 10 typhoons during the breeding season from 2004 to 2017 (Table 1), nine of which occurred during July–August (Figure 2).
Not only did we record nest abandonment repeatedly on Sheshan, we also documented using surveillance camera images that the mixed-species colony on Zhongdao in 2011 was abandoned during 14-15 June; 84% of the breeding GCT left Zhongdao, while the numbers of GCT on Tiejien increased gradually thereafter. In early July 2014, a relatively small mixed-species colony of GCT (about 700 individuals) and CCT (four individuals) on Tiejien abandoned their nests, and 79% of GCT and all CCT left the island. The main breeding colony of GCT and CCT at the MITR in 2014 was on Baimiao, which had not been occupied by the two tern species as a colony site since 2004.
In summary, in-season colony shifts occurred in 2004, 2007, 2011, 2013, 2014, 2016, and 2017; and were closely associated with nest abandonment events during 2008-2017 (Spearman’s r = 0.82, P = 0.004). During 2008-2017, chick:adult ratios for CCT in years when colony shifts occurred (0.163 ± 0.23 SD) were significantly less than in years when no colony shift occurred (0.237 ± 0.18 SD; t-test, P = 0.019).
Model selection
The chick:adult ratio for CCT declined significantly during the period 2004–2017 (Figure 3; slope = -0.029, R2 = 0.31, P = 0.021), while the ratio for GCT did not change significantly during 2011 to 2017.

Figure 3. Observed value (black line) of chick:adult ratios for Chinese Crested Terns (CCT) at the Matsu Islands Tern Refuge during 2004–2017 and predicted values (dashed line) based on intra-seasonal colony shifts and typhoon frequency in generalised linear models (see Table 2, model 1).
The best-fit model explaining variation in chick:adult ratios for CCT, given the data, indicated that both colony shifts and typhoon frequency explained a significant proportion of the variation in CCT productivity during 2004–2017 (Table 2, model 1). The chick:adult ratios for CCT declined when in-season colony shifts occurred coincident with typhoons, especially in 2012–2014 when typhoons struck the MITR and there was mass nest abandonment. This caused a dramatic decline in numbers of CCT chicks (Table 2, Figure 3). Chlorophyll-a concentration was included in the second most competitive model, but this explanatory variable was not significantly correlated with chick:adult ratios for CCT (Table 2, model 2). Due to the small sample size of chick:adult ratios for GCT (n = 7, data only available during 2011–2017), however, no explanatory variables explained a significant proportion of the variation in GCT chick:adult ratios.
Table 2. Comparison of generalized linear models explaining variation in chick:adult ratios of Chinese Crested Terns (CCT) at the Matsu Islands Tern Refuge during 2004-2017. Explanatory variables included average chlorophyll-a concentration (Chl-a, mg/m3) within foraging range of the colony in July-August, in-season Colony Shift to a different colony site (0/1), and Typhoon Frequency during the nesting season (0–2). Only competitive models with ∆AICc < 2 are shown. The P-values for each model were determined by comparing models via chi-square test to the null model.

The variation in annual maximum numbers of CCT was not significantly predicted by our explanatory variables (Table 3, model 1). The explanatory power of average concentration of chlorophyll-a within foraging range for variation in annual maximum numbers of GCT at the MITR during 2004–2017 was nearly significant, however (P= 0.057, Table 3, model 2). The number of GCT at the MITR was significantly correlated with chlorophyll-a concentration during 2008-2017 (R2 = 0.445, P = 0.021, Figure 4). Also, chlorophyll-a concentration had a significantly positive effect on annual maximum numbers of GCT in the second most competitive model (Table 3, model 3). On the other hand, typhoon frequency had a negative effect on the number of GCT, but this effect was not statistically significant (Table 3, model 3). In addition, in-season colony shifts were not significantly correlated with the observed maximum number of either GCT or CCT.
Table 3. Comparison of generalized linear models explaining variation in annual maximum numbers of Chinese Crested Terns (CCT) and Greater Crested Terns (GCT) at the Matsu Islands Tern Refuge during 2004–2017. Explanatory variables included average chlorophyll-a concentration (Chl-a, mg/m3) within foraging range of the colony, in-season Colony Shift to a different colony site (0/1), and Typhoon Frequency during the nesting season (0–2). Only competitive models with ∆AICc < 2 are shown. The P-values in models were determined by comparing models via F-test to null models.
