Introduction
The ability of birds to move through the landscape is a key parameter for ecological and conservation studies. Several conservation questions rely on our knowledge of avian movement ability, including the capacity to disperse and persist in fragmented landscapes, using elements that increase habitat connectivity, such as biological corridors (Castellón and Sieving Reference Castellón and Sieving2005, Uezu et al. Reference Uezu, Metzger and Vielliard2005, Lees and Peres Reference Lees and Peres2008) and stepping stones (Thiollay Reference Thiollay2005, Uezu et al. Reference Uezu, Beyer and Metzger2008). Empirical studies from the Neotropics have shown that the non-forest matrix can be a barrier to the movement of individuals between native vegetation patches, restricting local populations to patch boundaries and increasing the isolation effect (e.g. Andrade and Marini Reference Andrade, Marini, Albuquerque, Cândido, Straube and Roos2001, Van Houtan et al. Reference Van Houtan, Pimm, Halley, Bierregaard and Lovejoy2007, Hansbauer et al. Reference Hansbauer, Storch, Leu, Nieto-Holguin, Pimentel, Knauer and Metzger2008, Lees and Peres Reference Lees and Peres2009) or suggest that fauna can use isolated forest fragments or even isolated trees to increase their mobility in a mosaic habitat (Desrochers and Hannon Reference Desrochers and Hannon1997, Guevara et al. Reference Guevara, Laborde and Sánchez1998, Lima and Gascon Reference Lima and Gascon1999, Tubelis and Tomás Reference Tubelis and Tomás1999).
Natural forest patches embedded in a grassland matrix (Rizzini Reference Rizzini1979, Meguro et al. Reference Meguro, Pirani, Mello-Silva and Giulietti1996) are typical of many Brazilian regions, including the Pantanal (a major wetland) (Junk Reference Junk, Whigham, Dykjová and Hejný1993) and the Cerrado (Rizzini Reference Rizzini1979) and are an ideal location for evaluating avian movement among patches due to the existence of a patchwork of natural isolated forest patches (Damasceno et al. Reference Damasceno, Bezerra, Bortolotto and Pott1996). We are aware, however, of only two studies that evaluated movement of birds between natural forest patches in the Neotropical region: Andrade and Marini (Reference Andrade, Marini, Albuquerque, Cândido, Straube and Roos2001) and Yabe and Marques (Reference Yabe, Marques, Albuquerque, Cândido, Straube and Roos2001). This study examined the influence of distance between forest patches on the movement birds in the Pantanal and tested the following hypotheses: (1) the bird movement rate between forests patches decreases with inter-patch distance; and (2) large-bodied species move longer distances than small species.
Methods
Study area
The study was conducted in 1999 near the Vermelho River (19°36’S 56°56’W) in the Abobral sub-region of the Pantanal, municipality of Corumbá, state of Mato Grosso do Sul, Brazil. The Abobral sub-region (PCBAP 1997) is characterised by capões (herein ‘forest patches’) which are patches 1–2 m above the seasonally flooded landscape. These forest patches have arboreal-shrubby vegetation and are surrounded by natural grasslands. Vegetation on the edge of forest patches is composed of species from grasslands, flooded savanna or riparian forest. The central portion of the patch supports semi-deciduous species characteristic of non-flooded areas (Damasceno et al. Reference Damasceno, Bezerra, Bortolotto and Pott1996). The ecotones between forest patches and grasslands are steep and characterised by the presence of the palm Scheelea phalerata (Mart.) Bur, Arecaceae. The limit between the two types of vegetation is maintained by a small variation in topography and flood pulsation (Junk Reference Junk, Whigham, Dykjová and Hejný1993). The most important economic activities in the region are extensive cattle raising, fisheries and ecotourism. The study area lies in the centre of a cattle farm.
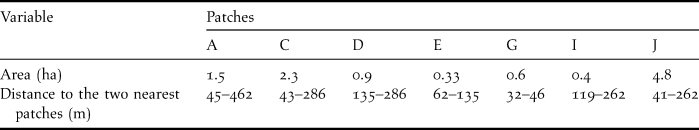
To detect bird movements we observed and captured birds in the seven largest patches of the study site (A, C, D, E, G, I, and J in Figure 1). These patches varied in size from 0.3 to 4.8 ha (Table 1). We measured the shortest distances between any two patches with a tape (distances < 300 m) or with program AutoCad version 14 (Autodesk Inc.) for distances > 300 m. Inter-patch distance between two adjacent patches varied from 32 to 462 m (mean = 164.5 ± 71.5 m) (Table 1).

Figure 1. Map of South America showing the location of the study area in the Pantanal region. Schematic drawing of the 11 forest patches in the Abobral sub-region of the Pantanal, state of Mato Grosso do Sul, Brazil.
