Introduction
Population declines of Gyps vulture species across south Asia have been well-documented since they were first reported in 1999 (Prakash Reference Prakash1999, Prakash et al. Reference Prakash, Pain, Cunningham, Donald, Prakash, Verma, Gargi, Sivakumar and Rahmani2003, Gilbert et al. Reference Gilbert, Watson, Virani, Oaks, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2006). As a result of these declines, the Oriental White-backed Vulture Gyps bengalensis, Long-billed Vulture Gyps indicus and the Slender-billed Vulture Gyps tenuirostris are all listed as ‘Critically Endangered’ (IUCN 2013).
The primary cause of these declines was the ingestion by vultures of livestock carcasses that had been recently treated with non-steroidal anti-inflammatory drugs (NSAIDs), principally diclofenac (Oaks et al. Reference Oaks, Gilbert, Virani, Watson, Meteyer, Rideout, Shivaprasad, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2004, Green et al. Reference Green, Newton, Shultz, Cunningham, Gilbert, Pain and Prakash2004). Since the identification of NSAIDs as the primary cause of vulture declines in south Asia, a range of conservation efforts have focused on the recovery of vulture populations. These have included the banning of veterinary diclofenac (Pain et al. Reference Pain, Bowden, Cunningham, Cuthbert, Das, Gilbert, Jakati, Jhala, Khan, Naidoo, Oaks, Parry-Jones, Prakash, Rahmani, Ranade, Baral, Senacha, Saravanan, Shah, Swan, Swarup, Taggart, Watson, Virani, Wolter and Green2008), the establishment of conservation breeding centres (Murn et al. Reference Murn, Khan and Farid2008, Bowden et al. Reference Bowden, Prakash, Ranade, Routh, Jakatt, Cuthbert, Rahmani, Green, Prakash and Parry-Jones2012), the identification of safe alternative veterinary drugs (Swarup et al. Reference Swarup, Patra, Prakash, Cuthbert, Das, Avari, Pain, Green, Sharma, Saini, Das and Taggart2007), efforts to remove diclofenac from the environment (Swan et al. Reference Swan, Naidoo, Cuthbert, Green, Pain, Swarup, Prakash, Taggart, Bekker, Das, Diekmann, Diekmann, Killian, Meharg, Patra, Saini and Wolter2006, Cuthbert et al. Reference Cuthbert, Taggart, Prakash, Swarup, Suchitra, Mateo, Chakraborty, Deori and Green2011) and the establishment of Vulture Safe Zones (Chaudhary et al. Reference Chaudhary, Subedi, Giri, Baral, Bidari, Subedi, Chaudhary, Chaudhary, Paudel and Cuthbert2012), which provide ‘safe’ food for vultures in designated areas and also use advocacy and lobbying to remove diclofenac from veterinary use and subsequently livestock carcasses. There is evidence that these conservation efforts are beginning to be successful, with residues of diclofenac in livestock carcasses having fallen in some areas (Cuthbert et al. Reference Cuthbert, Taggart, Prakash, Swarup, Suchitra, Mateo, Chakraborty, Deori and Green2011). As a result, the rate of population decline for Oriental White-backed Vultures has slowed, and for Long-billed Vultures, reversed (Prakash et al. Reference Prakash, Bishwakarma, Chaudhary, Cuthbert, Dave, Kulkarni, Kumar, Paudel, Ranade, Shringarpure and Green2012, Chaudhry et al. Reference Chaudhry, Ogada, Malik, Virani and Giovanni2012).
In Pakistan, the Oriental White-backed Vulture has been monitored extensively in some areas, particularly Punjab Province (Gilbert et al. Reference Gilbert, Watson, Virani, Oaks, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2006, Reference Gilbert, Watson, Ahmed, Asim and Johnson2007, Arshad et al. Reference Arshad, Chaudhry and Wink2009). The species is known to occur in Sindh Province, in south-east Pakistan (Roberts Reference Roberts1991), but there is relatively little reported information about the species from there. Gilbert et al. (Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood, Shah, Chancellor and Meyburg2004) recorded nests in several areas of Sindh, primarily in eastern and north-eastern districts, but numbers of nests were low (< 10). The range map for the species (Roberts Reference Roberts1991) does not extend to the far south-east of Sindh, in the Nagarparkar area of Tharparkar District, which is adjacent to the Great Rann of Kutch in the south-eastern corner of the province.
