Introduction
Conservation status of the world’s birds has continued to deteriorate in recent years (Butchart et al. Reference Butchart, Stattersfield, Bennun, Shutes, Akçakaya, Baillie, Stuart, Hilton-Taylor and Mace2004, BirdLife International 2008). Island endemic birds are particularly vulnerable to extinction because of the small population size and human activities such as habitat destruction and the introduction of mammalian predators (Johnson and Stattersfield Reference Johnson and Stattersfield1990, Newton Reference Newton1998, Biber Reference Biber2002, Blackburn et al. Reference Blackburn, Cassey and Duncan2004, Karels et al. Reference Karels, Dobson, Trevino and Skibiel2008). This situation also occurs in the Ryukyu Archipelago, a chain of islands in south-western Japan that includes a rich fauna and flora with high endemism (Biodiversity Center of Japan 2010). Many endemic species in the Archipelago have been threatened by human activities, and the Archipelago is regarded as an Endemic Bird Area (EBA) where habitat-based conservation is required (Stattersfield et al. Reference Stattersfield, Crosby, Long and Wege1998). The Ministry of the Environment (MOE) of Japan is currently planning to designate new national park areas in the Archipelago to protect the habitat of threatened species, with the intention of listing the Archipelago as a UNESCO World Natural Heritage Site in the near future.
Amami-Oshima in the Ryukyu Archipelago is an island that provides important habitats for many endemic, endangered bird species and subspecies (Biodiversity Center of Japan 2010). For example, the main populations of the Amami Woodcock Scolopax mira and Lidth’s Jay Garrulus lidthi are found on this island, and subspecies of the White-backed Woodpecker Dendrocopos leucotos owstoni and the Eurasian Scaly Thrush Zoothera dauma major exist only on the island (The Ornithological Society of Japan 2012), but see also Records Committee and Taxonomic Committee (2015) for errata. However, habitats of these native species were destroyed rapidly from the late 1950s to early 1970s due to large-scale forestry (clear-cutting of large areas of mature forests) that began in 1954 (Sugimura Reference Sugimura1988). Another threat to native species is an introduced mammal species, the small Indian mongoose Herpestes auropunctatus (Yamada Reference Yamada, Veitch and Clout2002, Yamada and Sugimura Reference Yamada and Sugimura2004, Watari et al. Reference Watari, Takatsuki and Miyashita2008, Ishida et al. Reference Ishida, Murata, Nishiumi, Takahashi and Takashi2015). In 1979, about 30 individuals were released in a forested suburb in Naze City (Naze area of Amami City at present) in the central part of the island to control a venomous snake, habu Protobothrops flavoviridis, and the mongoose expanded its population and distribution during the 1980s and 1990s (Ishii Reference Ishii2003, Yamada and Sugimura Reference Yamada and Sugimura2004). The population was estimated to be 6,141 (95% CI: 5,415–6,817) in 2000 but decreased drastically after the start of a mongoose eradication project in 2000 (Fukasawa et al. Reference Fukasawa, Hashimoto, Tatara and Abe2013a).
The conservation status of the subspecies Z. d. major was representative of sensitive island-endemic birds: the population size was thought to be slightly more than 58 birds in 1996 (AOC 1997), 74 pairs in 1999 (Khan and Takashi Reference Khan and Takashi2006), and 50–100 breeding pairs in the early 2000s (MOE 2002). All these estimates were based on song-count surveys conducted by a non-profit organization, the Amami Ornithologists’ Club (AOC). However, those estimates of population size were based on a limited research effort (i.e. the survey area was not large compared to the present study) and did not consider the possibility of thrush presence in areas where no research was conducted. It is plausible that more individuals existed in such areas, therefore, the population size presented in the past studies may be underestimated. In recent years, surveys showed a gradual population recovery (AOC 2008), where song counts recorded in 2013 documented 502 singing birds (see Table 1), although the whole population size has not been estimated.
Table 1. The number of singing Amami Thrushes recorded in two types of song-count surveys during each research year. Weather, temperature and wind velocity were recorded on the day of the LT survey each year.
