Introduction
Post-fledging survival is a key demographic gap in our knowledge of the life-history of many bird species (Cox et al. Reference Cox, Thompson, Cox and Faaborg2014). Whilst there are some exceptions to this (reviewed by Cox et al. Reference Cox, Thompson, Cox and Faaborg2014), the movements and habitat use of individuals post-fledging – after they leave the nest but before they disperse – are generally poorly studied, largely due to the difficulties of following small and mobile individuals in complex habitats once they have left their natal environment. Post-fledging survival can be estimated indirectly, either by ring recovery (Thomson et al. Reference Thomson, Baillie and Peach1999) or mark-recapture, but dispersal in first year birds is often very high and thus both these methods can underestimate survival (e.g. Gilroy et al. Reference Gilroy, Virzi, Boulton and Lockwood2012), and often do not allow separation of post-fledging mortality from mortality over a longer period (i.e. over-winter). However, estimates of post-fledging survival suggest this can be very low, and can therefore have a large influence on juvenile survival rate and subsequent recruitment to the breeding population (Cox et al. Reference Cox, Thompson, Cox and Faaborg2014).
Post-fledging mortality is mainly attributed to predation or exposure (Greño et al. Reference Greño, Belda and Barba2008; Davis and Fisher Reference Davis and Fisher2009; Hovick et al. Reference Hovick, Miller, Koford, Engle and Debinski2011). Fledglings may be an easy target for predators through reduced agility or lack of predator awareness (Baker et al. Reference Baker, Molony, Stone, Cuthill and Harris2008), and may be less able to deal with adverse weather conditions or other challenging environmental conditions due to inferior body condition or less efficient foraging abilities. Fledglings may have poor foraging skills or be unable to compete for a sparse food supply (Hetmański Reference Hetmański2007): indeed, prolonged parental care can substantially increase survival (Grüebler and Naef-Daenzer Reference Grüebler and Naef-Daenzer2010), but the provision of optimal parental care may not be possible for multi-brooded species (Grüebler and Naef-Daenzer Reference Grüebler and Naef-Daenzer2008).
Knowledge of factors affecting post-fledging survival is crucial for species of conservation concern, where habitat management may improve survival at this, and other life stages (Cox et al. Reference Cox, Thompson, Cox and Faaborg2014). Nesting and post-fledging habitat requirements may be similar for some species (e.g. Berkeley et al. Reference Berkeley, McCarty and Wolfenbarger2007), whereas for others, habitat that is good for nest survival may be poor for post-fledging survival (Shipley et al. Reference Shipley, Murphy and Elzinga2013). Fledglings may also require distinct habitats for shelter and foraging. Where this is the case, conservation management has the potential to provide habitats in close proximity, essential as birds can be reluctant to cross habitat gaps post-fledging (Desrochers and Hannon Reference Desrochers and Hannon1997).
Conditions in the nest have been linked to post-fledging survival across a range of species (Mitchell et al. Reference Mitchell, Guglielmo, Wheelwright, Freeman-Gallant and Norris2011). Heavier nestlings (Suedkamp Wells et al. Reference Suedkamp Wells, Ryan, Millspaugh, Thompson F and Hubbard2007), or those in better condition prior to fledging (Vitz and Rodewald Reference Vitz and Rodewald2011), often have higher post-fledging survival (Cox et al. Reference Cox, Thompson, Cox and Faaborg2014). It may be that body condition influences flight performance, whereby individuals in poorer body condition fly more slowly or are less agile and thus more susceptible to predation (Naef-Daenzer and Grüebler Reference Naef-Daenzer and Grüebler2008).