Through local fieldwork starting in 2009 and during a national survey of vultures in Pakistan in 2011, a small breeding colony of Oriental White-backed Vultures was recorded in the south-eastern corner of Tharparkar District in Sindh Province. This paper provides the first description of this previously unreported colony of Oriental White-backed Vultures. Based on fieldwork from 2011 to 2014 we describe the population size and associated spatial dynamics of the breeding colony, and discuss the future conservation of this colony.
Study area and methods
The study was carried out in the south-eastern corner of Tharparkar District, Sindh Province, approximately 10 km north-west of the Kharoonjar Hills (N24o20’ E70o43’) and the town of Nagarparkar (Figure 1). The region is arid and generally flat with areas of relief characterised by isolated granite outcrop hills. The loam soils and low rainfall provide for the main land-uses of low-density perennial livestock grazing and non-irrigated crop fields. Habitat is dry open scrub with scattered trees characterised primarily by stands of kandi Prosopis cineraria.
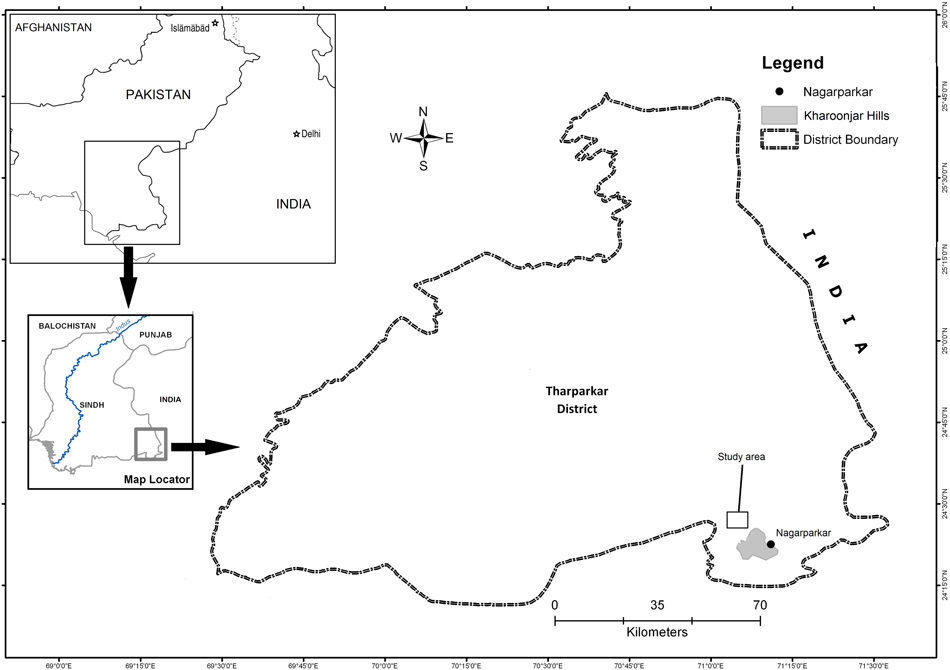
Figure 1. Location of the study area near Nagarparkar town in southeast Sindh Province, Pakistan.
Annual surveys of the study area were conducted from 2 February to 17 April 2011; 1 April to 15 April 2012; 1 March to 15 March 2013, and 24 January to 17 February 2014. Nests were located by thorough searches of the study area, by following flying birds to their roost locations in a 4x4 vehicle at the end of each day and by paying particular attention to areas with larger trees. Local residents were questioned for information about the locations of vulture nests or roosts. For roost sites that were located, numbers of birds were counted on four occasions during the annual survey visits. The number of roosting birds was counted between 15h00 and 18h00 and then again early the following morning at the same roost site. All positions were logged using a hand-held GPS. Nests were recorded as occupied if adults were in attendance or a chick was in the nest, but measures of breeding productivity were not possible because the survey was conducted only once each breeding season. Nest trees were identified with spray-painted numbers to avoid double-counting and for inter-year reference. The height of each nest tree was estimated by eye.
