Introduction
One potential conservation strategy is to select sites with a large number of species deemed at risk (e.g. Wege and Long Reference Wege and Long1995). There are many different ways one can assess ‘risk’ in a conservation context. Ideally, one would be able to conduct detailed studies of species’ behaviours, life histories, trophic linkages and autecological requirements, and integrate such knowledge into population models that could be used for risk assessment. In many situations, such intensive studies have not been conducted, and there is no guarantee that they will be, at least over the short time-scale that is relevant for conservation planning and decision-making in the face of rapid environmental change and degradation. An alternative approach that may be useful is to place local conservation assessment into a broader geographical context (e.g. Pimm and Raven Reference Pimm and Raven2000, Harris and Pimm Reference Harris and Pimm2004, Reference Harris and Pimm2008).
The total number of endemic species found in a site has been used to select priority areas for conservation (Fishpool et al. Reference Fishpool, Heath, Waliczky, Wege, Crosby, Adams and Slotow1998, Heath and Evans Reference Heath and Evans2000). Indeed, this was one of the original criteria developed by BirdLife International over 20 years ago, in order to select priorities for conservation at a global scale. Areas with high numbers of endemic species with overlapping distributions are denoted by BirdLife International as Endemic Bird Areas (EBAs), sites within them being selected as potential Important Bird Areas (IBAs) for conservation. IBAs were recently selected for all the states of the Brazilian Atlantic forest (Bencke et al. Reference Bencke, Mauricio, Develey and Goerck2006).
Vulnerability to fragmentation may also be affected by the position of a population within the geographical range of a species. Because populations at the edge of the range are generally smaller than those closer to the centre (Holt et al. Reference Holt, Keitt, Lewis, Maurer and Taper2005) and may have fewer nearby sources of colonists to rescue vacant patches, populations near the edges of their ranges may be more vulnerable to fragmentation (Lomolino and Channell Reference Lomolino and Channell1995, Reference Lomolino and Channell1998). Data from several taxa around the world (both animals and plants), however, do not always show a pattern of higher persistence of species near the centre of their ranges, although these analyses have occurred at a global rather than a local scale (Lomolino and Channell Reference Lomolino and Channell1995, Reference Lomolino and Channell1998; Channell and Lomolino Reference Channell and Lomolino2000a). Thus, it is not clear whether at a local scale, species may be more or less susceptible to fragmentation at the edges of their ranges.
In this study we examined whether species endemic to the Atlantic forest and those with small geographic ranges are more vulnerable to forest fragmentation, a particularly severe problem in that biome (Torezan Reference Torezan2003), using previously published data from forest fragments scattered throughout northern Paraná state, southern Brazil (Anjos et al. Reference Anjos, Zanette and Lopes2004, Anjos Reference Anjos2006). We tested sensitivity to fragmentation in the endemic species, taking into account their estimated range sizes. We also determined whether species at the periphery of their ranges are more vulnerable to habitat fragmentation than are non-endemic species closer to the centre of their geographic ranges within the same, highly disturbed landscape.
Methods
Study area and bird surveys
The effects of forest fragmentation on birds in northern Paraná have been investigated for several years (Anjos Reference Anjos2001a,Reference Anjos, Albuquerque, Cândido, Straube and Roosb, Reference Anjos2006, Anjos et al. Reference Anjos, Zanette and Lopes2004). In that landscape, where the matrix is composed mainly of agriculture and pasture, most remaining forest occurs in small, discrete patches. In a 50,000 km2 region around the city of Londrina, for example, there are only 17 forest fragments larger than 100 ha (Torezan Reference Torezan2003). Deforestation in this region mostly occurred in the last 60–70 years. The largest protected area among those fragments is Mata dos Godoy State Park (656 ha). In spite of this severe level of fragmentation, which is comparable to habitat loss documented in agricultural sections of the midwestern USA (Brawn and Robinson Reference Brawn and Robinson1996), several Atlantic forest endemics persist, even in quite small and isolated fragments. Indeed, some endemic species actually occur in higher abundances in smaller than in larger fragments (Anjos Reference Anjos2001a), whereas others are found only in larger fragments or their abundances are significantly lower in smaller and isolated fragments (Anjos Reference Anjos2001a). Anjos (Reference Anjos2006) used the data presented in Anjos et al. (Reference Anjos, Zanette and Lopes2004) to assign bird species to three levels of sensitivity to fragmentation (high, medium, and low). The levels of sensitivity for each bird species were based on point censuses of abundance in Mata dos Godoy State Park and two other larger forest fragments, which were used as reference sites. Eleven other forest fragments were categorized according to size (large: 180–570 ha or small: <180 ha) and degree of isolation (not isolated: connected by a forest corridor to another large fragment or when the distance to a fragment of similar size was less than 800 m; or isolated: >2,000 m from any other fragment of similar size). Bird species were assigned to one of three levels of sensitivity to forest fragmentation: (1) high, when the species occurred only in the reference fragments and in large, non-isolated patches; (2) medium, when the species occurred in the reference tracts, large and non-isolated tracts, small and non-isolated tracts, and large, isolated tracts, and (3) low, when the species occurred in all fragments, even in those that were small and isolated (see Anjos Reference Anjos2006 for details). We eliminated from the bird list presented in Anjos (Reference Anjos2006) a few species mostly found at forest edges as well as those that are known to occur in the matrix landscape (mostly agricultural areas and pastures); thus, we restricted the analyses presented here to 112 bird species that are fundamentally restricted to forests. A list of the bird species occurring in the forest fragments is given in the Supplementary materials.