Table 1. Area of the seven patches sampled in Pantanal, Brazil and the distances between them.
Data collection
We established two sampling points at the edge of each of the seven patches, each point facing the two closest adjacent patches. The points were established near the shortest distance between two patches, allowing good observation of bird movements, but with enough distance not to affect bird gap-crossing behaviour. For patch A, one of the two sampling points was between the patch and the riparian forest of the Vermelho River. Observations were conducted by one observer (RSY) during seven consecutive mornings in March, May, August, September and October 1999. Three hours of observations (1h30 for each edge) were conducted between 06h00 and 09h30, alternating 30 min observations between the two sampling points of a patch, with 5 min intervals for the observer to move between the points. This period was chosen due to greater bird movement than at other hours of the day. Since not all patches could be seen from the observation point, those that could were identified before starting observations. We used a 2-m high platform to have a free view of birds flying above the vegetation. For each individual bird, we recorded the source and the destination patches (= one observation) using 10 × 50 Nikon binoculars. The movement of each monospecific flock was counted as one observation. Each movement was classified either as movement between known or between unknown patches. Movements between known patches included those between identified source and destination patches, either adjacent or not. Movements between unknown patches are those between non-adjacent patches in which one of the patches, inside the study area or not, was not identified. We were able to identify most species; however, three species of Myiarchus flycatchers and two species of Turdus thrushes were identified at the genus level and hummingbirds (six species) at the family level. Thus, species from these groups were excluded from species-level analyses. Nomenclature followed Birdlife International’s taxonomy (Remsen et al. Reference Remsen, Cadena, Jaramillo, Nores, Pacheco, Robbins, Schulenberg, Stiles, Stotz and Zimmer2009).
We banded birds for 14 days (two days per patch) in January, February, April and September 1999, in the same patches where we carried out the observations. We used 6-m long by 2.6-m high mist-nets with 36 mm mesh size, opened for five hours from sunrise. This procedure was repeated on two consecutive mornings. The number of nets used varied with patch size: patches > 1 ha = 20 nets (10 in the interior and 10 at the edge) versus patches < 1 ha = 16 nets (eight in the interior and eight at the edge). All birds captured received a metal band, were weighed and immediately released < 5 m from the edge of the patch where they were captured.
Species were classified in relation to their level of dependence on forests according to Silva (Reference Silva1995) as: independent (lives in open vegetation), semi-dependent (lives in both open vegetation and forests), and dependent (lives mainly in forest habitat). We calculated the average distance travelled between two patches for each species, considering all possible combinations of patches (mean = sum of distances travelled divided by number of movements).
To test the hypothesis that bird movement rate (movements/hour) decreased with inter-patch distance we used a simple linear regression with log-log transformed data. We used the distances of paths between patches that were travelled by birds to run this test. To test the hypothesis that large-bodied species can move longer distances than small-bodied species, we correlated mean body mass against median distance moved and maximum distance moved using Spearman rank correlations. Body mass was based mainly on captured birds at the study site (Yabe Reference Yabe2001) and complemented with data from the literature (Marini et al. Reference Marini, Motta-Júnior, Vasconcellos and Cavalcanti1997, Piratelli Reference Piratelli1999, Sick Reference Sick2001, Antunes Reference Antunes2005, Dunning Reference Dunning2007) when sample sizes were smaller than five.
To analyse the association level of species with a given patch or group of patches, we used the Two-Way Indicator Analysis (TWINSPAN) classification method (Hill Reference Hill1979) using program PC-ORD version 4.17 (McCune and Mefford Reference McCune and Mefford1999). We analysed only forest-dependent and semi-dependent species with at least five observations.
Results
In total, 1,138 bird movements (105 hours of observation) were recorded including 842 movements between adjacent patches, 214 movements between non-adjacent patches and 82 movements with unknown origin or destination. Among these there were 55 movements by unidentified species, which were considered only in the movements/hour × distance correlation. Overall, 111 species observed and banded were identified in forest patches, including 20 forest-dependent, 49 semi-dependent and 42 independent. Since patches are usually very small, it is possible that other species from the region did not use them because of the low resources offered, even though they could be reached. Among these 111 species, we observed movement between patches of 14 forest-dependent species (229 movements), 28 semi-dependent species (496 movements) and 19 independent species (224 movements), for a total of 949 movements with identified species.
There was a significant negative correlation between overall movements/hour and distance (r 2 = −0.69, P < 0.01, n = 24 paths). This result was based on 1,056 movements, not considering the 82 movements with unknown origin or destination. The majority (55%) of these movements were over short (< 100 m) distances and performed by most (88%) species observed (Table 2). The results were similar when only forest-dependent and semi-dependent species were considered (Table 3), with a significant negative correlation between overall movements/hour and distance (r 2 = −0.67, P < 0.01, n = 23 paths; Figure 2). This result was based on 725 movements excluding the 82 movements with unknown origin or destination.