The European Turtle Dove Streptopelia turtur (hereafter, Turtle Dove) is in rapid decline across its European range (-78% 1980–2013; PECBMS 2015), with some of the fastest declines on the edge of its breeding range in the UK (-96% 1970–2012; Hayhow et al. Reference Hayhow, Conway, Eaton, Grice, Hall, Holt, Kuepfer, Noble, Oppel, Risely, Stringer, Stroud, Wilkinson and Wotton2014) and it is classified as ‘Vulnerable’ throughout Europe (‘Near Threatened’ within the EU27 countries) following a recent assessment (BirdLife International 2015). Within the UK, the species is classified as a farmland specialist although elsewhere is its range it is often associated with open woodland and forest borders with outlying wooded features (Cramp and Perrins Reference Cramp and Perrins1994). Nest survival has been monitored in two previous detailed autecological studies in the UK (Murton Reference Murton1968; Browne and Aebischer Reference Browne and Aebischer2004), but little is known about survival or habitat use post-fledging. Nestlings are thought to make their first excursions from the nest at 15–16 days old, although captive birds are capable of flight at 11–12 days, and parents are said to feed young until 28–30 days of age (Cramp and Perrins Reference Cramp and Perrins1994). A study of the congeneric Collared Dove Streptopelia decaocto found high post-fledging survival (61 ± 8 % during the first 13 weeks post-fledging; Eraud et al. Reference Eraud, Jacquet and Legagneux2011). However, Collared Doves are more likely to utilise anthropogenic habitats and resources whereas Turtle Doves rely more on agricultural or semi-natural habitats: within-species, Cohen and Lindell (Reference Cohen and Lindell2004) found post-fledging survival to be lower in agricultural habitats.
Here, we obtain the first estimates of post-fledging survival for this rapidly declining species, examining changes in daily survival rates and causes of mortality during the weeks following tagging in the nest. Second, through use of radio-tags and remote tracking loggers, we determine the duration of use of nest-site habitat, and assess ranging distances. Third, we examine habitat use by fledglings to determine which broad habitat types are important during this under-studied period of life history. Finally, we examine how foraging habitat around the nest may affect nestling growth and condition, and how nestling growth and condition may, in turn, affect post-fledging survival in order to inform conservation management.
Methods
Study sites and field methods
Data were collected at four farmland sites in the East of England, UK, during June–September 2014. Turtle Dove nests were located by cold-searching of suitable habitat and tracking of radio-tagged adults, as described by Stockdale et al. (Reference Stockdale, Dunn, Goodman, Morris, Sheehan, Grice and Hamer2015).
Once found, nests were visited every 3–4 days and the contents recorded; where adults were radio-tagged, nests were also monitored by deployment of an automated tracking station (DataSika Data Logging Receiver, Biotrack, UK) with an omni-directional aerial positioned within 5 m of the nest. The tracking station was configured to scan for the frequency of each radio-tag every 60 s continuously throughout the day and night. Field tests suggested that all adult tags and all but two nestling tags (with a lower range) were detected up to c.20 m from the tracking station. At five and seven days old, at the same time of day (± 1 hour), nestlings were weighed using a digital balance (± 0.1 g; Satrue, Taiwan) and standard morphometrics taken (minimum tarsus length and head-beak length; ± 0.1 mm; Redfern and Clark Reference Redfern and Clark2001). At seven days old, 15 nestlings from eight nests were tagged with 0.9 g radio-tags (tagging date range: 27 May– 2 August; median: 16 July; three first broods, three second broods, one third brood (single nestling), one unknown), with an intended battery life of up to five weeks, a line-of-sight range of 3 km and a ground-ground range of 300 m. Tags were attached to soft leather (0.8 mm thickness) leg rings using cotton secured with cyanoacrylate glue. Leg rings were sewn shut with cotton but were not further secured with cyanoacrylate, so the leg rings degrade and detach from the birds well before migration. Total weight of tag and leg ring was 1.2 g. Nestlings were tagged at seven days old when they met a minimum weight of 50 g (mean ±SE: 66.5 ± 3.5 g at tagging) but did not leave the nest until at least three days after this. As part of a separate experiment, the results of which are not reported here, one nestling from each brood was medicated with 2.5 mg carnidazole (Spartrix, Petlife Harkers, Suffolk, UK) under Home Office licence to reduce infection by Trichomonas parasites (Thomas et al. unpubl. data). We acknowledge that this treatment is likely to lead to an overestimate of the current population average post-fledging survival rates.
