Introduction
The Trindade Petrel Pterodroma arminjoniana is a vulnerable species that breeds on Trindade Island, 1,100 km away from the Brazilian coast, and on Round Island, in the South Indian Ocean (Brown and Jordan Reference Brown and Jordan2009, Brown et al. Reference Brown, Nichols, Faulkes, Jones, Bugoni, Tatayah, Gottelli and Jordan2010, BirdLife International 2015). Trindade Petrel breeds in natural cavities on rocky and steep slopes of Trindade Island (Antas Reference Antas and Croxall1991, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009), with two main populations breeding in at different seasons, one breeding in austral summer and the other in austral winter, with a few individuals probably breeding in between (Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). The breeding habitat hampers access to most of the breeding sites on the island. Consequently, the few population estimates of the species have a large degree of uncertainty. The population was estimated at c.15,000 individuals (BirdLife International 2015), but Fonseca-Neto (Reference Fonseca-Neto and Branco2004) estimated 6,500 individuals, based on counts of incubating adults, plus flying individuals sighted at the breeding areas. However, Fonseca-Neto (Reference Fonseca-Neto and Branco2004) did not specify any correction to prevent recounting of flying individuals. Using an extrapolation of nest counts per area to the total number of areas where Trindade Petrel was recorded, Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) obtained an estimate of 1,130 breeding pairs and doubled this number for the non-breeding population. They counted 377 nesting pairs, assumed that the unmapped nests corresponded to twice this value and that half the nests were occupied by two pairs within a given year, thus producing an estimated breeding population of 1,130 pairs.
Censusing seabird populations breeding on cliffs and steep slopes is often difficult or highly imprecise due to accessibility constraints. Thus, several authors developed habitat suitability maps for predicting nest/colony locations of a given species (e.g. Catry et al. Reference Catry, Campos, Segurado, Silva and Strange2003, Rayner et al. Reference Rayner, Hauber and Clout2007, Scott et al. Reference Scott, Moller, Fletcher, Newman, Aryal, Bragg and Charleton2009). Such Predictive Nest Habitat Modelling is based on (1) census of nesting birds in accessible areas, (2) modelling the habitat preferences of the species in accessible areas and (3) predicting the location and numbers of breeders for the remaining unsurveyed potential breeding area. In this study we provide the first breeding population estimative of Trindade Petrel based on nest-site mapping and nest counts in accessible breeding areas, and extrapolated the results to the whole area by the use of predictive nesting habitat modelling. Specifically, we tested the null hypothesis that predictive nest habitat modelling provides a similar population estimate to that of direct nest count methods used previously.
Methods
Study area
Trindade Island is one of the islands that rise from the Victoria-Trindade Ridge, a group of seamounts that originates at the coast and extends 1,100 km towards the Mid-Atlantic Ridge (Miloslavich et al. Reference Miloslavich, Klein, Díaz, Hernández, Bigatti, Campos, Artigas, Castillo, Penchaszadeh, Neill, Carranza, Retana, Astarloa, Lewis, Yorio, Piriz, Rodríguez, Yoneshigue-Valentin, Gamboa and Martín2011). The island is highly mountainous and the relief ranges from sea level to 600 m in a relative small area, thus the overall slope of the island is above 80° (Alves Reference Alves1998). The vegetation of the island was highly impacted by introduction of large herbivores, intensive logging and fire since its discovery by the year 1700 (Olson Reference Olson1981, Alves Reference Alves1998, Silva and Alves Reference Silva and Alves2011). Olson (Reference Olson1981) reports eventual sightings of feral cats and mice Mus sp. There are no records of any rats Rattus spp. on Trindade Island in the past (Olson Reference Olson1981) or presently. There is also a dense population of terrestrial crabs Johngarthia lagostoma that predate heavily upon seabird eggs (Fonseca-Neto Reference Fonseca-Neto and Branco2004, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009).
Nest mapping and density estimates
Trindade Petrel breeds on large rock cavities, and in contrast to most Pterodroma species which are ground burrowing breeders, nests of Trindade Petrels are quite exposed on the surface of such cavities. We searched for nests in areas described by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) and its surroundings. We used flying individuals as cues to find nesting sites. The distribution presented by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) was an outcome of a joint effort of several research surveys on Trindade Island between 1987 and 2007. They reported the locations of breeding areas found after exhaustive searching and exploration of the whole Island. After several years of inspection, they did not find breeding birds at high elevations (i.e. above 300m). Nevertheless, on two opportunities, we made excursions to the higher elevations of the island in search of nests.