Nest density (nests km-2) was calculated each year by dividing the number of nests by their spatial extent (km2), which was determined as the area of a polygon containing all occupied nests. Oriental White-backed Vultures nest in colonies (Roberts Reference Roberts1991) and clusters of nests are a feature of the species (BirdLife International 2014). To assess the spatial pattern of nests and change between years we calculated the mean nearest-neighbour distance (NND) between all nests each year. However, because more than one breeding pair of White-backed Vultures can nest in the same tree, we calculated two nearest-neighbour metrics: (a) the distance between trees with nests (tree-NND), and (b) the distance between nests (nest-NND), using a predetermined 3-m NND for nests in the same tree.
Mean NND (tree or nest) on its own is insufficient to describe differences between spatial point patterns because two point patterns with different characteristics might have the same mean NND. To assess the degree of clustering we calculated the ratio of the geometric mean (GMR) to the arithmetic mean of the squared NND (Brown and Rothery Reference Brown and Rothery1993, Murn et al. Reference Murn, Combrink, Ronaldson, Thompson and Botha2013). The maximum value for this statistic (GMR) is unity, where all NNDs are equal. Complete spatial randomness occurs at GMR = 0.5, whilst the minimum GMR value is unbounded. Therefore, increasingly smaller GMR values represent spatial patterns of nests with tighter clustering. We chose this metric because the spatial extent of the nests was discrete and as a result, there were no outlier nests that would have disproportionately affected the GMR. Similarly, the discrete spatial extent of the nests negated the need to account for edge effects – the existence of unknown nests marginally outside the study area – when measuring nearest-neighbour distances because the colony nests were the only nests in the entire study area. We expected mean values for tree-NND and nest-NND to reflect density, such that mean NND would decrease with increasing density and vice versa, but we held no a priori assumptions about the degree of nest clustering in relation to nest numbers or density.
NND data were transformed where necessary to stabilise variance and checked with Anderson-Darling tests, after which one way ANOVA was used for comparison of NNDs between years. Data that did not conform to parametric assumptions after transformation were subject to Kruskal-Wallis tests. Homogeneity of variance in sample ranks was checked with Levene’s test. Tests were performed in Minitab 16.
Results
Nests were located mainly in kandi Prosopis cineraria trees (all nests in 2011 and 2012), but neem Azadirachta indica (two nests in 2013), rohida Tecomella undulata and tamarind Tamarindus indica (one nest each in 2014) were also used. Nest trees were larger than surrounding trees and had a mean height of 11.6 (SD ± 2) m. A maximum of seven nests in one tree was recorded, and this large tree was located within one of the villages in the study area. The distribution of the nests across the four years was dynamic and although it was not possible to identify birds individually, several nests were occupied in each of the four years, whilst new nests were made each year (Figure 2).

Figure 2. The spatial pattern of nest trees in an Oriental White-backed Vulture colony, 2011 to 2014, Sindh Province, Pakistan.
The number of recorded nests increased each year and tripled during the study period (Table 1). The marked reduction in density from 2012 to 2013, despite a 40% increase in the number of active nests, reflects the spatial expansion of the colony (Figure 2). The number of trees containing more than one nest increased rapidly from 10.5% of trees in 2012 to 23.5% of trees in 2014, but despite the tree containing seven nests, the mean number of nests per multi-nest tree remained near 2.5 each year (mean = 2.55 nests tree-1, range = 2.4–2.75).
Table 1. Number of nests and some spatial characteristics of an Oriental White-backed Vulture Gyps bengalensis colony in Tharparkar District, Sindh, Pakistan.
