Introduction
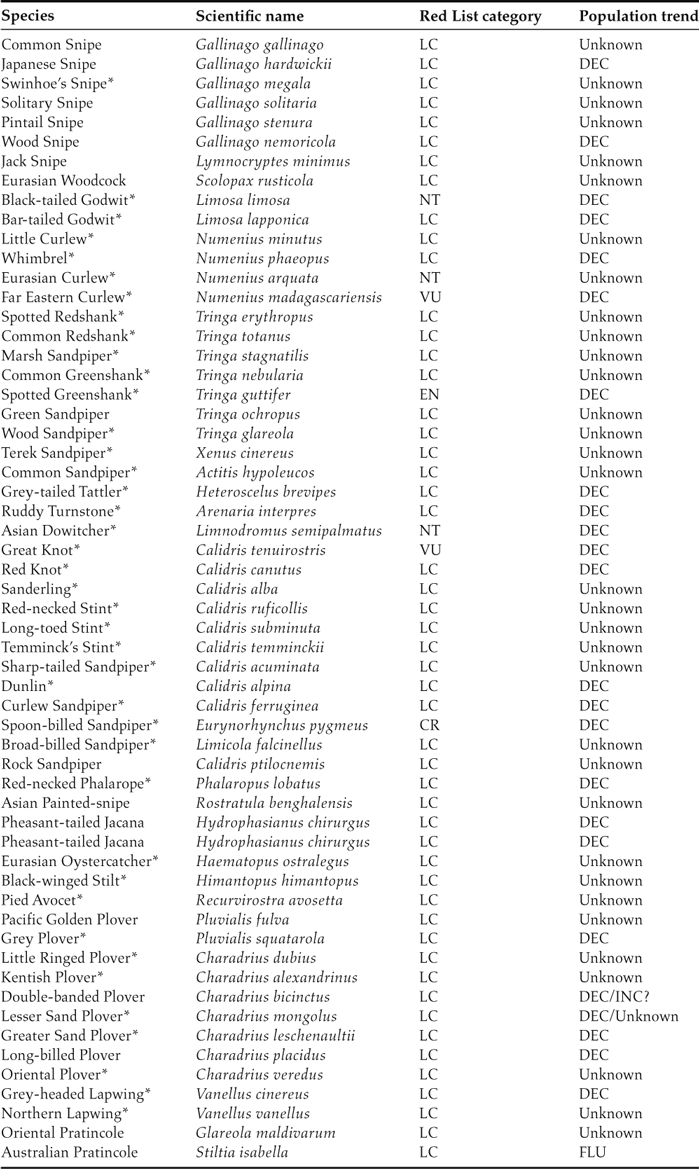
The migration of birds is an amazing natural phenomenon that is threatened by global change (Wilcove and Wikelski Reference Wilcove and Wikelski2008). Currently, one of the greatest crises of migratory birds is the widespread population decline of migratory shorebirds in the East Asian-Australasian Flyway (EAAF) (Kirby Reference Kirby2011, MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012). The EAAF covers a vast region stretching from the Arctic in Siberia and Alaska southwards through East and South-East Asia to Australia and New Zealand (Figure 1). Among the global flyways, the EAAF supports the highest numbers of shorebird species and individuals (58 species and five million individuals; Table 1) (Wilson Reference Wilson2003, Stroud et al. Reference Stroud, Baker, Blanco, Davidson, Delany, Ganter, Gill, González, Haanstra, Morrison, Piersma, Scott, Thorup, West, Wilson, Zöckler, Boere, Galbraith and Stroud2006) but also includes the highest number of threatened species and declining populations (Kirby Reference Kirby2011, Wetlands International 2012). Among the migratory species with known population trends, 88% (22 of 25 species) show population declines, and seven have been listed as threatened or Near Threatened in the IUCN Red List (Table 1; data from Wetlands International 2012). It is clearly important to determine the reasons for these population declines and that we take effective conservation measures.