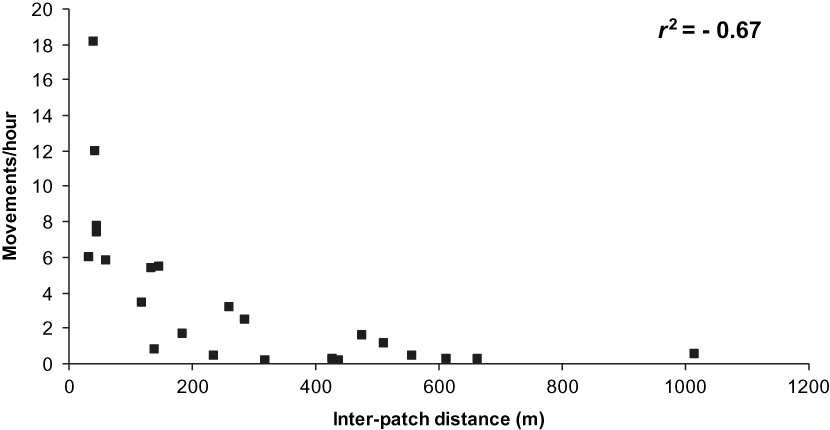
Figure 2. Relationship between number of movements/hour and inter-patch distance moved by forest dependent and semi-dependent birds flying between forest patches in the Pantanal.
Table 2. Number of observations (%), number of species (%), and the average number of movements per hour of all species for three distance classes for all forest patches in Pantanal, Brazil.

Table 3. List of forest dependent (D) and semi-dependent (SD) birds that moved between forest patches in Pantanal, Brazil, with corresponding body mass, median and maximum distance traveled and patches with higher association level (ND: species with non-preferential occurrence).

Large species usually moved longer distances than small species. A significant positive correlation was found between body size and median distance travelled (rs = 0.49, P = 0.004, n = 34 species) and maximum distance travelled (r s = 0.33, P = 0.057, n = 34 species). When we analysed only the 16 Passeriformes with body mass up to 100 g (mean = 41.5 ± 16.71 g), there was no significant correlation between body mass and either median or maximum distance (both r s < 0.08, P > 0.23). No small-bodied bird moved more than 286 m, as was observed for medium and large-bodied birds (Table 3).
The patch destination for small-bodied species was always detected because small birds flew shorter distances, making it easier to observe their movements among patches. However, as expected, large birds were easier to identify than the small ones. Thus, records of unidentified birds correspond only to the movements of those < 60 g.
The TWINSPAN analysis, based on 1,127 observations, created two groups of patches (Eigenvalue = 0.36), those with area larger than or equal to 1.5 ha (patches A, C and J) and those smaller than 1 ha (patches D, E, G, and I). Twenty of the 30 species analysed revealed a greater association with the large patches (> 1 ha), seven showed no distinction between patch size and only five showed a higher association with small patches (< 1 ha) (Table 3).
Banding (4,960 mist-net hours) of 368 birds of 69 species (69 recaptures of 19 species; 42 recaptures in the same patch where the birds were banded and 27 recaptures in other patches) in the forest patches revealed that at least four forest-dependent and eight semi-dependent species used more than one forest patch as part of their home ranges. Birds of these species were captured in at least two or three forest patches within a nine month period (Table 4).
Table 4. Forest dependent and semi-dependent species whose banded individuals moved between forest patches in Pantanal, Brazil. Asterisk indicates that the same individual used different paths to travel between patches.

Evidence of forest-dependent and semi-dependent species using patches as stepping stones was obtained via recaptures and observations. For example: a) a banded Red-billed Scythebill Campyloramphus trochilirostris travelled from patch A to patch C (distance = 148 m) and then from patch C to patch J (1,014 m). As the maximum distance observed for this species was 286 m (median = 32 m; n = 21 observations), this individual is likely to have used the other patches between patches C and J to reach patch J; b) a banded Pale-crested Woodpecker Celeus lugubris travelled from patch J to G (distance = 477 m). This species was observed six times moving between adjacent patches and the maximum distance travelled was 262 m (median = 82 m). Hence, this individual most likely used patches H and I to reach patch G; c) a banded Little Woodpecker Veniliornis passerinus travelled between patches, as follows: from G to D (distance = 680 m), from D to I (distance = 520 m) and from I to E (distance = 337 m). As the maximum distance observed was 138 m (median = 46 m; n = 51 observations), this individual most likely reached the patches where it was recaptured using the other patches between them.