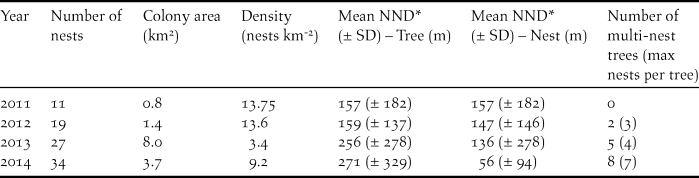
* Nearest-neighbour distance
The two nearest-neighbour metrics revealed different aspects of the growth of the colony, and neither was correlated with colony area or nest density. Mean tree-NND increased each year and was significantly different between years (one-way ANOVA F 3,87 = 2.81, P = 0.04), possibly reflecting the decreasing density. Mean nest-NND decreased each year (Kruskal-Wallis H = 11.92, P = 0.008), which is most likely a function of the increasing number of trees with multiple nests. Across all years, mean tree-NND was 230 m (± SE 28 m) and mean nest-NND was 110 m (± SE 20 m).
In each year, the spatial pattern of trees with nests was clustered (GMR < 0.25). But despite increasing numbers of nests, increasing mean tree-NND and decreasing density, the GMR for nest trees remained in the region of 0.20 between 2012 and 2014 (Figure 3).
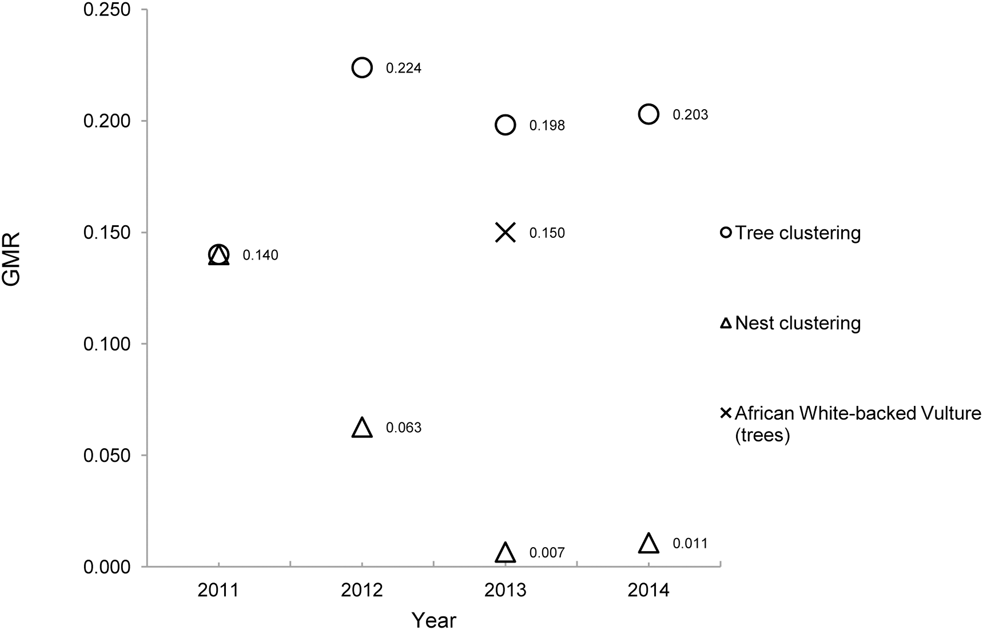
Figure 3. Spatial dynamics of an Oriental White-backed Vulture breeding colony over four years. The clustering characteristics of nest trees and nests are analysed as spatial point patterns. GMR is the extent of clustering; lower values occur with tighter clustering of a spatial point pattern. A similar tree-nesting vulture species, the African White-backed Vulture Gyps africanus, is provided for comparison.
Maximum roost counts during each survey period were 39 birds in 2011 (one site located near the active nests), 102 birds in 2013 (two sites) and 145 birds in 2014 (two sites). No roost counts were conducted in 2012. The roost sites were in the same location each day, and did not change between years. In 2014 the approximate age proportions were 60% adults, 14% sub-adults and 25% juveniles. Assuming that 1) one adult of a breeding pair (34 pairs) will remain at an active nest overnight; 2) the other breeding adult (34 birds) joins a communal roost and 3) that non-breeding adults (approximately 54 birds) and immature birds (57 birds) were also part of communal roosts, we estimate that the population of this colony during the 2014 breeding season was approximately 180 individuals. Thus, approximately 30% of the adult population are estimated to be non-breeding birds.