Monitoring of tagged birds
Fourteen nestlings from seven nests were monitored in the vicinity of the nest using an automated tracking station, as described above for adults (one single nestling in the eighth nest was not monitored due to limited availability of tracking stations). Whilst for some birds it was possible to distinguish from the signal strength whether the bird was on the nest or moving around nearby, this was not possible for two lower-powered tags so we did not distinguish between these two states within our analyses.
Once tagged, all 15 birds were relocated daily during the first week and then until they were recovered dead, the battery on their tag ran out or they left the area. Birds were initially triangulated, and if found in the same place on two consecutive days, were sighted to confirm they were still alive. When a bird was found dead, we examined crop contents and the oesophageal tract to rule out trichomonosis as the cause (where the crop would be empty and yellow caseous lesions present in the oesophagus; Stockdale et al. Reference Stockdale, Dunn, Goodman, Morris, Sheehan, Grice and Hamer2015), and examined the body for any signs of predator activity. We assumed a bird to have been killed by a predator when either the transmitter or metal leg ring was found on a severed leg, with body remains, or surrounded by cut or chewed feathers. In two cases the body was submitted to the Garden Wildlife Health Initiative (GWH; ZSL, UK) for gross necropsy.
Habitat use and foraging distances
We mapped field-scale habitat within 3 km of each nest and classified this into four broad categories designed to differentiate between habitat structure and seed availability: cereals; non-cereal arable break crops; seed-rich (including semi-natural grassland, low-intensity (mostly horse) grazing, quarries and fallow), and other largely unsuitable habitats (amenity grassland, woodland, hay and silage crops). Whilst hay meadows were historically used as foraging sites by turtle doves (Murton et al. Reference Murton, Westwood and Isaacson1964), those within our study area tended to contain tall and dense vegetation making them unsuitable for foraging turtle doves (Browne and Aebischer Reference Browne and Aebischer2003).
We obtained more than one foraging fix (where a foraging fix is defined as a relocation of a bird >50 m from its nest site) for eight of the radio-tagged nestlings. All foraging fixes (n = 113) were assumed to have an accuracy of ± 50 m based on re-sightings and calibrations from nest observations. Thus, we calculated the composition of utilised habitat from a 50 m radius of each foraging point using the ‘gIntersection’ command in the ‘rgeos’ package (Bivand et al. Reference Bivand, Rundel, Pebesma and Hufthammer2014) in R (R Core Team 2014).
First, we calculated mean foraging distance across all nests during each week post-tagging. There was a clear break point in mean foraging distances between weeks 1–3 and weeks 4–7, so we analysed habitat selection within these two time periods separately. During weeks 1–3, foraging distances were relatively small so we used a circle with radius 329 m (95th percentile of foraging distances during this period), centred on the nest site, to estimate available foraging habitat. Subsequently, we used a circle of radius 2.92 km (95th percentile of foraging distances during this period) to represent habitat available 4–7 weeks post-tagging.
Statistical analysis
All statistical analyses were carried out in R version 3.1.2 “Pumpkin Helmet” for Mac (R Core Team 2014).
Post-fledging survival
We calculated mean daily survival rate for the first four weeks post-tagging. Where birds were lost from follow-up due to radio-tag battery failure (radio-tag had given weak and intermittent signal for five days prior) or were known to have left the area (last located ∼6 km from nest site) (n = 2, at 26 and 31 days post-tagging, respectively), we assumed survival. Mean daily survival was calculated as 1 – (number of deaths / number of bird days monitored) for each week separately (Heisey and Fuller Reference Heisey and Fuller1985).