We mapped the accessible breeding sites of the Trindade Island with a hand-held GPS receiver GARMIN GPSMAP® 60CSX (average precision ±4 m) and counted the active nests in those areas between September and November 2014, during the incubation period. Active nests were considered as those with adults, chicks or eggs, following Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). Additionally, we used the areas and the number of nests given by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) for the other areas that we could not access (Figure 1). Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) counted 377 active nests with adults, eggs or chicks, between December 2006 and April 2007. In ArcGis 10.2 we created polygons from the mapped areas, and randomly added the number of nests of each breeding sites within the polygons. We excluded the South Islet presented by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) because it has the higher density of nests in a relatively small area; probably the isolation from the main island resulted in a protection against past threats. This islet would bias the result towards those characteristics that are not representative of the rest of the island.

Figure 1. Location of Trindade Island (star) above, on the right, and the Trindade Petrel Pterodroma arminjoniana nesting areas mapped during this study (cross hatch) and by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) (simple hatch), overlapped on a 3D Digital Elevation Model topography.
Geographic information system data
Trindade Petrel nests are associated with steep slopes and cliffs from low to intermediate elevations (Fonseca-Neto Reference Fonseca-Neto and Branco2004, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). Hence, we used topography to evaluate nest habitat suitability. A contour line shapefile was provided to us by the Brazilian Navy through the “Secretaria da Comissão Interministerial para os Recursos do Mar” (SECIRM). We created a Digital Elevation Model (DEM) through a Triangulated Irregular Network (TIN) 3D interpolation of the contour lines on ArcGis v10.2 (Figure 1). TIN interpolation allows for a more detailed estimation of elevation in comparison to other methods (Ewans et al. Reference Ewans, Kirkpatrick and Townsend2001, Tucker et al. Reference Tucker, Lancaster, Gasparini, Bras and Rybarczyk2001). Altitude, slope (terrain inclination) and aspect (the direction, in degrees) images were generated from the DEM. The altitude raster was used to evaluate finer measures of relief inclination associated with precipitation movement and soil runoff, the flow length and the flow drop, using the hydrology toolbox of the Spatial Analyst on Arc Gis 10.2 (Figure 2). Flow length measures the downstream distance for precipitation moving from one grid cell to another, and flow drop is a measure of the probability of precipitation movement to a given grid cell. These variables should be important in characterising breeding areas because sites with severe runoff and high precipitation speed may be unsuitable or disadvantageous for nesting.
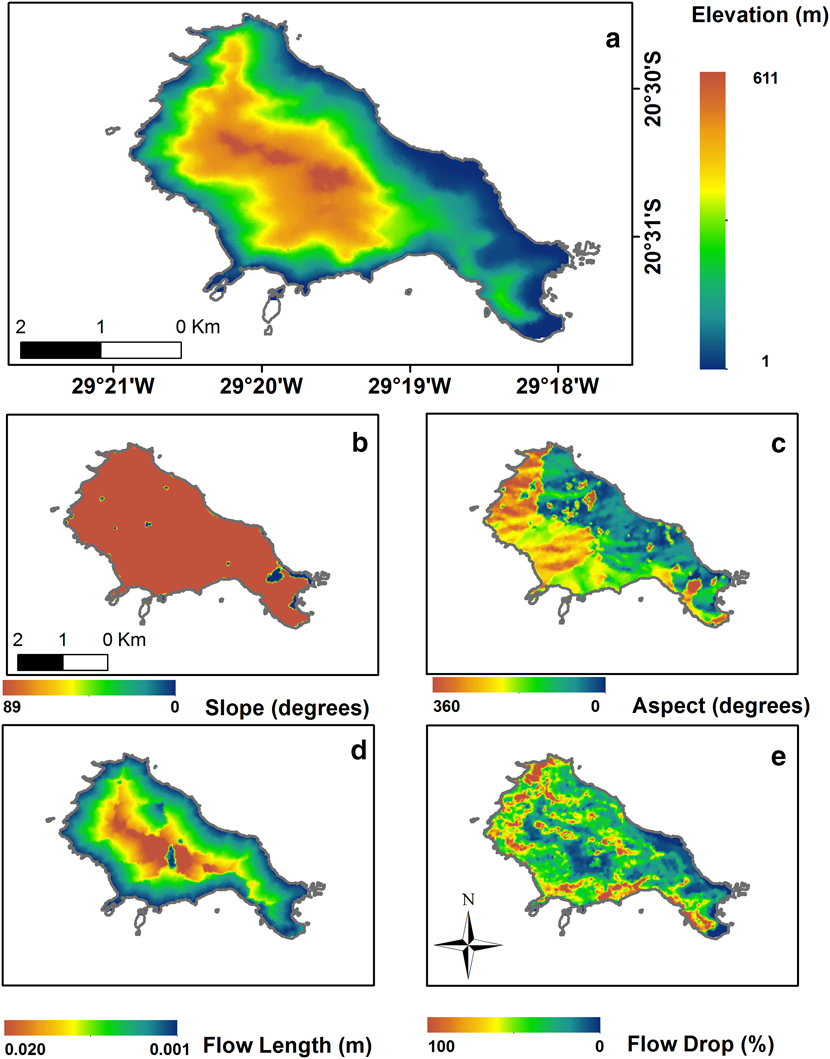
Figure 2. Environmental variables used to evaluate the Predictive Nest Habitat Modelling of Trindade Petrel Pterodroma arminjoniana with the MaxEnt modelling technique.