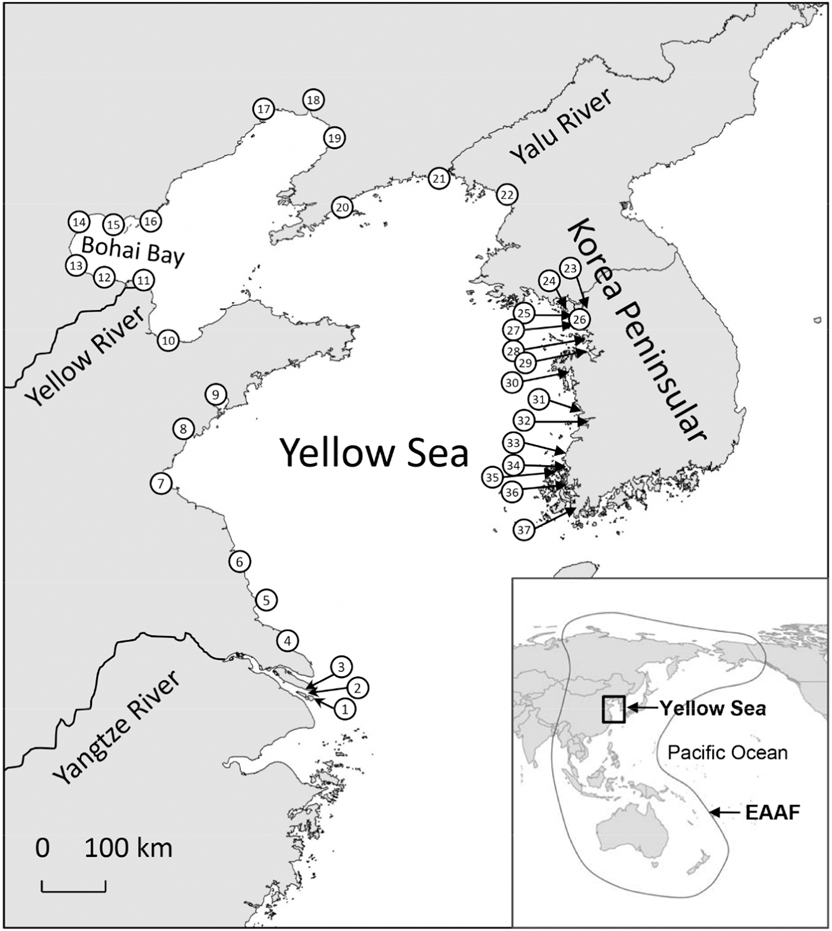
Figure 1. Location of sites that support at least one shorebird species that meets the 1% criterion of Ramsar sites (the site is visited by > 1% of the total population in the flyway). Site names in the figures: 1: Jiuduansha Nature Reserve; 2: Hengsha East Shore; 3: Chongming Dongtan Nature Reserve; 4: Rudong Coast; 5: Dongsha Islands; 6: Yancheng Nature Reserve; 7: Haizhou Bay; 8: Qizi Bay; 9: Jiaozhou Bay; 10: Laizhou Bay; 11: Yellow River Delta Nature Reserve; 12: South Bohai Bay; 13: Southwest Bohai Bay; 14: Northwest Bohai Bay; 15: Luannan Coast; 16: Shi Jiu Tuo/Daqing He; 17: Linghe Estuary; 18: Shuangtaizihekou Nature Reserve; 19: Northeast Liaodong Bay; 20: South Dalian Peninsula; 21: Yalu Estuary Nature Reserve; 22: Mundok Migratory Bird Wetland Reserve; 23: Han-Imjin Estuary; 24: Ganghwa Island; 25: Yeongjong Island (south); 26: Song Do; 27: Daebu Island; 28: Namyang Bay; 29: Asan Bay; 30: Cheonsu Bay; 31: Geum Estuary; 32: Saemangeum Area; 33: Paeksu Tidal flat; 34: Hampyeong Bay; 35: Muan-Gun Tidal flats; 36: Aphae Island; 37: Haenam Tidal flats. Data are absent for North Korea except for limited information from Mundok Migratory Bird Wetland Reserve. Barter (Reference Barter2002) included Suncheon Bay and Nakdong Estuary along the southern coast of the Korean Peninsula among 27 sites that meet the 1% criterion of Ramsar sites. We did not include these two sites because they are outside of the traditional Yellow Sea region. Data sources: Barter (Reference Barter2002), Moores (Reference Moores2006), Yang (Reference Yang2006), and China Coastal Waterbird Census Group (2009, 2011).