To establish any linear temporal trends in foraging distance and proportion of time spent at nest, we constructed two linear mixed-effect models using the ‘lmer’ and ‘glmer’ functions within the ‘lme4’ package (Bates and Maechler Reference Bates and Maechler2009). Response variables were the distance from the nest of each separate foraging trip (log transformed) within a linear mixed-effects model (LMM), and the proportion of each day (using a simple proportion) for which each bird’s radio-tag was detected in the vicinity (within ∼20 m) of the nest (using a binomial generalised linear mixed-effects model; GLMM). Within each model we specified nested random effects of bird within nest to account for non-independence of multiple foraging points from individual birds, and multiple birds from the same nest. We established the significance of any trends over time by comparing models with and without nestling age (in weeks) designated as a fixed factor, using F statistics (LMM) and χ2 statistics (GLMM) to determine significance.
We used residuals from a linear regression of body mass on tarsus length at seven days old to give an index of condition (Labocha and Hayes Reference Labocha and Hayes2012). To determine whether body condition or weight at seven days influenced survival within 30 days post-tagging, we constructed two binomial GLMMs with status at the end of the time period (alive = 1 or dead = 0) as the response variable. Nest ID was designated as a random effect to control for non-independence of siblings, and we included day of tagging as a continuous covariate to control for season differences in survival. We examined whether or not 95% confidence intervals of model parameter estimates overlapped zero as an indication of statistical significance.
Foraging habitat selection
As previously described, we separated foraging analyses into weeks 1–3 (n = 8 fledglings; 44 foraging locations) and weeks 4–7 (n = 6 fledglings; 69 foraging locations) post-tagging. To compare available habitat (as defined above) with used habitat within our four broad habitat categories for each time period, we used the ‘compana’ function in the ‘adehabitatHS’ package (Calenge Reference Calenge2006) to perform a compositional analysis of habitat use (Aebischer et al. Reference Aebischer, Robertson and Kenward1993). This analysis assumes independence of data points, which is violated with our data where we potentially have two data points per nest. However, we found no evidence of siblings foraging together and we had movement data from multiple fledglings for only two nests at 1–3 weeks and one nest at 4–7 weeks; thus we treat nest-mates within our sample as independent. We expressed habitat categories for each fledgling as a proportion of the total used or available area, respectively, with totals summing to 1. We then replaced any zero values in the matrix with 0.0001: zero values can bias the test as log-ratio differences cannot be computed. As the arbitrary quasi-zero value selected can also influence results, we repeated our analysis with two additional values (0.001 and 0.00001) to confirm the consistency of our results. First, we tested the significance of habitat selection using a Wilks’s lambda test. If habitat selection was significant, habitat types were ranked independently of availability according to the number of positive differences between pairs of habitat types.
Foraging habitat and survival
To assess whether available foraging habitat influenced metrics of fledging survival (nestling weight at seven days, nestling condition at seven days and survival after 30 days), we constructed one binomial GLMM (status at end of monitoring period) and two LLMs (weight and condition, with Gaussian error structure). As we found birds selecting seed-rich habitat for foraging during the first three weeks post-tagging, we used the proportion of seed-rich habitat within available foraging habitat as a predictor variable. Nest ID was designated as a random effect to control for within-nest variation and parental quality. We examined whether or not 95% confidence intervals predicted from the model overlapped zero as an indication of statistical significance.
Results
Fifteen birds were radio-tagged in the nest of which 11 fledged successfully. Four nestlings from three nests were found dead and had not been seen > 2 m away the nest whilst alive; we suspect these were predated at or around the time of fledging. One nestling dropped its tag at fledging: whilst circumstantial, a ringed but untagged young bird was seen subsequently, foraging with the radio-tagged adult from this nest, suggesting this bird survived; however, we do not consider this individual any further. A further four nestlings were found dead post-fledging. All four nestlings were thought to have been predated by a mammal, based on location of carcasses (usually underneath dense vegetation), and the presence of chewed feathers; GWH confirmed this in both cases submitted to them. We ruled out post-mortem scavenging following death from other causes for two reasons: three carcasses were intact and did not appear to have been stashed by a predator, suggesting scavenging had not occurred; two of these were submitted to GWH to rule out other causes of death. In the fourth case, where the carcass had been dismembered, the bird had been sighted and appeared healthy and active the previous day. Seven nestlings were followed until either the battery on their radio-tag ran out, they left the area, or monitoring ceased (during the first week of September).