Data analysis
We examined the number of species in each category of sensitivity in northern Paraná, contrasting Atlantic forest endemic versus non-endemic species. We used the list of endemic species presented in Bencke et al. (Reference Bencke, Mauricio, Develey and Goerck2006). In order to examine whether species at the edge of their ranges are more sensitive to forest fragmentation, we used the range maps based on del Hoyo et al. (Reference del Hoyo, Elliot and Sargatal1992–2006) and Ridgely and Tudor (Reference Ridgely and Tudor1994) and for each species categorized whether northern Paraná is close to the known border of its range. We considered northern Paraná as the border of a species' range if it was within the outer 10% of its distribution (Table 1).
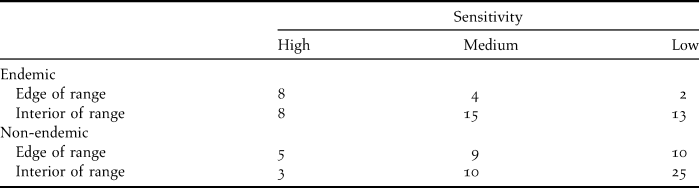
Table 1. Number of bird species classified into one of three categories of sensitivity to forest fragmentation in northern Paraná, Brazil, based on their endemism (endemic and non-endemic) and whether the fragmented studied landscape of northern Paraná was at the edge of their ranges or the interior.
Harris and Pimm (Reference Harris and Pimm2008) estimated the original range of endemic bird species of Atlantic forest. We used those estimated values to evaluate if highly sensitive species were more likely to have smaller ranges than species with medium and low sensitivity. We recognize, however, that these are actually upper limits on current species’ distributions, which can be a shrunken shadow of their former ranges (see Harris and Pimm Reference Harris and Pimm2008) given that the lowland Atlantic forest has been reduced to c. 4–6% of its original forest cover in the state of Paraná.
Statistical analysis
An RxC test for independence (G-test) was used to evaluate significance (α = 0.05) between number of species in different categories of sensitivity (high, medium, and low) and range categories (range-restricted and not range-restricted; Table 1). We evaluated the differences among the medians of the endemic species ranges in those categories of sensitivity using Mann-Whitney U-tests (α = 0.05).
Results
Our results show that endemic bird species were indeed more sensitive to forest fragmentation than non-endemics (G = 9.66, df = 2, P < 0.1). The endemic species Bertoni's Antbird Drymophila rubricollis and Dusky-tailed Antbird D. malura, for example, had high sensitivity to forest fragmentation, whereas Variable Antshrike Thamnophilus caerulescens and Rufous-winged Antwren Herpsilochmus rufimarginatus, non-endemics, had low sensitivity (Appendix 1). Median range size was significantly smaller in species with high sensitivity to fragmentation than those with medium sensitivity (U = 0.007); none of the other comparisons were significant.
Species at the edges of their ranges were also more sensitive to forest fragmentation (G = 6.45, df = 2, P < 0.05, Table 1). The Black-necked Aracari Pteroglossus aracari, which is at the southern border of its range in northern Paraná, for example, was more sensitive to fragmentation than were Red-breasted Toucan Ramphastos dicolorus and Spot-billed Toucanet Selenidera maculirostris, both of which were found further south down to northern Rio Grande do Sul and Argentina (Supplementary materials).