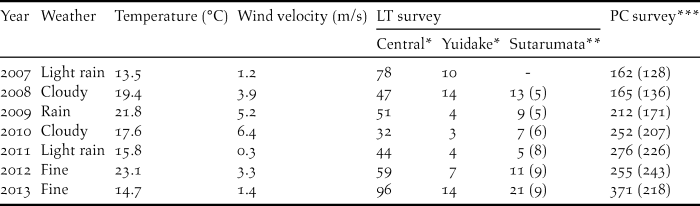
* Number of 2 km transect lines along these roads are 41 (Central Forest Road) and 5 (Yuidake Forest Road), respectively.
** Number of 2 km transect lines along Sutarumata Forest Road in each year are different and shown in parentheses.
*** Number of transect points in the PC survey in each year are different and shown in parentheses.
The song count method is a common and useful technique for monitoring bird populations, especially for forest-dwelling species that are sometimes difficult to observe visually. When estimating a bird’s population based on song count, two factors must be considered. One is the environmental conditions, such as forest type, topography, etc., which may affect the distribution of birds. The song distribution recorded in the count survey may be affected by such environmental conditions in the survey area. The other factor is intensity of research effort, which may affect the detectability of song. Because bird song is degraded as it propagates through a natural environment (Dabelsteen et al. Reference Dabelsteen, Larsen and Pedersen1993), the detectability of song decreases with distance between the birds and researchers. Taking distance and detectability into consideration is important in song data sampling (Buckland et al. Reference Buckland, Anderson, Burnham and Laake1993). The intensity of research effort (i.e. length of line-transects, number of point-transects, etc.) may affect the detectability of song because a high intensity reduces the distance between birds and researchers. In the present study, we attempted to evaluate environmental conditions affecting distribution of Z. d. major and estimated the number of singing males using the results of the AOC’s song-count surveys conducted from 2007 to 2013. Based on these results, taking both environmental conditions and research intensity related to song detectability into account, we re-evaluate the recent conservation status of Z. d. major.
Methods
Study area and species
Amami-Oshima is the second largest island (712.5 km2) in the Ryukyu Archipelago (Figure 1). Z. d. major is a forest-dwelling bird endemic to this island. It prefers old-growth broad-leaved evergreen forests in the island for its breeding habitat (Mizuta Reference Mizuta2014). This bird was formerly considered to be the distinct species Z. major (BirdLife International 2000, Clement and Hathway Reference Clement and Hathway2000) and has been commonly called the Amami Thrush. In this paper, we use this common name to denote Z. d. major. When the Amami Thrush was considered to be a distinct species, it was assessed as ‘Critically Endangered’ on the IUCN Red List (BirdLife International 2000). This bird is now treated as a subspecies of the Eurasian Scaly Thrush (Collar Reference Collar2004), which is categorised as ‘Least Concern’, although the threatened status of this subspecies is recognised (BirdLife International 2012).

Figure 1. Map of Amami-Oshima Island in the Ryukyu Archipelago of south-western Japan. Three transect lines in the LT survey were indicated on the map: Amami Central Forest Road (Central, white line), Yuidake Forest Road (Yuidake, black line) and Sutarumata Forest Road (Sutarumata, black line). A shaded rectangle in the north-eastern part of the island indicates the area where no Amami Thrushes have been detected.
Song-count survey
The Amami Thrush is usually hard to observe because of its shy and highly elusive behaviour, but its loud and melodious song is remarkable and distinct from those of other songbirds on the island. The song is mainly heard for a short period before sunrise during the breeding season, especially in March and April. The AOC has been conducting a public participation survey for song counts of the Amami Thrush since 1994 (AOC 1997, 2008, Khan and Takashi Reference Khan and Takashi2006). This line transect survey (hereafter called the “LT survey”) was carried out on a single day every March. In the LT survey, a 42 km transect line was set along the forest road that passes through the central part of the island (Amami Central Forest Road), and a 6 km line was set in the southern part of the island (Yuidake Forest Road). An approximately 10 km line (Sutarumata Forest Road) has been set in the central part of the island since 2008 (Figure 1). The participants (more than 100 volunteers) were divided into pairs, and each pair walked a 2 km stretch of line along each of the three transect lines. All pairs started walking at the same time (approximately one hour before sunrise) in the same direction, and made a round trip (total 4 km) in 60 minutes. The starting point of each pair was set at intervals of 1 km along the transect line. Therefore, the whole transect line was walked by two pairs, except for the first and last 1 km stretches, which were walked by one pair. The repetition of the survey (walked by two pairs, making a round trip) enabled us to minimise missed detections. The length of one transect line, Sutarumata Forest Road, varied among years depending on the number of participants. Pairs fixed their location on a map as they walked along the transect line. It was usually difficult for researchers to sight singing thrushes because of the dark surroundings. Therefore, when observers heard the song of the thrush without sighting it, they recorded the approximate location of the song on the map based on the direction and the loudness of the sound. If the song stopped and another song was heard from that location within a c.300 m radius of the previous song, those two songs were considered to be by the same individual (AOC 2008). The song is characteristic and easy to differentiate from that of other birds, but at least one member of each pair was selected from the participants who had taken part in this survey in the past. Meteorological conditions, such as weather, temperature and wind velocity on the day of LT survey were checked on the website of the Japan Meteorological Agency (http://www.data.jma.go.jp/obd/stats/etrn/index.php) because such conditions may affect the results of the song count.