Cumulative post-fledging survival for 14 Turtle Dove nestlings is displayed in Figure 1, with a post-fledging survival estimate within our population of 42.9 % over 35 days. This assumes that two nestlings for whom monitoring ceased before 35 days had elapsed since tagging (one whose radio-tag ceased to function but was last detected foraging in a farmyard 1.0 km from its nest site 26 days post-tagging, and one that was detected 6.2 km away from its nest site at the beginning of September, 31 days post-tagging) both survived to this point. During the first three weeks post tagging, mean daily survival rates were 0.989, 0.944, and 0.940, respectively, levelling out after this point (Figure 1).
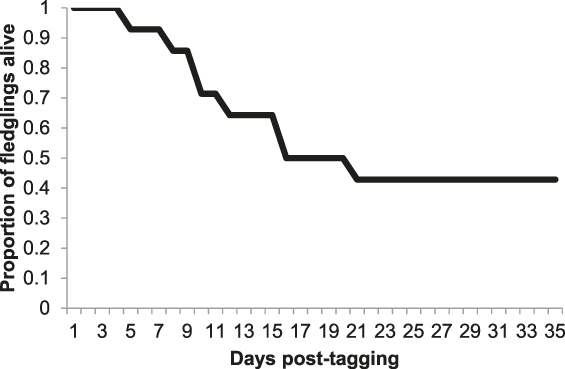
Figure 1. Cumulative post-fledging survival for 14 Turtle Dove fledglings up to 35 days post-tagging.
From the 10 birds confirmed to have left the nest successfully together with their radio-tag, between six and 44 radio-tag relocations were triangulated (mean ± SE: 23.40 ± 4.94 relocations). These birds were followed for between 13 and 49 days (29.9 ± 3.31 days) until they were either recovered dead, the battery on their tag ran out, or they left the area. As nestling age increased, the distance of foraging points from the nest increased (LMM, χ2 = 56.736, P < 0.001; Figure 2) and the amount of time spent in the vicinity (within ∼20 m) of the nest decreased (GLMM, χ2 = 92.29, P < 0.001; Figure 3).
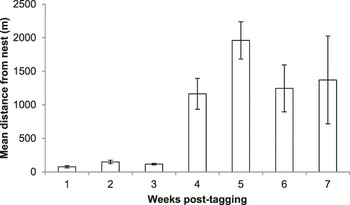
Figure 2. Mean ± SE foraging distances of radio-tagged Turtle Dove fledglings up to seven weeks post-tagging.
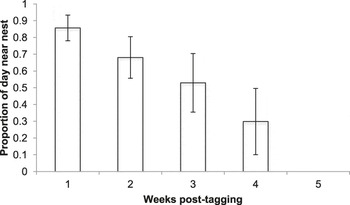
Figure 3. Mean ± SE percentage of time spent in the vicinity (within ∼20 m) of the nest by Turtle Dove fledglings up to five weeks post tagging.
During the first three weeks post-tagging, foraging distances remained relatively short, with a mean ± SE foraging distance of 127 ± 84 m from the nest (based on 44 foraging triangulations; Figure 2). During this period, fledglings remained in the vicinity (within ∼20 m) of the nest for over 50% of the time (Figure 3). Comparison of selected foraging habitats with those available suggested significant habitat selection (Wilks’s λ = 0.22, P = 0.02), with seed-rich habitats being preferentially selected and non-cereal arable break crops avoided (Figure 4a).