Table 1. Checklists of migratory shorebirds in the Yellow Sea. Species that are only occasionally recorded in the Yellow Sea but are abundant in other flyways are not included. Species that meet the 1% criterion of Ramsar sites (the site is visited by > 1% of the total number in the flyway) at one or more sites in the Yellow Sea are marked with an asterisk (*). LC, NT, VU, EN, and CR indicate the IUCN Red List categories of Least Concern, Near Threatened, Vulnerable, Endangered, and Critically Endangered, respectively. Under “Population trend,” DEC, INC, and FLU indicate that the population has been decreasing, increasing, or fluctuating, respectively. Data are from Barter (Reference Barter2002), Chen (Reference Chen2006), Moore (Reference Moores2006), Bamford et al. (Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008), and China Coastal Waterbird Census Group (2009, 2011). Population trends are from Wetlands International (2012).
Because the environment in shorebird breeding and non-breeding grounds has been relatively stable, the population decline of migratory shorebirds in the EAAF has been linked to the loss and degradation of stopping sites (including staging and stopping sites) en route (reviewed in MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012). Most shorebirds depend on intertidal wetlands at stopping sites and non-breeding grounds. Many researchers have concluded that the major cause of population declines in the EAAF is the loss of intertidal wetlands in the Yellow Sea (Figure 1), which contains the most important stopping sites in the flyway (e.g. Rogers et al. Reference Rogers, Hassell, Oldland, Clemens, Boyle and Rogers2009, Amano et al. Reference Amano, Székely, Koyama, Amano and Sutherland2010, Wilson et al. Reference Wilson, Kendall, Fuller, Milton and Possingham2011, Szabo et al. Reference Szabo, Butchart, Possingham and Garnett2012).
The Yellow Sea (including Bohai Bay) is a semi-enclosed, shallow sea between the Korean Peninsula in the east and the Chinese mainland in the north and west (Figure 1). It extends for 1,000 km from north to south and 700 km from east to west. The total area of intertidal wetlands in the Yellow Sea is about 20,000 km2 (Chen Reference Chen2006). Field surveys have revealed that the Yellow Sea region is critical for migratory shorebirds in the EAAF (Barter Reference Barter2002, Chen Reference Chen2006, Moores Reference Moores2006, Bamford et al. Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008). Annually, over two million shorebirds, or about 40% of the total birds in the flyway, stop over in the Yellow Sea during their northward migration, and about one million during their southward migration (Barter Reference Barter2002, Bamford et al. Reference Bamford, Watkins, Bancroft, Tischler and Wahl2008). At least 41 shorebird species occur in “internationally important numbers” (> 1% of the total number in the flyway) at one or more sites in the Yellow Sea (Table 1), and 37 sites support (or until recently supported) at least one shorebird species with internationally important numbers (Figure 1). Moreover, more than 30% of the estimated flyway breeding populations of 18 shorebird species stop over in the Yellow Sea during their northward migration, and the region supports almost the entire flyway breeding population for at least six species (Barter Reference Barter2002).
Many shorebird species use intertidal wetlands, and the intertidal specialists are restricted to these habitats except when breeding. However, intertidal wetlands in the Yellow Sea have suffered serious pressure from increases in the human population and rapid economic development. About 600 million people (> 8% of the global population) live in the Yellow Sea region in China, North Korea, and South Korea (Chen Reference Chen2006) and this region is experiencing rapid economic development. This has caused dramatic environmental changes, including the loss of intertidal wetlands, spread of invasive smooth cordgrass Spartina alterniflora on intertidal flats, an increase in pollution and an increase in human disturbance. All of these have seriously harmed migratory shorebirds (reviewed in MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012).
Developing effective measures for the conservation of shorebirds will depend on understanding their current status, population trends and the effects of environmental change. Research can provide solid evidence of the actual effect of threats on shorebirds and predict what will happen in the future, thus offering guidance for conservation actions. This paper reviews recent studies on shorebirds in the Yellow Sea and discusses key research on six topics required for prioritizing conservation objectives, setting targeted actions and evaluating conservation effectiveness (Table 2): 1) confirming the population consequences of loss of stopping sites; 2) estimating migration timing and numbers of shorebirds at stopping sites; 3) determining the differing abilities of species to use alternative habitats; 4) understanding intra- and interspecific differences in the use of stop-over sites; 5) maintaining and expanding surveys of shorebirds and their habitats; 6) Identifying threats to shorebirds beyond habitat loss by reclamation.