Predictive nesting habitat modelling
There are several methods of modelling species distribution (e.g. Peterson et al. Reference Peterson, Papes and Eaton2007, Oppel et al. Reference Oppel, Meirinho, Ramírez, Gardner, O’Connell, Miller and Louzao2012). We chose to use the Maximum Entropy (MaxEnt) approach (Phillips et al. Reference Phillips, Anderson and Schapire2006) as it is regarded one of the best mechanistic methods for modelling species distributions based on presence-only data (Elith et al. Reference Elith, Graham, Anderson, Dudik, Ferrier, Guisan, Hijmans, Huettmann, Leathwick, Lehmann, Li, Lohmann, Loiselle, Manion, Moritz, Nakamura, Nakazawa, Overton, Peterson, Phillips, Richardson, Scachetti-Pereira, Schapire, Soberon, Williams, Wisz and Zimmermann2006, Oppel et al. Reference Oppel, Meirinho, Ramírez, Gardner, O’Connell, Miller and Louzao2012), and it relies on non-linear relations of presence-only data and habitat variables (Elith et al. Reference Elith, Phillips, Hastie, Dudík, Chee and Yates2011, Merow et al. Reference Merow, Smith and Silander2013). For instance, Austin (Reference Austin2002, Reference Austin2007) emphasises the need to use non-linear methods in habitat modelling. Furthermore, MaxEnt is robust to the use of correlated variables in the modelling procedure to predict probability of presence in each cell (Royle et al. Reference Royle, Chandler, Yackulic and Nichols2012), as it is a machine learning method (Elith et al. Reference Elith, Phillips, Hastie, Dudík, Chee and Yates2011, Merow et al. Reference Merow, Smith and Silander2013). We set aside 10% of presence localities as set points to calculate model accuracy. The multidimensional environmental similarity surface method (MESS) was used to evaluate where the environment had values out of range (Merow et al. Reference Merow, Smith and Silander2013). We used a bootstrap resampling procedure (n = 50) to obtain a prediction of the average distribution (Edrén et al. Reference Edrén, Wisz, Teilmann, Dietz and Söderkvist2010). The MaxEnt model produces a logistic output as a measure of habitat suitability (from 0 to 1) for predicting nest locations of breeding Trindade petrels, as proposed by Merow et al. (Reference Merow, Smith and Silander2013).
Predicting breeding areas
We inspected how the resultant entropy (as a measure of how the final presence probabilities differentiate from those expected using the model) of the 50 resamplings was related to the maximum sensitivity plus specificity threshold outputs, and chose the higher threshold value that minimised the entropy. Then we selected only the pixels that were above this threshold value to calculate the area of the island where we expected the Trindade Petrel nests to occur. We extrapolated the density of nests in the known and mapped nesting sites to the area calculated using this threshold.
Results
The accuracy of the models was high (bootstrap mean AUC = 0.977 ± 0.004) meaning that the model was able to predict almost all of the 10% of data set aside for testing. The variables with higher contribution to the models were elevation and flow drop, which together represented 67.6% of the contribution to models and 82.9% of permutation importance (the percentage of AUC reduction on models without the variables) (Table 1). The Trindade Petrel had a higher estimated probability of presence in low to medium elevations, in areas where slope varied from flat to steep, but the probability of presence decreased considerably on slopes above 80° (Figure 3). Such factors indicated that the higher presence probabilities were restricted mostly to the outer edges of the island (Figure 4).
Table 1. Mean ± SD percentage contribution and permutation importance of the environmental variables (for explanation of variables see methods) from the 50 bootstrapped MaxEnt model, predicting the probability of presence of Trindade Petrel Pterodroma arminjoniana nests in Trindade Island.