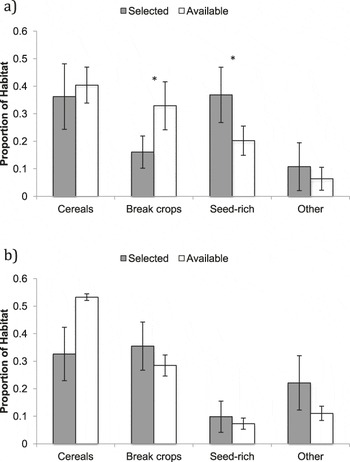
Figure 4. Habitat available to and selected by Turtle Dove fledglings at a) 1–3 weeks and b) 4–7 weeks post-tagging. * indicates selection at P < 0.05.
During weeks 4–7 post-tagging, foraging locations became further from the nest, being on average 1,440 ± 60 m away (based on 69 foraging triangulations; Figure 2). Datalogger data suggested that by this stage, fledglings had largely abandoned the nest vicinity (Figure 3). There was no evidence for significant habitat selection (Wilks’s λ = 0.56, P = 0.67), although there was a non-significant avoidance of cereals (Figure 4b).
Heavier nestlings at seven days old, and those in better body condition, had an improved chance of survival to 30 days post-tagging (weight χ2 1 = 11.94, P < 0.001, Figure 5a; condition χ2 1 = 10.81, P = 0.001, Figure 5b). Skeletal body size did not influence survival (tarsus length: χ2 1 = 0.27, P = 0.60; mean ± SE survived: 18.40 ± 0.34; died: 17.56 ± 0.46 mm).
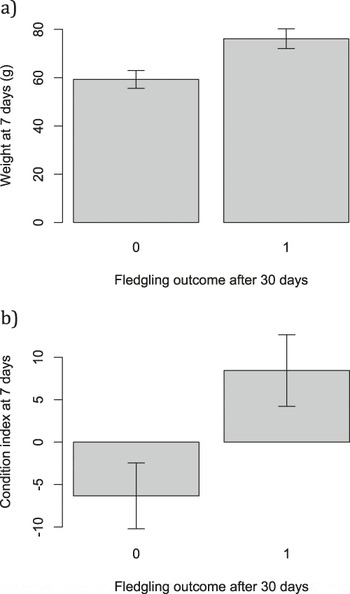
Figure 5. Mean ± SE a) fledgling weight and b) fledgling body condition for fledglings that did and did not survive 30 days post-fledging.
The proportion of available seed-rich habitat was associated with both nestling weight (L. ratio4,5 = 8.60, P = 0.003; Figure 6a) and condition at seven days old (L. ratio4,5 = 6.99, P = 0.008; Figure 6b). There was some indication of an association between seed-rich habitat and survival to 30 days post-fledging (Figure 6c), although this fell short of statistical significance (χ2 1 = 1.93, P = 0.16).
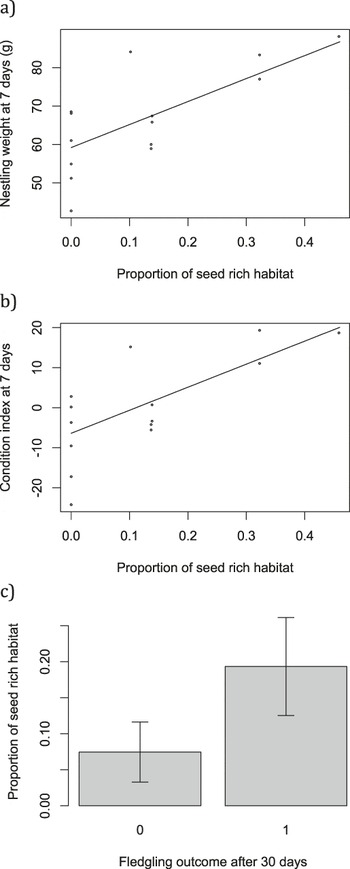
Figure 6. a) nestling weight and b) nestling condition were associated with the proportion of seed rich habitat. Points show raw data and lines are predicted from the minimal model with median value for day of tagging (23: 19 July); c) mean ± SE proportion of available seed-rich habitat for fledglings that did and did not survive 30 days post-fledging.