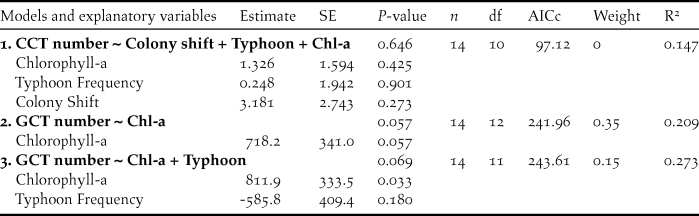

Figure 4. Annual maximum numbers of Greater Crested Terns (GCT, black lines) at the Matsu Islands Tern Refuge in relation to the average chlorophyll-a concentration (mg/m3) within a 40-km foraging range of the colony during July–August, 2004-2017 (grey lines). The correlation between chlorophyll-a concentrations and numbers of GCTs was significant during 2008–2017 (R2 = 0.445, P = 0.021).
Discussion
Trends in colony size
Combined with the annual numbers of CCTs reported in Zhejiang, China (Shui-Hua Chen pers. comm. 2017) and South Korea (Song et al. Reference Song, Lee, Lee, Lee, Kim, Choi, Shin, Park, Lee and Kim2017), we note that since 2008 the annual percentage of the known global population of nesting pairs at MITR ranged from 28% to 76%. CCTs nest together with GCTs at the MITR, as they do at the other two colonies in Zhejiang (Chen et al. Reference Chen, Fan, Chen and Lu2011). The overlap in the breeding period and the significant positive correlation between numbers of GCT and CCT at the MITR requires more study on the timing of breeding and potential interspecific competition for nesting habitat and forage fish. The numbers of breeding CCT and GCT at the MITR have been relatively stable over the past decade, but the mean numbers of CCT (10 ± 4) at the MITR indicated that the species is still on the brink of local extinction in the Matsu Archipelago. Further research is required to understand what may be limiting colony size (e.g. food or habitat availability, predators, interspecific competition).
The 2015 discovery of the new CCT colony in South Korea was remarkable in part because it represented a major expansion of the breeding range for the species, and because it was the first example of CCTs breeding within a colony of Black-tailed Gulls and in the absence of breeding GCT (Song et al. Reference Song, Lee, Lee, Lee, Kim, Choi, Shin, Park, Lee and Kim2017). In contrast, the Matsu Archipelago breeding colony of Black-tailed Gulls is about 50 km east of the CCT and GCT breeding islands (26°21’19.47’’N, 120°29’8.45’’E), and there are few records of Black-tailed Gulls observed within the GCT and CCT colonies at the MITR. The new information on CCT nesting with Black-tailed Gulls in South Korea has encouraged us to expand our search areas and the list of species that CCT will associate with in looking for potential new CCT breeding sites in East Asia.
From 2008 to 2017, the annual number of GCT nesting at the MITR was significantly correlated with chlorophyll-a concentration within foraging range. The annual maximum numbers of GCTs decreased by 45% in 2010–2011 and 40% in 2014–2016, while over the same period chlorophyll-a also decreased (Figure 4). This implies that GCT colony size at the MITR was primarily influenced by food availability, because phytoplankton productivity and larval fish abundance are associated with chlorophyll-a concentrations (Kim et al. Reference Kim, Kang, Oh, Suh and Hwang2005). In addition, the high chlorophyll-a concentrations around the Matsu Archipelago reflect the high nutrient inputs of the Ming River (Figure 5), the same phenomenon that Gong et al. (Reference Gong, Chen and Liu1996) reported in the Yangtze River estuary.
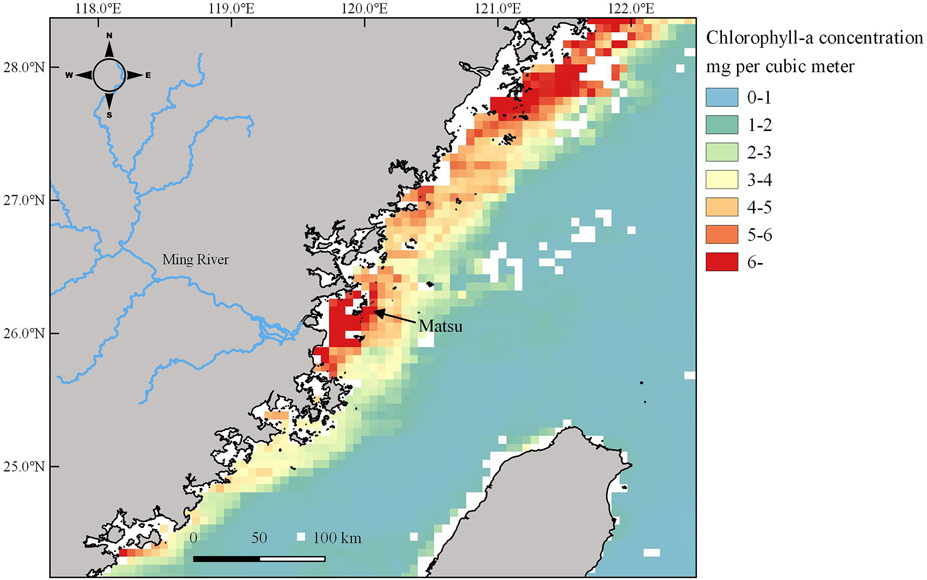
Figure 5. Monthly average of chlorophyll-a concentrations (mg/m3 at 4-km resolution) obtained from MODIS (July–August 2010) around the Matsu Archipelago showing the high primary productivity near the Ming River estuary.