Although LT surveys were conducted in the main habitat of the Amami Thrush on the island (AOC 1997), survey protocols were apparently insufficient to cover all potential habitat on the island. Therefore, a point count survey was also conducted at a considerable number of points scattered around the island (see Table 1 for number of points in each research year), except for the north-eastern part of the island, where no thrushes have been detected (Figures 1, 2). This point count survey (hereafter called the “PC survey”) was conducted by members of the AOC and the Amami Wildlife Conservation Center (AWCC) on several days in March and early April. In this survey, researchers went to their own survey points approximately one hour before sunrise and stayed there for 50 minutes to record the approximate location of each song on a map. The LT survey was carried out on a fixed day regardless of weather conditions because many volunteers were gathered on that day. In contrast, the PC survey avoided days with rough weather.
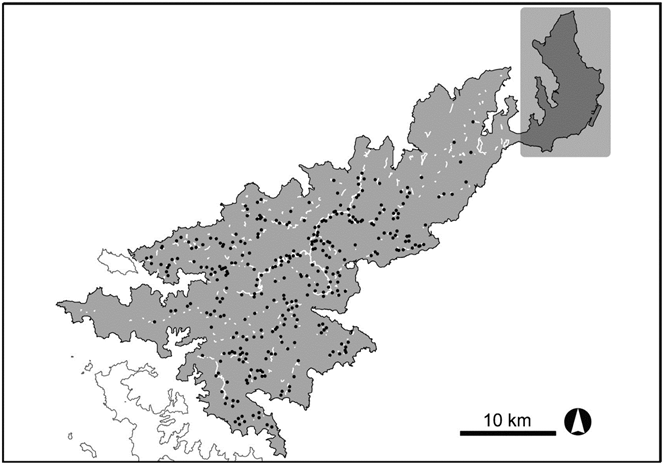
Figure 2. A mapped example of the results of the song-count survey conducted in 2012. Black dots indicate the locations of singing Amami Thrushes, and white lines and dots indicate the locations of the LT and PC surveys. A shaded rectangle in the north-eastern part of the island indicates the area where no Amami Thrushes have been detected.
Double-counting of singing individuals was carefully eliminated in both surveys. If two singing individuals were plotted on the map by different researchers within a 300 m radius and even if these songs were not recorded at the same time, they were regarded as the same individual (AOC 2008). A 300 m radius was determined based on the focal observation of individuals which moved at most 300 m during singing (AOC 2008). The number of songs counted during the survey indicated the minimum number of singing thrushes detected by researchers.
The results of both surveys from 2007 to 2013 were used in the present analysis. Surveys were also conducted in the years before 2006, but those data were not used in the analysis because few surveys were conducted during this time. The number of singing Amami Thrushes recorded in each year, number of line- and point-transects in the survey, and total number of people who participated in both surveys are shown in Table 1, along with the meteorological conditions on the day of the LT survey. The results of the 2012 survey and locations of the line- and point-transects are illustrated in Figure 2.
Population estimates
Based on plot data of singing Amami Thrushes, we estimated the population for the entire island. First, the map was divided into 600 m grid cells, and the number of singing thrushes located in each grid cell was counted. Potential thrush habitat (island area except for the north-eastern part of the island) was covered by a total of 2,099 grid cells. We then developed a simple statistical model that incorporates distance-dependent detection probability and environment-dependent population density, as follows:
where Y i is the number of individuals in the ith grid cell recorded in LT and PC surveys, λ i is the mean abundance (mean number of singing individuals) in the grid cell, and π i is song detectability (i.e., probability that a singing individual in the ith grid cell is detected) of the survey.