Discussion
We provide the first empirical estimates of survival during the post-fledging period – between leaving the nest and dispersing - in the rapidly declining Turtle Dove. We find evidence that seed-rich habitat influenced both nestling weight and condition, which in turn positively influenced survival to 30 days. Juveniles selected seed-rich habitat near to the nest during the first three weeks post-fledging, highlighting the importance of a combination of suitable nesting and foraging habitat in close proximity.
During the early post-fledging period, we found evidence for avoidance of non-cereal arable break crops and selection of seed-rich foraging habitats, comprising fallows, semi-natural grasslands, quarries and low-intensity grazing (mostly horses). Many of these semi-natural/low intensity grazing grasslands and fallows are eligible for payments under agri-environment schemes in England and elsewhere in Europe. Sward structure in these habitats tends to be patchy, with areas of bare ground, similar to habitats favoured by foraging adult Turtle Doves (Browne and Aebischer Reference Browne and Aebischer2003). Given the prevalence of oil seed rape (OSR) in nestling Turtle Dove diet from a previous study (Browne and Aebischer Reference Browne and Aebischer2003), the avoidance of break crops by fledglings is surprising. However, the dense structure of OSR crops prior to harvest is likely to render seeds inaccessible, especially to relatively inexperienced flyers and it is possible that OSR seeds in both studies were being taken from sources other than the standing crop (e.g. from spillages in farmyards, or supplementary feeding of game or wild birds). A formerly important source of spilled seed, crop stubbles after harvest, may have continued to decline in suitability due to more efficient combine harvesters and the short duration of stubbles, many of which are sprayed and tilled soon after harvest in preparation for the next crop. Fledglings left the vicinity (within ∼20 m) of the nest around four weeks post-fledging, when recorded distances from the nest became larger. We found no foraging habitat selection during this time, but as we did not distinguish between foraging habitats and those used for shelter, this is not surprising: during this later period multiple sites were used for shelter, unlike in the early period when the nest area was used. Habitats providing shelter and those used for foraging are likely to be distinct as foraging habitats tend to be open (Browne and Aebischer Reference Browne and Aebischer2003), whereas sheltering habitats are likely to be formed from large hedgerows, woodland and scrub.
Nestling weight and condition at seven days of age (approximately a week prior to fledging) significantly influenced the likelihood of survival until 30 days after this. This corresponds to previous studies (Suedkamp Wells et al. Reference Suedkamp Wells, Ryan, Millspaugh, Thompson F and Hubbard2007, Mitchell et al. Reference Mitchell, Guglielmo, Wheelwright, Freeman-Gallant and Norris2011, Vitz and Rodewald Reference Vitz and Rodewald2011): it may be that individuals in poorer body condition or with lower energy reserves move more slowly and thus are more susceptible to predation (Naef-Daenzer and Grüebler Reference Naef-Daenzer and Grüebler2008), or that birds in poorer condition prioritise foraging over vigilance. Furthermore, nestling weight and condition were both strongly influenced by the proportion of seed-rich habitat available near the nest, highlighting the importance of providing a combination of suitable dense nesting cover and seed-rich foraging habitat (Dunn et al. Reference Dunn, Morris and Grice2015a) in close juxtaposition for Turtle Doves, via agri-environmental measures or other means. In the English Countryside Stewardship agri-environment scheme, with agreements starting from 1 January 2016, a management package for Turtle Doves will recommend this combination of habitats with a maximum separation of 300 m.