Although we did not find the same correlation between chlorophyll-a concentrations and breeding numbers for CCT, we provide here the first evidence of a link between ocean condition variables and seabird colony size in the East China Sea. However, the knowledge gaps between the availability and variability in prey stocks for both tern species, plus the spatiotemporal relationship between seabirds, their prey fish, and ocean productivity, will require further evaluation and long-term investigation.
Breeding productivity
Based on model selection, typhoon frequency and in-season colony shift were important factors affecting annual chick:adult ratios for CCT. During 2008–2017, within-season colony shifts were usually associated with nest abandonment events. Although a new colony was usually started on another island soon after nest abandonment, CCT chick:adult ratios were significantly lower in years when the colony shifted in-season.
In the MITR, within-season shifts to a new breeding island after nesting failure delays nesting chronology and increases breeding asynchrony; breeding synchrony is important for social facilitation, matching with peaks in prey availability, and reduction in chick mortality due to predators (Gochfeld Reference Gochfeld1979, Hatchwell Reference Hatchwell1991, Regular et al. Reference Regular, Hedd, Montevecchi, Robertson, Storey and Walsh2014). However, average chlorophyll-a concentration within foraging range of the colony during July–August was not a significant explanatory factor for variation in CCT chick:adult ratios, based on model selection. Also, Gong et al. (Reference Gong, Wen, Wang and Liu2003) indicated that maximum primary production in the East China Sea occurs in mid-July. Thus, the mismatch of peak food demands during nesting with the peak in prey availability may not explain the low breeding productivity of CCT when colonies shifted between islands. Nevertheless, the energy invested by terns in the first nesting attempt may predispose breeding adults to have lower breeding productivity during the second nesting attempt following the colony shift (Monaghan and Nager Reference Monaghan and Nager1997, Arnold et al. Reference Arnold, Hatch and Nisbet2004).
Nine of the 10 typhoons that stuck the Matsu Archipelago during 2004–2017 did so during July-August, causing lower success of later nests and/or relaying by CCT at the MITR. The predicted values of chick:adult ratios based on the model with the best fit declined from 0.54 to 0.21 when one typhoon occurred and declined further to 0.08 when two typhoons occurred in a season. In particular, when two typhoons stuck the MITR after abandonment of the initial nesting attempts, as occurred during the early stages of the 2012 and 2013 breeding seasons, the predicted chick:adult ratios of GCT and CCT approached zero.
Chen et al. (Reference Chen, Fan, Roby, Lu, Chen, Huang, Cheng and Zhu2015) indicated that most typhoons affected China’s coastal areas (Zhejiang and Fujian provinces) during July-September, and pre-fledged chicks at tern colonies with delayed nesting chronology were more susceptible to severe weather during the typhoon season. This scenario might worsen under climate change because the frequency of typhoons impacting the Zhejiang and Fujian coasts has been increasing from 1950 to 2012 (Chen et al. Reference Chen, Fan, Roby, Lu, Chen, Huang, Cheng and Zhu2015). In the northern Great Barrier Reef, however, Devney et al. (Reference Devney, Short and Congdon2009) found that breeding colony size of GCT in the long term was not related to direct disturbance from typhoons, presumably because periods between typhoons were of sufficient duration for terns to re-nest successfully. Therefore, sufficient time for terns to complete their breeding cycle before the onset of the typhoon season is crucial for maintenance of population size.
In summary, asynchronous breeding at colonies associated with abandonment of early-stage nesting colonies and the prolongation of the breeding period into the peak of the typhoon season apparently caused lower hatching success and chick survival for CCT nesting at the MITR, but not for GCT. Consequently, the additive effects of in-season colony shifts and typhoons may be responsible for the lower than expected breeding productivity of CCTs in the MITR during 2004–2017.
Other possible threats
During 2008–2017, four out of seven nest abandonment events occurred on Sheshan. Terns settling on Sheshan to nest tended to abandon their nests in June, even though their eggs were close to hatching. We speculate that this phenomenon is associated with the tradition of local fishermen who periodically land on islands to collect shellfish or seaweed during spring tide series. Furthermore, the timing of spring tide series coincided with nest abandonment events on 16 June 2011 and on 19 June 2012. We believe that there is an association between the type and magnitude of human disturbance and tern nest abandonment events in the MITR, but the relationship was not measured in this study. The nest abandonment phenomenon on Sheshan was perhaps more frequent because this island is connected to a larger, human-inhabited island (Xiju) at low tide during spring tide series. Some ground predators may also have invaded Sheshan during that period.