We assumed that the log mean abundance is explained by a linear model of environmental factors, X:
where β 0 is the intercept, and β j is the regression coefficient of jth environmental factors. The following environmental factors in the grid cell were examined to confirm their influence on mean abundance in each grid cell: mean forest age (FAGE; range: 0–130 years old), area of open habitat that is not suitable for breeding thrushes (AOH; range: 0–0.35 km2), mean height above sea level (HASL; range: 0–633.13 m), and ground ruggedness (Slope-Aspect Ruggedness Index: SARI; range: 0–6.56, see Nellemann and Fry Reference Nellemann and Fry1995, Jepsen et al. Reference Jepsen, Madsen, Karlsson and Groth2005). The ground ruggedness is assumed to influence the distribution of earthworms, which are the most important food source for nestlings (Mizuta Reference Mizuta2014). The introduced small Indian mongoose may also have a serious impact on the thrush population. Therefore, the relative density of mongoose (RDM) was also considered as one of the environmental factors that may affect thrush abundance. The RDM was calculated from trapping results of the mongoose eradication project (MOE, unpubl. data). See Appendix S1 in the online supplementary material for details.
Song detectability in a grid was affected by the intra-grid mean of the minimum distance from the survey line or point, D (see below), and the binary variable of survey method, M. If the survey method is LT and PC, M is 0 and 1, respectively. The attenuation rate of song detectability was considered to follow an exponential power function of D, and the song detectability of ith mesh by kth (k = 1, 2) survey method was expressed as follows,
where α 0 (< 0) is the parameter of baseline attenuation rate, α 1 is the effect of survey method on attenuation rate, which is expected that α 0 + α 1 M k < 0. The n is a power exponent that determines the shape of the attenuation function. D, the intra-grid mean of the minimum distance from the survey line or point, was determined as follows: each 600 m grid cell was divided into 100 m sub-grid cells (36 sub-grid cells in a 600 m grid cell). Distance from the centre point of each sub-grid cell to the nearest survey line/point was then measured, and the mean value of these distances in each 600 m grid cell was calculated and called “D,” which is smaller when the research effort in the grid cell is more intense. The detectability of singing individuals in a grid cell was considered to attenuate with increasing values of D.
From equations (1), (2) and (3), we obtain the following model:
The form of equation (4) is similar to a generalised linear model (GLM) with Poisson error and log link. However, this model has an unknown parameter n and can be considered to be a non-linear model. To estimate n and other parameters, we calculated profile likelihoods, where the value n was fixed to 26 equally-spaced values from 0.5 to 3.0 with intervals of 0.1 and substituted for D
n
, which was included in the GLM analyses (mentioned below). Effect of survey method on attenuation rate corresponds to the interaction term
![]() ${\alpha _1}D_{ik}^n{M_k}$
, which we denote by D
n:METH hereafter. The value α
0 and α
1 is a coefficient estimated in the GLM analyses. To obtain a model that explains the number of thrushes recorded in the grid cell among 26 models with different D
n
, the estimation was performed for each research year. The number of singing thrushes recorded in each grid cell was treated as a response variable, and five environmental factors (mentioned above) and the index of the intensity of research effort (the fittest D
n
) were treated as explanatory variables. The land area in the grid cell was treated as an offset term. Among 26 different values of n (0.5 to 3.0 with intervals of 0.1), the model with the lowest AIC was selected to determine the value n of the fittest attenuation rate and the combination of explanatory variables (i.e. the five environmental factors and D
n:METH). Higher-ranked models (with the value of ∆AIC being less than 2) were listed to examine the factors important for improved prediction of thrush abundance in each research year.