Table 2. Key research issues and their main targets for conservation actions

1) Understanding the population consequences of loss of stopping sites
Although many studies have linked shorebird population declines in the EAAF to the loss of stopping sites in the Yellow Sea, solid evidence is still lacking. A frequently mentioned piece of evidence is that the population decline of Great Knots Calidris tenuirostris in their non-breeding grounds followed reclamation at the Dongjin and Mangyeong Estuary (Saemangeum) on the east coast of the Yellow Sea. This reclamation, which caused a decline of nearly 90,000 Great Knots in the enclosed and surrounding area during northward migration (Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008), just matched the population decline of Great Knots on the main non-breeding grounds in north-west Australia (Rogers et al. Reference Rogers, Hassell, Oldland, Clemens, Boyle and Rogers2009). At the same time, however, the Great Knot wintering population was increasing in South-East Asia, perhaps because of a northward shift in their non-breeding distribution (Round Reference Round2006, D. Melville pers. comm.).
Loss of stopping sites, especially refuelling sites, can have two important consequences for shorebird populations: 1) Birds may not be able to store sufficient fuel for the ongoing migratory flight and thus fail to arrive at their destination; and 2) birds can arrive at their destination but perform poorly because of the carry over effects of inadequate refuelling en route, which could reduce reproductive success (Morrison et al. Reference Morrison, Davidson and Wilson2007, Vézina et al. Reference Vézina, Williams, Piersma and Morrison2012).
Comparing population survival rates at different life history stages is an effective method for elucidating the population consequences of loss of stopping sites. Over the past two decades in the EAAF, hundreds of thousands of shorebirds have been individually marked with traditional metal rings, engraved leg-flags, and combinations of colour rings (Minton Reference Minton and Straw2005, Tang et al. Reference Tang, Xue, Ma and Feng2011), providing an opportunity to estimate population survival rates at different stages in the annual cycle according to the recapture and resighting of marked individuals (Leyrer et al. Reference Leyrer, Lok, Brugge, Spaans, Sandercock and Piersma2013, Lok et al. Reference Lok, Overdijk, Tinbergen and Piersma2013, Piersma et al. in prep.). Although monitoring population status is difficult at breeding grounds, where birds generally disperse over a large area, shorebirds concentrate in large flocks at stopping and wintering sites. The increasing numbers of birdwatchers and volunteers in the Yellow Sea region has led to a substantial increase in the reporting rate of marked individuals (e.g. Minton et al. Reference Minton, Gosbell, Johns, Fox and Afanasyev2011a, China Coastal Waterbird Census Group 2011).
Moreover, the combination of survival modelling with monitoring the age structure of populations (e.g. the ratio of first-year birds to total birds in non-breeding grounds) may provide the first indications of change in populations, which will be helpful in detecting the effects of loss of stopping sites on reproductive success and/or population dynamics of migratory shorebirds (Minton et al. Reference Minton, Jessop, Collins, Gosbell and Straw2005, Robinson et al. Reference Robinson, Clark, Lanctot, Nebel, Harrington, Clark, Gill, Meltofte, Rogers, Rogers, Ens, Reynolds, Ward, Piersma and Atkinson2005, Rogers et al. Reference Rogers, Rogers, Barter and Straw2005). Most shorebird species have differences in moult between age classes (e.g. adult vs. first-year birds); this makes it feasible to record the age ratio in flocks through visual scans (Lemke et al. Reference Lemke, Bowler and Reneerkens2012). Monitoring age structure also helps to understand the effects of environmental changes on different age classes (e.g. Gill et al. Reference Gill, Alves, Sutherland, Appleton, Potts and Gunnarsson2014).