Combining information on both endemism and position in their geographic range, we found evidence for an interaction effect. Endemic species at the periphery of their geographic ranges were much more susceptible than non-endemic or endemic species in their range interiors. (Table 2). There was also a small, but statistically significant, effect of endemism on sensitivity for interior species (Table 2). This may explain why Spot-breasted Antvireo Dysithamnus stictothorax (an endemic species at the border of its range) is highly sensitive to forest fragmentation whereas its congener, Plain Antvireo D. mentalis (non-endemic and not at the periphery of its range) has low sensitivity (see Appendix S1, Supplementary materials).
Table 2. RxC test of independence (G-test; α = 0.05) of species and various combinations of endemism (endemic and non-endemic) and position in the species' ranges.

Discussion
We found that both range size and position within geographic range were significantly associated with vulnerability to forest fragmentation in Atlantic forest bird species. There are several possible reasons for these results. First, species at the edge of their range may have fewer nearby populations available to provide the immigrants necessary to rescue patches once they have lost their local populations. Second, there is often a broad correlation between local abundance and the extent of a species' range (Gaston Reference Gaston2003). All else being equal, if endemic species have low average abundance, they may be more vulnerable to a reduction in the area of suitable habitat. Holt et al. (Reference Holt, Lawton, Gaston and Blackburn1997) proposed a model of the relationship between range size and local abundance; one of the biological assumptions in this model was that some species have lower maximal growth rates. All else being equal, this can lead to a positive range-abundance correlation. A reduced maximal growth rate would mean there is a smaller demographic margin for error to help a species cope with unfavourable changes in its environment.
Some bird species that showed high or medium sensitivity to forest fragmentation in northern Paraná may not be as sensitive in other regions of the Atlantic forest. Swallow-tailed Manakin Chiroxiphia caudata and Plain Parakeet Brotogeris tirica, for example, are apparently much less sensitive to fragmentation closer to the centre of their ranges in São Paulo state than they are in Paraná (Uezu et al. Reference Uezu, Metzger and Vielliard2005). Channell and Lomolino (Reference Channell and Lomolino2000b) predicted that local extinctions would first occur in peripheral populations and then proceed to more central populations. Our results generally support this prediction, as species seem more likely to become extinct in small patches near the periphery of their range.
Geographic variation in sensitivity to forest fragmentation may also be related to local habitat suitability. The Atlantic forest is not a homogenous biome. It is composed of several types of forest; perhaps the seasonal semi-deciduous forests of northern Paraná and the dense ombrophilous forests of eastern São Paulo differ in their overall suitability for species such as C. caudata which has significantly higher populations in the latter forest type than the former (Anjos et al., in prep.). Low populations have been suggested as a factor that should increase sensitivity of species to habitat fragmentation (reviewed in Henle et al. Reference Henle, Davies, Kleyer, Margulis and Settele2004).
Our results suggest that both endemics and those at the edge of larger ranges might need larger tracts to ensure their continued existence within that region. Paradoxically, managers seeking to prevent the loss of biodiversity from their political units (e.g., those trying to preserve total diversity in the state of Paraná) might need to spend more resources on maintaining populations of species that barely make it into their geographic regions. State wildlife biologists face this problem routinely; should they worry more about populations of widespread species that are only marginally present within their geographic area, or should they spend more of their resources on those species for which their region offers prime habitat near the centre of their range? Fortunately, larger reserves benefit both endemic species and those at the periphery of large ranges. Collectively, these results point to the great importance of continuing to preserve the largest tracts of habitat within a political unit.
Nevertheless, it is also noteworthy that there are at least two examples of endemic species in the study that were not especially vulnerable to fragmentation, some of which were actually more abundant than their wider-ranging congeners in small fragments. Surucua Trogon Trogon surrucura and Rufous-capped Spinetail Synallaxis ruficapilla, both restricted-range species (Bencke et al. Reference Bencke, Mauricio, Develey and Goerck2006), tended to be more abundant in the study area and were more tolerant of forest fragmentation than were their wider-ranging congeners, Black-throated Trogon T. rufus and Grey-bellied Spinetail S. cinerascens. As predicted by Holt et al. (Reference Holt, Lawton, Gaston and Blackburn1997) there are some circumstances in which a positive range-abundance correlation is not observed at all. For such species, even smaller forest fragments may have considerable conservation value – especially if the species is able to use secondary/edge habitats in small patches where competitors and predators may be absent. Therefore, we should not ignore the value of preserving small fragments for the conservation of some endemic species such as Araucaria Tit-spinetail Leptasthenura setaria, which is strongly associated with forests found further south of the study area, but is locally abundant in some small patches (Anjos and Boçon Reference Anjos and Boçon1999).