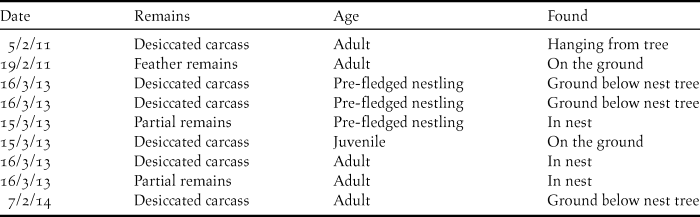
Nine dead vultures were found between 2011 and 2014 (Table 2), although systematic surveys to locate dead birds were not conducted. The cause of death for these birds could not be established due to advanced decomposition of the bodies.
Table 2. Details of dead vultures found during breeding colony surveys of Oriental White-backed Vultures Gyps bengalensis in Tharparkar District, Sindh, Pakistan.
Discussion
The nest densities in 2011 and 2012 (Table 1) are comparable with and slightly higher than pre-decline (1980s) densities of 12.2 nests km-2 recorded in Keoladeo National Park, India (Prakash and Rahmani Reference Prakash and Rahmani1999), higher than pre-decline densities of 2.5–5 nests km-2 reported from coastal mangrove areas of southern Bangladesh (Sarker Reference Sarker1987) and approaching the higher densities of 15 nests km-2 reported for Changa Manga forestry plantation in Punjab Province near Lahore (Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood and Shah2002). This last population was experiencing rapid decline from diclofenac poisoning during the monitoring period (Gilbert et al. Reference Gilbert, Watson, Virani, Oaks, Ahmed, Chaudhry, Arshad, Mahmood, Ali and Khan2006), and so nest densities could have been lower than a potential maximum. Apart from differences in habitat (arid Nagarparkar, wetland-dominated Keoladeo, coastal mangroves Sundarbans, and forest plantation Changa Manga), the manner in which spatial extent of the breeding areas was calculated and the availability of trees in each study area may provide another explanation for these variations in nest density. Regardless of the reasons for this variation, the nest densities found in our study are within the range of densities reported for a number of different locations prior to, and during, the decline of south Asian vulture populations (Prakash and Rahmani Reference Prakash and Rahmani1999, Sarker Reference Sarker1987, Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood and Shah2002). However, none of these densities even remotely approaches historical accounts for this species of ‘up to 15 nests in one tree’ and 100 nests in a 250m diameter circle (Hume and Oates 1889-1890), although Roberts (Reference Roberts1991) describes up to six nests occurring in one tree.
Despite an increasing mean tree-NND each year and a moderate level of nest tree clustering (Figure 3), the decreasing mean nest-NND and the increased clustering of nests in a growing breeding population highlights the strong colonial tendencies of this species. In comparison, there are two other tree-nesting Gyps vultures, the Slender-billed Vulture and the African White-backed Vulture Gyps africanus. We are unaware of any nearest-neighbour analyses for the relatively less-studied Slender-billed Vulture, so a direct comparison is not possible. However the species is reported to nest singly (one breeding pair per tree) in relatively small colonies of 7–8 pairs (BirdLife International 2014) although more than one nest in a tree has been recorded occasionally (Mathews Reference Mathews1918).