2) Estimating migration timing and numbers of shorebirds at stopping sites
Understanding the importance of a stopping site depends on accurate estimates of the numbers of birds that use the site. Since the 1980s in South Korea and since the mid-1990s in China, shorebird surveys conducted along the coasts of the Yellow Sea have provided valuable data concerning the importance of this region for migrating shorebirds in the EAAF (Table 1, Figure 1). Most of these surveys are “peak counts”, i.e. they are conducted at the peak of migration. However, because arrival and departure times differ among individuals, the peak number of shorebirds that use a site will be consistent with the actual number only when departure occurs after all the individuals have arrived. Otherwise, peak counts will underestimate the number of birds by missing either the early departures or the late arrivals. Such underestimation is common at temporary stop-over sites where the migration period of the population is much longer than the length of stay of individual birds. At Chongming Dongtan in the southern Yellow Sea, for example, the migration period of Great Knots lasts for one month while the length of stay of individual birds is shorter than three days (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013). As a consequence, many more birds pass through Chongming Dongtan than are detected by peak counts during migration.
Comparing the migration periods of species and length of stay of individuals is helpful in estimating the population turnover rate and thus the total number of birds that a site supports. Although the migration periods of species can be determined by bird counts, length of stay of individual birds is difficult to determine. Determining the arrival and departure times of individual birds has become increasingly possible because of the recent miniaturisation of remote-tracking equipment (satellite tags can weigh < 5 g) (e.g. Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell, Schuckard, Melville and Riegen2012). Light-level geolocators also provide information of time schedules, especially for small and medium-sized species, and have been used for shorebirds in the EAAF (Minton et al. Reference Minton, Gosbell, Johns, Christie, Klaassen, Hassell, Boyle, Jessop and Fox2011b, Conklin et al. Reference Conklin, Battley and Potter2013). However, the migration routes of only a few species along the EAAF have been established with satellite tags (e.g. Bar-tailed Godwit Limosa lapponica, Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell, Schuckard, Melville and Riegen2012; Far Eastern Curlew Numenius madagascariensis, Driscoll and Ueta Reference Driscoll and Ueta2002) or light-level geolocators (e.g. Ruddy Turnstone Arenaria interpres and Greater Sand Plover Charadrius leschenaultia, Minton et al. Reference Minton, Gosbell, Johns, Christie, Klaassen, Hassell, Boyle, Jessop and Fox2011b).
Length of stay of individual birds can also be estimated by the resighting of marked individuals in the field and the use of a capture-recapture model (Schaub et al. Reference Schaub, Pradel, Jenni and Lebreton2001, Verkuil et al. Reference Verkuil, Wijmenga, Hooijmeijer and Piersma2010, Masero et al. Reference Masero, Santiago-Quesada, Sánchez-Gusmán, Villegas, Abad-Gómez, Lopes, Encarnação, Corbacho and Morán2011) and, when modelled together with regular count data, researchers can estimate the total number of birds passing through (e.g. Gillings et al. Reference Gillings, Atkinson, Baker, Bennett, Clark, Cole, González, Kalasz, Minton, Niles, Porter, Serrano, Sitters and Wood2009). In addition, modelling of continuous bird count data can also be used to estimate the actual number of birds at the stopping sites (Thompson Reference Thompson1993, Rogers et al. Reference Rogers, Yang, Hassell, Boyle, Rogers, Chen, Zhang and Piersma2010, Choi et al. Reference Choi, Battley, Potter, Rogers and Ma2014a). This approach can be used on all species, in contrast to satellite telemetry (only possible on larger species) or geolocators (only practical for species with high recapture rates to retrieve geolocators).
Changes in migration timing at stopping sites (including changes in the rates of mass gain or a change in body condition at departure, see below) might reflect habitat changes and could be an early warning of population decline. For example, when food is insufficient at a site, birds might depart later and/or leave the site with lower fuel store than usual (Baker et al. Reference Baker, González, Piersma, Niles, de Lima Serrano do Nascimento, Atkinson, Clark, Minton, Peck and Aarts2004). Against the background of global climate change, migration timing of shorebirds may not keep pace and phenological mismatch between breeding and times of peak food availability might occur (e.g. Pearce-Higgins et al. Reference Pearce-Higgins, Yalden and Whittingham2005). At the moment there is still a lack of studies on the population consequences of changes in shorebird migration timing at stopping sites in the EAAF.