Discussion
The majority of forest-dependent and semi-dependent birds inhabiting the Pantanal seem to be able to cross gaps between forest patches, although a number of species that did not cross due to an increase in distance between patches, as was observed by Lees and Peres (Reference Lees and Peres2009). Despite these frequent movements, most birds did not cross large open areas, because of higher energy needs, metabolic costs, or potential predatory risk (Grubb and Doherty Reference Grubb and Doherty1999).
Movements among various patches allow individuals to secure resources (e.g., food, mates) that may be rare in small patches due to the reduced area. Multiple patch use was detected in forest-dependent birds in the gallery-forest/grassland landscape in the Cerrado (Andrade and Marini Reference Andrade, Marini, Albuquerque, Cândido, Straube and Roos2001). Large bodied species (> 200 g) are probably using forest patches for feeding or dispersing through the landscape. Blue-fronted Amazon Amazona aestiva, Crested Oropendola Psarocolius decumanus and Toco Toucan Ramphastos toco were observed crossing savannas to reach forests more than 1,000 m away in the Brazilian Cerrado (Tubelis et al. Reference Tubelis, Cowling and Donnelly2004). Toco Toucan was also observed in the southern Pantanal flying over large open areas searching for food resources, and moving around a habitat mosaic according to fruit availability in different vegetation types (Ragusa-Netto Reference Ragusa-Netto2006).
This study found that several species, both medium (> 100 g and < 200 g) and large-bodied birds with varying diets, were able to cross distances longer than 400 m. This is in agreement with Lees and Peres (Reference Lees and Peres2009) who found that body mass was consistently the most important predictor variable of the movement of birds across gaps, with large-bodied birds crossing gaps more frequently than small-bodied birds. Some species were able to move very large distances (i.e. 1,014 m) and this was typically associated with frugivores which are extremely vagile and able to cross large distances to secure patchy resources. Medium to large-bodied canopy birds that make daily long-distance foraging forays, flying high above the canopy show little or no evidence of reduced movement rates across wider gaps (Lees and Peres Reference Lees and Peres2009). Nevertheless, the size of patches is critical since a shortage of resources is expected in small ones (Willis Reference Willis1979, Silva and Tabarelli Reference Silva and Tabarelli2000). However, it is also possible that medium to large-bodied frugivores will use a group of patches as a single unit therefore increasing their available space and maintaining resource needs (Whitcomb et al. Reference Whitcomb, Whitcomb and Bystrak1977, Yabe and Marques Reference Yabe, Marques, Albuquerque, Cândido, Straube and Roos2001).
The spatial arrangement and the size of forest patches may affect species’ movements. Most species were more associated with large patches in the study area. The small patches located in the central part of the study site are probably being used as stepping stones, mainly by the small-bodied species, increasing their chances of exploring resources in the landscape. As most small birds seemed to prefer to fly between adjacent forest patches, the semi-linear arrangement in a clustered sequence may increase the functionality of patches so that they act like a biological corridor (Dunning et al. Reference Dunning, Borgella, Clements and Meffe1995, Rosenberg et al. Reference Rosenberg, Noon and Meslow1997, Beier and Noss Reference Beier and Noss1998) and allow several species to reach patches that otherwise could not be reached. Other large-bodied species, however, such as Blue-throated Piping-guan Pipile cumanensis, may use only the large forest patches, since Pipile species are large forest-dependent frugivores associated with extensive continuous forests (e.g. riparian forests; del Hoyo et al. Reference del Hoyo, Elliot and Gardatal1994) or cordilheiras (Tubelis and Tomás Reference Tubelis and Tomás1999).
In conclusion, our results showed that both fragment size and distance between forest patches may affect the use of patches by birds. Small forest patches may be inappropriate habitat for many large-bodied species and more isolated forest patches are more difficult to use by small-bodied species. Thus, the size and spatial arrangement of habitat patches may act as a filter to species occurring in the landscape. The findings of this study indicate that stepping stones can provide a viable alternative to continuous biological corridors if the latter is not possible. Also, responses of Neotropical birds to predicted climate-driven change in the geographical distribution (Marini et al. Reference Marini, Barbet-Massin, Lopes and Jiguet2009) are dependent on birds’ ability to move through the landscape, as has been shown here. However, the type of species in an area, the size and the distance between vegetation patches are all critical factors that must be considered in projects that aim to use forest patches as stepping stones to increase or maintain local bird biodiversity.
Acknowledgements
We thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for the master fellowship to R.S.Y. We also thank CNPq and Graduate Program in Ecology and Conservation of Universidade Federal de Mato Grosso do Sul for equipment and logistical support. We thank K. DeMatteo and D. P. Tubelis for help with a previous version and B. Schwantes Marimon for help with TWINSPAN program. The manuscript was benefited from the comments of Camila Donatti and an anonymous referee.