${\alpha _1}D_{ik}^n{M_k}$
, which we denote by D
n:METH hereafter. The value α
0 and α
1 is a coefficient estimated in the GLM analyses. To obtain a model that explains the number of thrushes recorded in the grid cell among 26 models with different D
n
, the estimation was performed for each research year. The number of singing thrushes recorded in each grid cell was treated as a response variable, and five environmental factors (mentioned above) and the index of the intensity of research effort (the fittest D
n
) were treated as explanatory variables. The land area in the grid cell was treated as an offset term. Among 26 different values of n (0.5 to 3.0 with intervals of 0.1), the model with the lowest AIC was selected to determine the value n of the fittest attenuation rate and the combination of explanatory variables (i.e. the five environmental factors and D
n:METH). Higher-ranked models (with the value of ∆AIC being less than 2) were listed to examine the factors important for improved prediction of thrush abundance in each research year.
Finally, the equation for the best model with the lowest AIC was used to estimate population size. The estimated number of singing individuals in each grid cell,
![]() ${\hat \lambda _i}$
, was calculated using equation (2), and the whole number of singing individuals on Amami-Oshima Island was calculated by summing up
${\hat \lambda _i}$
, was calculated using equation (2), and the whole number of singing individuals on Amami-Oshima Island was calculated by summing up
![]() ${\hat \lambda _i}$
across all of the grid cells. A Monte-Carlo 95% CI of the estimated population size was calculated using coefficients of the model equation that were randomly generated 100 times from the multinomial normal distribution obtained by Laplace approximation of the likelihood function.
${\hat \lambda _i}$
across all of the grid cells. A Monte-Carlo 95% CI of the estimated population size was calculated using coefficients of the model equation that were randomly generated 100 times from the multinomial normal distribution obtained by Laplace approximation of the likelihood function.
Because it is plausible to presume that females do not sing, the result of the song count was regarded as number of males that are capable of breeding and was simply doubled to obtain the estimated size of the breeding population, based on the assumption that the sex ratio does not deviate from 1:1. Although bird banding surveys conducted by one of the authors confirmed that first-year males do sing (H. Torikai pers. obs.), the estimation method adopted here does not account for the presence of floater-males even if such individuals exist in the population. Therefore, we emphasise that the population estimates shown in the present study indicate the number of males and females that are capable of breeding.
R version 3.1.2 (R Core Team 2014) was used for statistical analyses. The library “mgcv” was used for the GAM analysis, and “MASS” and “MuMIn” were used for the GLM analysis. The library “mvtnorm” was used to calculate the 95% CI. Geographical analyses were conducted using ArcGIS 10.2 (Esri Inc.). Vegetation maps were provided by the sixth and seventh Vegetation Surveys of the National Survey on the Natural Environment conducted by the MOE. Data concerning forest age were obtained from the forest register data maintained by Kagoshima Prefecture, which were provided for research use.
Results
In the LT survey, the number of singing thrushes did not change significantly until 2012, but the record suddenly increased in 2013 (Table 1). The number of singing thrushes counted in the PC survey increased during the seven years, with an increasing number of survey points (Table 1). A sudden increase was also recorded in 2013 in this survey, even though the number of transect points decreased in comparison to the previous year (Table 1).
In the LT survey, wind velocity was a possible factor affecting the number of singing thrushes counted by researchers; the count tended to be lower when the wind velocity was high (Table 1).
In the GLM analyses, to better predict the number of singing thrushes recorded in a grid cell, the variables adopted in the models with ΔAIC < 2 varied across years (Table 2). The best models for different years also contain different combinations of variables, but FAGE (mean forest age) and AOH (area of open habitat) were adopted in the models for nearly all years (Tables 2, 3). The sign of each estimated coefficient of the variables indicated that singing thrushes were recorded more abundantly in grid cells that included older-aged forest and less open habitat. Both the density and distribution of the small Indian mongoose decreased drastically in recent years (Figure 3) due to the progress of the mongoose eradication project. In the GLM analyses, the RDM (relative density of mongoose) was adopted in many of the models for the early research years (2008–2011), suggesting that singing thrushes were more abundant in the grid cells with lower mongoose density before the early 2010s.
Table 2. Ranking of models in each research year for explaining the number of singing Amami Thrush recorded in a grid cell, with all possible combinations of D n and the environmental factors. The models are arranged in order of increasing Akaike information criterion (AIC). ΔAIC is the difference in AIC from that of the best model, and models with ΔAIC < 2 are shown.