Figure 3. Estimated probability of Trindade Petrel Pterodroma arminjoniana nest presence in relation to the different environmental variables included in the MaxEnt Predictive Nest Habitat Modelling (grey areas) and a smoothed loess tendency (black line).

Figure 4. Mean predictive probability of presence (a) and standard deviation (b) of the predictions of Trindade Petrel Pterodroma arminjoniana nests locations at Trindade Island. Outputs of a MaxEnt Predictive Nest Habitat Modelling.
The entropy on the 50 bootstrapped models described a parabola in relation to the maximum sensitivity plus specificity thresholds (Figure 5). The minimum entropy would match a threshold near zero, but this would use almost the entire area of the island, which is an unreal assumption. As the estimated entropy starts to decrease when the threshold reaches 0.20 of presence probability, we chose to use the higher value of 0.29, rounded to 0.30. By applying a threshold value of 0.30 the potential nesting area of Trindade Petrel on the island (Figure 6) occupied 5,346 m2 (Table 2). Within this area 1,048 nests are expected to occur. Adding the numbers from the South Islet which were previously excluded, we reached an estimate of 1,228 nests for Trindade Island.

Figure 5. The entropy in relation to the logistic threshold values of the 50 bootstrapped models. We chose the higher value of threshold with the potential lower value of entropy (Logistic threshold = 0.30).
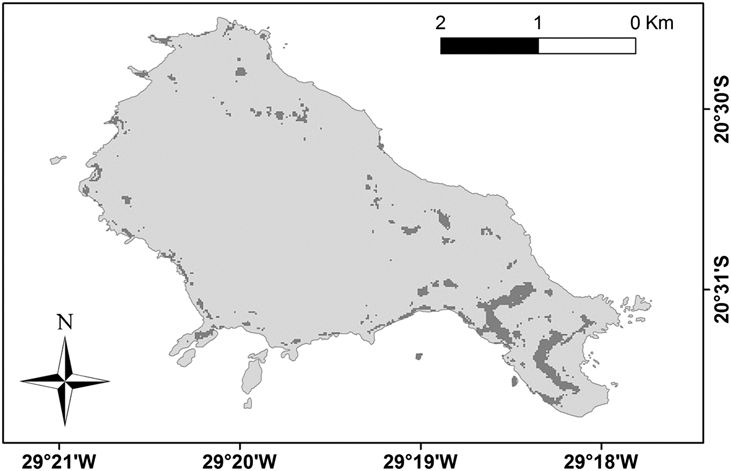
Figure 6. Expected distribution of Trindade Petrel Pterodroma arminjoniana colonies based on a logistic threshold of 0.30. See the methods section for the full MaxEnt Predictive Nest Habitat Modelling procedure.
Table 2. Number of Trindade Petrel Pterodroma arminjoniana nests on the mapped areas and extrapolation to the estimated areas using the Predictive Nest Habitat Modelling (excluding the South Islet) with a logistic threshold (probability of occurrence) of 0.30.

Discussion
We found a breeding population value relatively similar to the value given by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009), thus corroborating the size of the breeding population proposed by them. They suggested a population of 1,130 breeding pairs, while our modelling resulted in 1,228 breeding pairs. Information regarding species abundance in the past (Murphy Reference Murphy1936, Olson Reference Olson1981) is mostly anecdotal, but a population decline between the mid-1970s and mid-1990s can be inferred from a reduction of sightings in the non-breeding areas (Lee Reference Lee1999, Reference Lee2000). It is worth noting that our count was conducted during the austral summer (September–November). Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009) included year-round data in their counts, so by using their data we reduced any potential error due to Trindade Petrels having subpopulations with different breeding periods. However, we would be cautious and assume that our population values are a potential minimum population size. Furthermore, this species shows nest site fidelity; e.g. Fonseca-Neto (Reference Fonseca-Neto and Branco2004) observed that seven out of 18 individuals returned to the same nests, and eight to the same cavities. Krüger et al. (Reference Krüger, Paiva, Colabuono, Petry, Montone and Ramos2016) found that 16 out of 40 birds returned to the same cavities.