Ground animals such as brown country rats and king rat snakes Elaphe carinata are potential predators on tern eggs and chicks at the MITR. Brown country rats are commonly recorded on Zongdao, however, no rats were recorded on Sheshan during extermination attempts from 2010 to 2017. King rat snakes have only been recorded on larger, human-inhabited islands in the Matsu Archipelago. Since they are excellent swimmers, it is very likely that king rat snakes have been a factor in the tern colony abandonment events that have occurred repeatedly at the MITR. In addition, the aerial predator of terns at the MITR is the Peregrine Falcon Falco peregrinus, which is commonly seen year-round in the Matsu Archipelago. Although we found no direct evidence that CCT chicks or adults were being preyed upon during this study, predators should be considered a possible factor in the recorded episodes of nest abandonment and colony shifts.
The effects of these possible threats on tern colony size and breeding productivity at the MITR still need more monitoring and evaluation, and should be included in future conservation projects. Twenty-four hour observations using surveillance cameras and/or by researchers would help determine if the presence of predator and human disturbance reduces nesting success of CCTs in the MITR.
Future prospects for conservation at the Matsu Islands Tern Refuge
We found evidence that in-season colony shifts prolonged the tern nesting period into the peak typhoon season, which severely impacts CCT breeding productivity. The long-term decline in CCT breeding productivity at the MITR during the study period is a crucial explanation for the low numbers of CCTs nesting in the Matsu Archipelago and the continued low global population of CCT. It is urgently necessary to minimise human disturbance and the impact of predators at all the islands where CCT are nesting, especially the early in the nesting season. Also, researchers should not be prohibited from landing on protected tern nesting islands, as data on the factors limiting tern colony size and nesting success are crucial for effective conservation and restoration. For instance, a small bird blind for researchers was set up on Tiejien in 2017; this helped to minimise the risk of disturbance to nesting terns by researchers during observation periods.
We found that CCT and GCT attempting to nest on Sheshan in the MITR experienced nest abandonment episodes several times, apparently due to human disturbance. Jones and Kress (Reference Jones and Kress2012) reviewed a number of attempts to relocate seabird colonies away from human development, marine pollution, and fisheries conflicts to more favourable locations by using the techniques of social attraction (i.e. decoys, audio playbacks, mirror panels). In addition, bird scaring lines (tori lines) are used by longline fisheries to effectively deter albatrosses and petrels from ingesting baited hooks (Domingo et al. Reference Domingo, Jiménez, Abreu, Forselledo and Yates2017). A combination of social attraction at islands in the MITR that are free of human disturbance, and ropes, stakes, and flagging at Sheshan would be effective tools for dissuading tern nesting on Sheshan early in the breeding season and attracting GCTs and CCTs to colonise other islands instead.
Apart from the previous records of illegal harvest of tern eggs in Zhejiang, there were no observations of direct exploitation of CCT or GCT by humans at the MITR during our study period. However, photographers, tourists, fishermen, fishing boats and rock fishing activities are the main sources of human disturbance to breeding terns at the MITR. Lienchiang County designated eight protected islands as core areas of the MITR, including a 100 m buffer around each island where behaviors such as blaring boat horns, setting off firecrackers, and feeding seabirds are all prohibited. Nevertheless, the boundaries of the buffer zones are not clearly marked and local authorities were unable to enforce the prohibitions. We suggested the use of buoys and ropes to identify the boundaries of the buffer zones at each island in the MITR, as well as proper education programmes for local people to raise public awareness of seabird conservation in Matsu.
Furthermore, research on the role of predators, interactions with other sympatric species, prey availability, impacts of fisheries on prey availability, climate change, and impact of human disturbance are essential, as this species is still on the brink of extinction. More rigorous monitoring of tern breeding colonies and the creation of public education opportunities are recommended to combat the current significant decline of CCT nesting success rates within the MITR. These actions could greatly improve the future conservation success of this critically endangered species.
Acknowledgements
This research was funded by the Forestry Bureau (No. 106-08.1-25), Lienchiang County Government (No. Tern106002), and the Ministry of Science and Technology of Taiwan (No. 105-2621-B-002-003-MY3). We thank Shui Hua Chen, Vice President of the Zhejiang Museum of Natural History for providing valuable data and suggestions. We also thank the Wild Bird Society of Taipei and the Wild Bird Society of Matsu for providing great help in the field, especially Jin Sung Ruan and Siou Liang Du. We are particularly grateful for the kind assistance given by Hong Guan Wu, Captain of the “Queen Plum Flower” in Matsu. We would also like to thank Thomas A. Gavin, Professor Emeritus, Cornell University for help in editing the English in this paper.