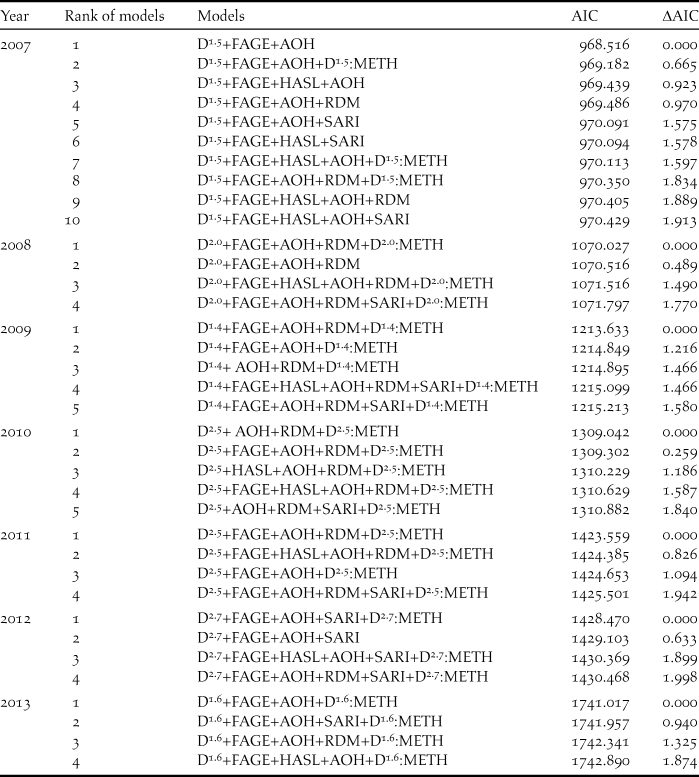
Table 3. Parameter estimates and SE of the best model in each research year for explaining the number of singing Amami Thrushes recorded in a grid cell, with all possible combinations of D n and the environmental factors.
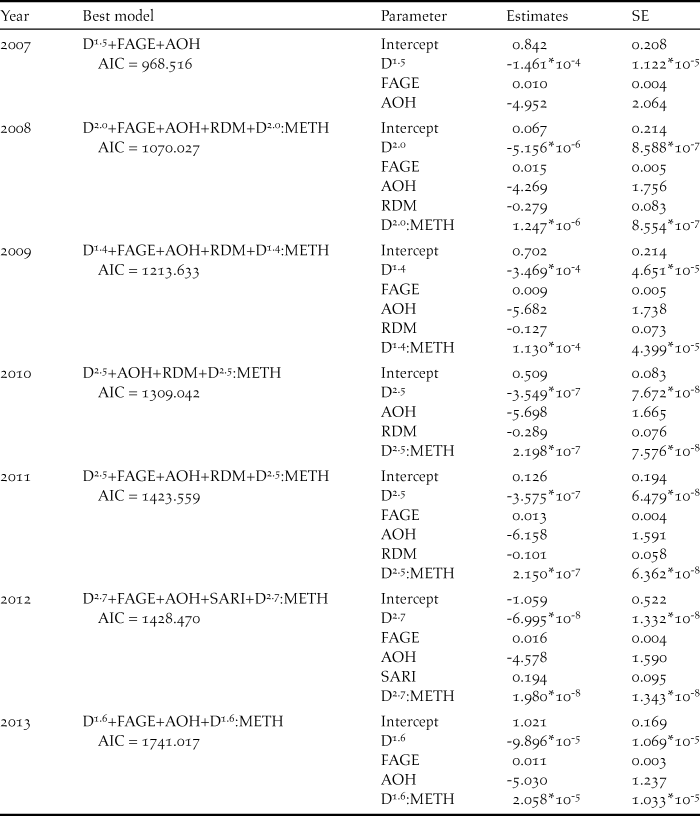
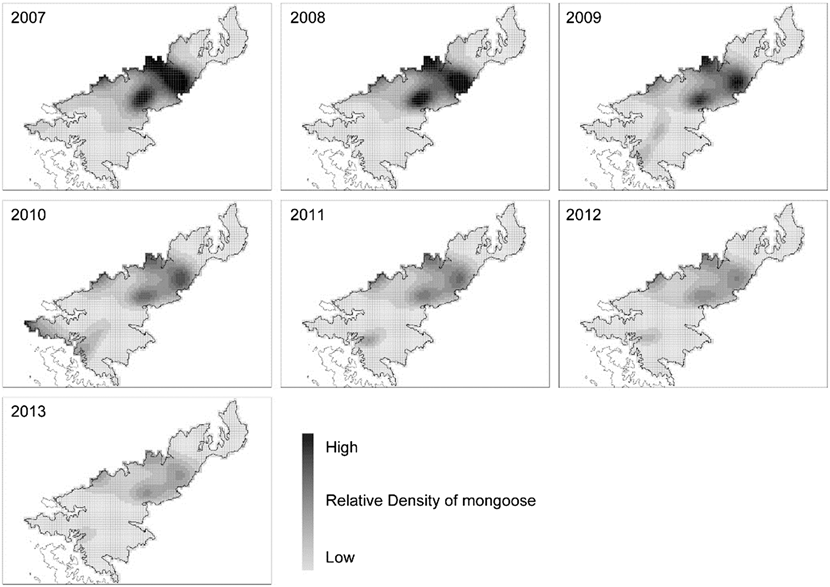
Figure 3. Annual change in the relative density and distribution of small Indian mongoose on Amami-Oshima Island, calculated using the generalised additive model based on the CPUE data from the mongoose eradication project. The size of each grid cell is 600 m x 600 m.