African White-backed Vultures nest in what have been termed ‘loose colonies’ (Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992), usually with only one breeding pair per nest tree. In savanna areas (i.e. not linear riparian nests) a mean NND distance has been reported as 697 m (± SD 913 m, n = 217) with a GMR of 0.15 (Murn et al. Reference Murn, Combrink, Ronaldson, Thompson and Botha2013). Although the mean tree-NND for African White-backed Vultures is significantly higher (T = 6.85, P < 0.001) than the Oriental White-backed Vultures in this study, the African species still shows a tendency for clustered nest trees (Figure 3). Given that a tightly-clustered pattern (GMR ∼ 0.01) of Oriental White-backed Vulture nests can occur in nest trees that are moderately clustered (GMR ∼ 0.20), it is likely that the colonial-nesting and clustering tendencies of this species reflect the sufficient availability of trees large enough to support multiple nests. Although not all trees in a colony will be large enough or of suitable canopy structure to accommodate more than one nest, a characteristic spatial arrangement (i.e. clustering) of nests may occur at a threshold that is related to the availability of suitable trees. For example, Satheesan (Reference Satheesan1995) reported large (pre-decline) numbers of roosting Oriental White-backed Vultures on very large trees in Agra City, India, but did not record any more than three nests in one tree in the same area. Similarly, in post-decline breeding populations, numbers of nests per tree are still in the range of 1–3 (Baral et al. Reference Baral, Gautam and Tamang2005, Roy and Shastri Reference Roy and Shastri2013). It is therefore possible that the tight nest-clustering characteristics favoured by this species can be achieved with a relatively small number of nests per tree if the available trees are of a suitable size, structure and spatial pattern (GMR∼ 0.2). This may suggest that an optimum level of nest tree clustering exists to support a range of colony sizes and nest densities.
Monitoring and conservation implications
Despite not being able to assess breeding productivity we do consider that, given the limitations of an annual survey, the number of nests recorded each year was representative of the breeding colony size for two reasons. Firstly, the number of inactive nests was low, suggesting that the number of breeding pairs that started a breeding attempt and failed is also low. Secondly, the survey dates of January to April each year covered the main part of the breeding season; most breeding pairs that did make an attempt would have made nests and been incubating, whilst early breeders would still be either in late stage incubation or with a chick in the nest.
Although it is encouraging that the number of recorded nests has tripled since 2011, this is tempered by a slowing of the rate at which the colony is growing (Table 1) and the fact that a number of queries and research priorities remain. Firstly, it is unknown from where the additional breeding birds have arrived. In the absence of additive mortality, Oriental White-backed Vultures are generally long-lived birds with an estimated generation time of 16 years (BirdLife International 2014). Using a conservative estimate of birds not reproducing until their fourth year, the increase in the breeding population observed in our study has almost certainly been enhanced by immigration, as the demographics of such a small population with this length of generation time do not support the observed rapid increase in the number of nests. It is also possible however, that following the 2006 ban on veterinary diclofenac, there has been an increasing rate of survival to maturity for age cohorts hatched post-2006, and that these birds are the source of the additional birds.
Secondly, the risks and effects of mortality (such as NSAID poisoning) need to be assessed. Although a number of dead birds were found without a concerted search effort, four of the nine birds were of pre-fledging or recently-fledged age, and high mortality in this age group of vultures is not unusual (Mundy et al. Reference Mundy, Butchart, Ledger and Piper1992). The five dead adults that were found offer more cause for concern, as adult survival is one of the most sensitive demographic parameters for vultures (Oro et al. Reference Oro, Margalida, Carrete, Heredia and Donázar2008, Margalida et al. Reference Margalidia, Colomer and Oro2014) and suggests that this age class is potentially still at risk. Even with the goal of complete removal of diclofenac and other harmful non-steroidal anti-inflammatory drugs from the environment, it is possible that residual quantities of diclofenac remain in livestock carcasses and are a threat to vultures. The establishment of a Vulture Safe Zone (VSZ) in the study area in 2012 saw the beginning of a new phase of environmental monitoring and conservation to address this. Across the approximately 8,000 km2 VSZ, a range of activities such as livestock health camps, awareness-raising sessions in villages and consultations with veterinary dispensaries are all aimed at highlighting the risks to vultures from diclofenac and emphasising the need to maintain the ban on its use in livestock.