As with other species of gadfly petrels (Pterodroma spp.) (Harris Reference Harris1970, Bretagnolle and Attié Reference Bretagnolle and Attié1991, Zino et al. Reference Zino, Oliveira, King, Buckle, Biscoito, Costa Neves and Vasconcelos2001) Trindade Petrel nests on steep slopes and cliffs, but unlike other species (Zino et al. Reference Zino, Oliveira, King, Buckle, Biscoito, Costa Neves and Vasconcelos2001, Rayner et al. Reference Rayner, Hauber and Clout2007, Pinet et al. Reference Pinet, Salamolard, Probst, Russel, Jaquemet and Le Corre2009), they do not associate with higher elevations. Species such as Zino’s Petrel Pterodroma madeira (Zino et al. Reference Zino, Oliveira, King, Buckle, Biscoito, Costa Neves and Vasconcelos2001), Cooks’s Petrel Pterodroma cookii (Rayner et al. Reference Rayner, Hauber and Clout2007) and Barau’s Petrel Pterodroma baraui (Pinet et al. Reference Pinet, Salamolard, Probst, Russel, Jaquemet and Le Corre2009) breed in association with remnant vegetation restricted to higher mountain areas and cliffs where human activity, trampling and grazing by introduced large herbivores are restricted. The nesting distributions of these three species have had limited habitat modification, as they are associated with vegetation at higher elevations where native vegetation remains. Trindade Petrel seems to present the opposite response, as it is a surface breeder not associated with dense vegetation, therefore avoiding higher elevations (Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009).
It has been suggested that Trindade Petrels were limited to cliffs and slopes due to the combined effects of feral goat trampling, feral cats and intense egg predation by crabs (Alves Reference Alves1998, Fonseca-Neto Reference Fonseca-Neto and Branco2004, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). In pristine conditions Trindade Petrels were probably able to nest on the ground close to the coast, but there is no description of the nesting habitat before habitat modification on the island. On two occasions during field surveys, one of the authors (LK) was able to participate in exploration of the high peaks of the island, and found no signs of Trindade Petrels there or near the remnant patches of vegetation, something also highlighted by Luigi et al. (Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009).
Lee (Reference Lee1999, Reference Lee2000) suggested that this species has suffered a severe decline, after analysing historical sightings in the North Atlantic. This suggestion, added to the population size of 15,000 individuals (BirdLife International 2015), raised some questions concerning the current population status of this species and a possible negative effect of the feral goats inhabiting the Island for two centuries, occasional feral cats and egg predation by crabs (Alves Reference Alves1998, Fonseca-Neto Reference Fonseca-Neto and Branco2004, Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). Furthermore, researchers on early expeditions recorded the presence of feral cats at very low densities (Olson Reference Olson1981, Williams Reference Williams, Croxall, Evans and Schreiber1984), which may also have contributed to the decrease of the Trindade Petrel, and for the restriction of breeding sites to steep cliffs of the island. There are several examples of feral cat predation reducing populations of seabirds (Carlile et al. Reference Carlile, Priddel, Zino, Natividad and Wingate2003, Pinet et al. Reference Pinet, Salamolard, Probst, Russel, Jaquemet and Le Corre2009, Ratcliffe et al. Reference Ratcliffe, Bell, Pelembe, Boyle, Benjamin, White, Godley, Stevenson and Sanders2009), but this type of impact over Trindade Petrel is only implied, since there is no confirmation of cat predation in the past, and currently cats are absent from the Island.
Conclusion
To our best knowledge, this is the first study evaluating the population size of Trindade Petrel using the Predictive Nesting Habitat Modelling technique. We found that our up-to-date estimate of the breeding population likely matches another recently reported figure (Luigi et al. Reference Luigi, Bugoni, Fonseca-Neto, Teixeira, Mohr, Castro, Costa and Alves2009). Furthermore, we were able to generate a picture of the distribution of breeding areas that can be verified in future field studies, and can be used to guide decisions for the protection of the breeding habitat of this vulnerable species.
Acknowledgements
This study is part of the project “Distribution and trophic ecology of birds of Trindade Island and Saint Peter and Saint Paul Archipelago: data for the evaluation of contamination by organic pollutants in ocean ecosystems” (CNPq405416/2012-1). Logistical support was provided by the Brazilian Secretary of the Inter-Ministry on Marine Resources (SECIRM). We would like to thank the Capitão de Fragata Sidnei Costa-Abrantes and the Secretaria da Comissão Interministerial para os Recursos do Mar (SECIRM) for providing the elevation curve lines of the island. We thank SECIRM by field survey support. L.K. acknowledges the National Council of Technological and Scientific Development CNPq for his PhD scholarship (Programa Ciência sem Fronteiras processo 245540/2012-1). This study benefited from the strategic program of MARE, financed by FCT (MARE—UID/MAR/04292/2013).