In general, however, endemic species near their range limits seem particularly sensitive to habitat fragmentation. It would be instructive to look at this association in a wider context, as for example the entire Atlantic forest. Moreover, studies of global extinction risk (Purvis et al. Reference Purvis, Gittleman, Cowlishaw and Mace2000) have identified range size as one broad correlate of such risk. Our results suggest that the relationship between range attributes and conservation risk scale down to local landscape levels.
Acknowledgements
Financial support for this study was obtained from CNPq (Brazilian Council for Development of Science and Technology) through the Mata Atlântica Program and research grants for the first author (305593/07-2 and 201476/07-0). We thank the Instituto Ambiental do Paraná (IAP, Brasil) for permission to conduct research in the Godoy State Park, and the owners of private lands where the forest fragments are located. We also thank the three reviewers and Mike Barfield, whose suggestions improved the final version the manuscript, and the University of Florida Foundation for its support.
Appendix 1. Bird species grouped according to endemism to the Atlantic forest and levels of sensitivity to forest fragmentation. *indicates those species for which northern Paraná is near the edge of their ranges.
Endemic and high sensitivity: Tinamus solitarius; Brotogeris tirica*; Pionopsitta pileata; Triclaria malachitacea*; Pulsatrix koeniswaldiana; Pteroglossus bailloni; Campephilus robustus; Dysithamnus stictothorax*; Drymophila rubricollis*; Drymophila malura*; Terenura maculata; Hylopezus nattereri*; Campylorhamphus falcularius*; Hemitriccus obsoletus*; Hylophilus poicilotis; Euphonia pectoralis.
Non-endemic and high sensitivity: Micrastur semitorquatus*; Dromococcyx pavoninus*; Trogon rufus*; Nonnula rubecula*; Pteroglossus aracari*; Grallaria varia; Oxyruncus cristatus; Vireo olivaceus.
Endemic and medium sensitivity: Pyrrhura frontalis; Trogon surrucura; Baryphthengus ruficapillus; Ramphastos dicolorus; Selenidera maculirostris; Melanerpes flavifrons; Psilorhamphus guttatus*; Scytalopus indigoticus*; Dendrocincla turdina; Xiphorhynchus fuscus; Cranioleuca obsoleta*; Philydor lichtensteini; Mionectes rufiventris; Hemitriccus diops*; Chiroxiphia caudata; Schiffornis virescens; Turdus subalaris; Pyrrhocoma ruficeps; Saltator fuliginosus.
Non-endemic and medium sensitivity: Patagioenas speciosa*; Trogon viridis*; Dryocopus lineatus*; Chamaeza campanisona*; Sittasomus griseicapillus; Xiphocolaptes albicollis; Synallaxis cinerascens; Philydor rufum; Poecilotriccus plumbeiceps*; Colonia colonus; Sirystes sibilator; Tityra inquisitor*; Tityra cayana*; Pachyramphus validus; Cyanocorax chrysops; Pipraeidea melanonota; Dacnis cayna*; Cacicus haemorrhous*; Euphonia violacea.
Endemic and low sensitivity: Aramides saracura; Phaetornis eurynome; Picumnus temminckii*; Veniliornis spilogaster; Piculus aurulentus; Hypoedaleus guttatus; Mackenziaena severa; Pyriglena leucoptera; Conopophaga lineata; Synallaxis ruficapilla; Automolus leucophthalmus; Heliobletus contaminatus*; Myiornis auricularis; Tachyphonus coronatus; Basileuterus leucoblepharus.
Non-endemic and low sensitivity: Crypturellus obsoletus; Crypturellus parvirostris*; Penelope superciliaris; Herpetotheres cachinnans*; Leptotila rufaxilla*; Geotrygon montana*; Forpus xanthopterygius*; Pionus maximiliani; Piaya cayana*; Anthracothorax nigricollis*; Colaptes melanochloros; Thamnophilus caerulescens*; Dysithamnus mentalis; Herpsilochmus rufimarginatus; Dendrocolaptes platyrostris; Xenops rutilans; Leptopogon amaurocephalus; Todirostrum cinereum*; Myiopagis caniceps*; Phylloscartes ventralis; Lathrotriccus euleri; Myiodynastes maculatus; Empidonomus varius; Myiarchus swainsoni; Pachyramphus viridis; Pachyramphus castaneus; Pachyramphus polychopterus; Turdus albicollis; Trichothraupis melanops; Habia rubica; Hemithraupis guira; Saltator similis; Parula pitiayumi; Basileuterus culicivorus; Euphonia chlorotica.