Intensity of research effort in a grid cell (the intra-grid mean of the minimum distance from the survey line or point, D) certainly affects the number of recorded thrushes in the grid cell, so D n was included in all models (Table 2). The fittest n varied from 1.4 to 2.7 across years, and the sign of the coefficient of D n in the models was always negative. Therefore, the detectability of singing thrushes decreased with increasing values of D, showing an inverse-sigmoid shape.
The interaction term between D n and METH (survey method) was also adopted in most of the models (Table 2). This indicated that methodological differences (LT or PC) affected the number individuals detected in the survey. Estimated number of singing thrushes in each year based on song-count survey data varied from 945 (95% CI: 827–1105) in 2010 to 2,512 (2,173–2,897) in 2013, using the best models in the GLM analyses. The whole population size (number of males and females that are capable of breeding) was therefore estimated to range from 1,890 (1,654–2,210) in 2010 to 5,024 (4,346–5,794) in 2013 (Figure 4).
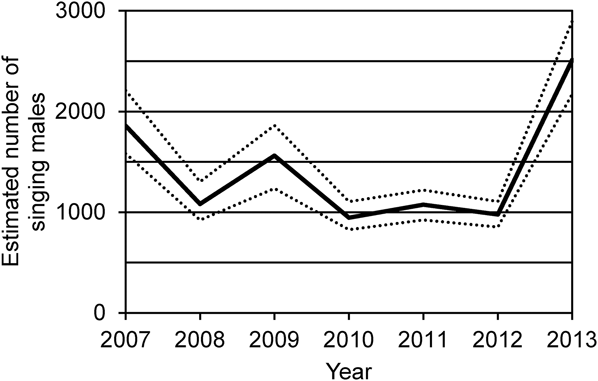
Figure 4. Estimated number of singing male Amami Thrushes based on the results of the song-count surveys conducted from 2007 to 2013. The 95% CI is shown by the dotted lines.
Discussion
The number of singing Amami Thrushes counted in the PC survey increased each year as the number of transect points increased (Table 1). The number of singing thrushes counted in the LT survey across years tended to be lower when the wind velocity was higher (Table 1). The sound of rustling leaves made by strong wind may prevent the researchers from detecting the song, and/or the thrushes may be reluctant to sing in a strong wind. However, the PC survey (including more research effort than the LT survey) was not conducted under rough weather condition, so difference of weather condition among years should not have a serious effect on the estimates. Song count data were affected by several factors such as the amount of research effort, indicating that annual fluctuation in the number of singing thrushes recorded during the survey does not necessarily reflect population fluctuations across years. However, in 2013, both the LT and PC surveys showed a substantial increase in singing thrushes (Table 1). This increase is not a result of a change in the amount of research effort, because the number of transect lines/points was not increased, rather decreased, from 2012 to 2013. The increase in singing thrushes probably reflects a population expansion that occurred between 2012 and 2013, although the reason for this expansion is unclear.