Thirdly, an important next step in the monitoring of this colony is to determine breeding success. Comparing breeding success with pre-decline populations (Prakash Reference Prakash1999) and those that were suffering acute mortality from diclofenac poisoning (Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood and Shah2002) could offer an indication of what levels of additive mortality exist for this population. Similarly, comparison of breeding productivity with the nearby colony of Long-billed Vultures (Chaudhry et al. Reference Chaudhry, Ogada, Malik, Virani and Giovanni2012) will be important to see if the colonies are both (or neither) affected by similar rates of mortality.
Finally, dispersal behaviour of birds from this population must also be assessed. Oriental White-backed Vultures can range over vast distances (Gilbert et al. Reference Gilbert, Watson, Ahmed, Asim and Johnson2007), so it is not unlikely that birds may be dispersing across a wide area, in the same way that birds may have arrived to the Nagarparkar colony from adjacent areas such as Gujarat in India.
The description of breeding colony spatial dynamics outlined here has important implications for the conservation of Oriental White-backed Vultures, particularly in areas (such as our study area or other arid zone areas) where the number of suitable nest trees may be limited and a lack of suitable trees may limit clustering of nests and hence potential colony size. Large trees in particular should be preserved and protected, whilst conservation management and sustainable harvesting of timber for fuel stock and/or building materials can be focused on smaller trees. Where nest trees do not appear to be a limiting factor, Oriental White-backed Vulture colonies can reach very high numbers such as the 700–800 nests recorded at Changa Manga (Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood and Shah2002), although the number of multi-nest trees and extent of nest clustering has not been reported for this colony.
Based on a recent national survey of vultures (Iqbal et al. Reference Iqbal, Khan and Murn2011), this colony of Oriental White-backed Vultures is currently the only known extant breeding population in Pakistan, in addition to being previously unreported in the general literature (Roberts Reference Roberts1991, Gilbert et al. Reference Gilbert, Virani, Watson, Oaks, Benson, Khan, Ahmed, Chaudhry, Arshad, Mahmood, Shah, Chancellor and Meyburg2004, Chaudhry et al. Reference Chaudhry, Ogada, Malik, Virani and Giovanni2012 ). Lending additional importance to our study area is the nearby colony of Long-billed Vultures breeding in the Kharoonjar Hills (Chaudhry et al. Reference Chaudhry, Ogada, Malik, Virani and Giovanni2012), which is the only breeding colony of this species recorded for Pakistan (Roberts Reference Roberts1991). The Vulture Safe Zone in Sindh Province is thus a major step towards the long-term conservation of these two species in the south Asian region.
Diclofenac is not the only NSAID that is toxic to vultures - there are other veterinary drugs that represent a threat such as ketoprofen (Naidoo et al. Reference Naidoo, Wolter, Cromarty, Diekmann, Duncan, Meharg, Taggart, Venter and Cuthbert2010), aceclofenac (Sharma Reference Sharma2012) and flunixin (Zorrilla et al. Reference Zorrilla, Martinez, Taggart and Richards2014), which are all still legal and in circulation. In this regard, we support fully the continued development and maintenance of Vulture Safe Zones in south Asia as a means of ongoing progression towards the conservation objective of restoring viable populations of vultures to areas where they once occurred. However, the long-term conservation value of a Vulture Safe Zone will be reduced if there are limited opportunities for vultures to nest in the spatial patterns that optimise the dynamics of their breeding colonies. Based on the results presented here, and in addition to the removal of unsafe veterinary drugs, a key component of Vulture Safe Zone work should be the preservation of nest tree distributions that can support large colonies of clustered nests of Oriental White-backed Vultures.
Acknowledgements
U.S. Fish and Wildlife Service for a grant enabling establishment of Vulture Safe Zone, the Parkar Foundation for continued advocacy in the Sindh Vulture Safe Zone. A grant from the National Birds of Prey Trust helped fund the 2011 national survey and WWF-Pakistan’s small grants programme supported the 2014 breeding season survey. Muhammad Humza and Irfan Ashraf from WWF-Pakistan’s GIS laboratory provided mapping support. Antoni Margalida and two other reviewers provided feedback that improved the manuscript.