Our data suggest post-fledging survival rates of 42% during the first 35 days post-tagging in this rapidly declining species, towards the lower-mid range of 23% to 87% survival during the first three weeks post-fledging reported by Cox et al. (Reference Cox, Thompson, Cox and Faaborg2014) in passerines and below the higher 61% survival rate during the longer 13 weeks post-fledging for sympatric Eurasian Collared Doves (Eraud et al. Reference Eraud, Jacquet and Legagneux2011). It is possible that the rates in our study may be an over-estimate of population-average survival as half the nestlings in our dataset were medicated to treat the parasite Trichomonas gallinae, widespread within our study population and a known cause of nestling mortality (Lennon et al. Reference Lennon, Dunn, Stockdale, Goodman, Morris and Hamer2013, Stockdale et al. Reference Stockdale, Dunn, Goodman, Morris, Sheehan, Grice and Hamer2015) so we recommend that this figure be treated with some caution. However, we do not anticipate the treatment influencing subsequent post-fledging behaviour. Survival was lowest during the first three weeks post-fledging, where birds made only short forays from the nest site. This is similar to post-fledging behaviour in passerines, where the highest mortality is during the first three weeks post-fledging (Cox et al. Reference Cox, Thompson, Cox and Faaborg2014) and dispersal from the nesting area starts around the 3rd week post-fledging (Kershner et al. Reference Kershner, Walk and Warner2004), but seemingly earlier dispersal than for Eurasian Collared Doves, which have a larger initial exploratory range (∼500 m) and disperse at around 38 days after fledging (Eraud et al. Reference Eraud, Jacquet and Legagneux2011).
All post-fledging mortality was attributed to mammalian predation, consistent with previous studies of post-fledging survival (Greño et al. Reference Greño, Belda and Barba2008, Davis and Fisher Reference Davis and Fisher2009, Hovick et al. Reference Hovick, Miller, Koford, Engle and Debinski2011). Potential mammalian predators in our study area include stoat Mustela erminea, least weasel Mustela nivalis, red fox Vulpes vulpes, brown rat Rattus norvegicus and domestic cat Felis catus. Three of the four oldest predated nestlings that were located had not been significantly damaged or eaten, and domestic cats (and no other potential mammalian predators) had either been observed or caught on Bushnell Trophy Cam camera traps (Bushnell, Kansas City, MO) placed within 20 m of the nest as part of separate monitoring work. A study of Collared Doves attributed approximately half of predation events to domestic cats (Eraud et al. Reference Eraud, Jacquet and Legagneux2011). Some studies of the population dynamics of birds in urban areas indicate that cats are a significant predator of birds (Baker et al. Reference Baker, Molony, Stone, Cuthill and Harris2008), although their role as predators in the wider countryside in Europe is largely unknown (but see Woods et al. Reference Woods, Mcdonald and Harris2003).
Overall, our data highlight the importance of breeding habitat in close proximity to good foraging habitat (Dunn et al. Reference Dunn, Hamer and Benton2015b). This may be especially important early in the season when adults re-nest rapidly after nestlings fledge, sometimes starting to build the next nest while feeding nestlings in the first nest (Dunn et al. unpubl. data). The small ranging distances of birds during the first three weeks post-fledging, along with the large proportion of time spent in the vicinity (within ∼20 m) of the nest site, suggest that recently fledged young either don’t have the ability to forage over large areas or don’t need to. The strong impact of nestling weight and condition on subsequent survival, along with the influence of seed-rich habitat on both these metrics, also highlights the importance of good foraging habitat available to foraging adults while feeding young. We suggest that habitat management to improve post-fledging survival in this species should focus on providing a combination of suitable foraging and nesting sites in close proximity. Further research should examine potential impacts of food quality, as well as quantity, on nestling growth and subsequent survival post-fledging, as well as examining the relative contributions of survival at this, and other points in the annual cycle.
Acknowledgements
Thanks to Alexander Ball, Kerry Skelhorn and Rebecca Thomas for assistance with ringing and radio-tagging nestlings, and to the aforementioned as well as Emma Cutten, Connie Miller and Louis O’Neill for help monitoring nests and radio-tracking nestlings post-fledging. This work was jointly funded by RSPB and Natural England under the AfBiE (Action for Birds in England) partnership. Nestlings were ringed and tagged under licence from the British Trust for Ornithology following approval from the Unconventional Marks Panel.