AOC (2008) inferred that the population of the Amami Thrush had gradually increased since the early 2000s due to the recovery of forest conditions as a consequence of slowing forest-cutting and to the progress of the mongoose eradication project. Our results appear to support this view. Variables adopted in the best model in the GLM analyses (Tables 2, 3) suggested that singing thrushes were abundant in older forest with less open habitat. Therefore, the recovery of forest conditions appears to be an important factor that led to the observed increase in the thrush population in recent years. Large-scale forestry (clear-cutting of large areas of mature forests) peaked in the early 1970s and thereafter decreased (Sugimura Reference Sugimura1988), so forest condition may have become favourable for the Amami Thrush from the early 2000s to the present. Relative mongoose density was also adopted as a variable in the best models in 2008–2011 (Table 3), indicating that the mongoose likely influenced thrush abundance when its density was relatively high before the early 2010s (see Figure 3).
It is important to note that the topography of surroundings may have a possible effect on the detectability of song recognised by researchers. Although many studies have conducted population estimates using sound distance sampling (e.g. Marques et al. Reference Marques, Thomas, Martin, Mellinger, Ward, Moretti, Harris and Tyack2013), the effect of topography on the song detectability is poorly understood. Because local topography could cause small-scale variation in sound attenuation in a landscape, we considered that the effect of topography on estimates on the whole island is limited. However, careful attention should be paid when we discuss the effect of variables on the model selection in the present study. Songs may attenuate more rapidly in an environment with steep topography in mountain area with higher altitude. Therefore, it is possible that we underestimated the significance of some variables such as the ground ruggedness and the mean height above sea level. Although these effects should be taken into account, nonetheless the variables adopted in most models (mean forest age and relative density of mongoose) are considered to have an actual effect on the distribution of the Amami Thrush and are therefore important for the present estimates.
The estimated numbers of the singing males were 945–1,858 before 2012, followed by a sudden increase to 2,512 in 2013 (Figure 4). The increase in singing thrushes recorded in 2013 (Table 1) is reflected in this sudden increase in the estimate, although the population parameters affecting this increase is not clear. Compared to the present estimates, past studies estimated extremely small population sizes (a few more than 58 birds in 1996: AOC 1997, 74 pairs in 1999: Khan and Takashi Reference Khan and Takashi2006, 50–100 breeding pairs in early 2000s: MOE 2002). Although population recovery did occur, these numbers from past studies may be underestimates because those studies estimated population size based on a limited research effort and did not consider the possibility of thrush presence in areas where no research was conducted (see Introduction). The present estimation improved these insufficiencies: we attempted to conduct the PC survey at as many points as possible and also considered song detectability based on the intensity of research effort by applying the distance sampling method. We believe that these improvements enabled more accurate population estimates for the Amami Thrush.
This study shows that the Amami Thrush has increased in number from the early 2000s to the present and now has a stable or increasing population due to the recovery of forest conditions and a decrease in the mongoose population. This result is good news for this island endemic bird, which was formerly regarded as ‘Critically Endangered’ (BirdLife International 2000). However, it is always necessary to keep in mind that island birds are susceptible to catastrophes, such as severe storms and disease epidemics (MOE 2014). Population monitoring is therefore essential for the conservation of island birds like the Amami Thrush. The song-count survey managed by the AOC with public participation has been conducted since 1994; this survey is one of the most ideal approaches for monitoring the thrush population. This survey also has important implications for raising awareness and increasing environmental education among the inhabitants of the island. We intend to continue this public participation survey for many years as a mean of promoting further conservation of the Amami Thrush. Also to conserve the Amami Thrush, we would like to stress that old-aged forests are an important habitat and should be maintained and the mongoose eradication project must be completed.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S095927091600023X
Acknowledgements
We are most grateful to all of the volunteers who participated in the Amami Thrush song-count survey. We are also grateful to the leading members of the AOC and the Rangers of AWCC for their management of the survey. Part of this study was conducted under the Protection/Propagation Program (PPP) for the Amami Thrush that was formulated by the Ministry of the Environment of the Japanese Government. Special thanks are due to members of the PPP Committee, namely S. Hattori, K. Ishida, N. Ishii, H. Sakamoto, F. Yamada, and M. Yokota, for their useful suggestions on the research. We are intellectually indebted to K. Ishida, who gave us insightful comments on the early manuscript.