3) Determining different abilities of species to use alternative habitats
Although natural intertidal wetlands have been rapidly lost over the past three decades in the Yellow Sea (Ma et al. Reference Ma, Melville, Liu, Chen, Yang, Ren, Zhang, Piersma and Li2014, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014), the area of artificial wetlands has increased, at least in China, because large areas of intertidal wetlands have been enclosed and changed into aquaculture ponds, saltworks, and paddyfields (Niu et al. Reference Niu, Zhang and Wang2012). Many studies have indicated that artificial wetlands provide both foraging and roosting habitats for various shorebirds (Masero et al. Reference Masero, Pérez-Hurtado, Castro and Arroyo2000, Barter et al. Reference Barter, Riegen and Xu2003, Ma et al. Reference Ma, Li, Jing, Tang and Chen2004, Moores Reference Moores2006) and may mitigate the adverse effects of loss of intertidal wetlands. Effective management plays a critical role in enhancing the habitat quality of artificial wetlands for shorebirds (Elphick Reference Elphick1996, Erwin Reference Erwin2002, Ma et al. Reference Ma, Cai, Li and Chen2010). Unfortunately, artificial wetlands cannot completely substitute for natural intertidal wetlands as shorebird habitats (Ma et al. Reference Ma, Li, Jing, Tang and Chen2004). Specialists that rely exclusively on intertidal wetlands as foraging habitats are more likely to suffer from the loss of intertidal wetlands than habitat generalists.
A recent study indicated that, in the Africa-Eurasian flyway, Ruff Philomachus pugnax changed their migration route in response to the loss of refueling sites along their original migration route (Verkuil et al. Reference Verkuil, Karlionova, Rakhimberdiev, Jukema, Wijmenga, Hooijmeijer, Pinchuk, Wymenga, Baker and Piersma2012). Similarly, the use of new refueling sites by Black-tailed Godwit Limosa limosa islandica in the Netherlands and eastern England appears to be a response to environmental changes on their migration route (Alves et al. Reference Alves, Gunnarsson, Potts, Gélinaud, Sutherland and Gill2012). For those intertidal specialists in the EAAF, however, change in migration route is unlikely because the Yellow Sea is irreplaceable along their migration. Two case studies indicated that the response to the loss of intertidal wetlands in the Yellow Sea might be different for intertidal specialists (like Red Knot Calidris canutus and Great Knot) than for other shorebirds: first, after the surrounding intertidal wetlands had been lost on the Luannan Coast, Red Knots concentrated in the small area of intertidal wetlands that remained (Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011); second, the local population of Great Knots dramatically decreased after reclamation of the Saemangeum area (Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008). This suggests that population declines caused by reclamation of stopping sites may vary according to the condition of surrounding habitats: the very abundant food along the Luannan Coast (Yang et al. Reference Yang, Chen, Ma, Hua, van Gils, Zhang and Piersma2013) can still support large numbers of Red Knots, while high quality foraging habitats might be limited in the regions surrounding Saemangeum. Monitoring the population dynamics and spatial distribution of intertidal specialists will help us understand the response of shorebirds to habitat loss and help answer questions such as “Will some species refuel in the middle or southern Yellow Sea if their refuelling sites in the north have been lost?” Satellite tracking is an effective method to detect flexibility in habitat use (or the shift of stopping sites) after the loss of intertidal habitats. Moreover, population monitoring will also help researchers identify those forces that drive the evolution of migration routes and strategies.
4) Understanding intra- and interspecific differences in the use of stopping sites
Stopping sites can be classified as staging sites, where birds deposit large amounts of fuel, or as stop-over sites, where birds deposit smaller amounts of fuel or do not refuel at all (Warnock Reference Warnock2010). As critical refuelling sites for migratory birds, staging sites are a conservation priority. Although field surveys on the species and numbers of individuals at different stopping sites have provided valuable data for illustrating the importance of the Yellow Sea for shorebirds (Barter Reference Barter2002, Chen Reference Chen2006, Moores Reference Moores2006), the functions of different stopping sites for shorebirds are still largely unexplored. For the northward migration of Great Knots, recent studies have indicated that the northern Yellow Sea provides critical refuelling sites, while the southern Yellow Sea supports temporary stop-over sites for weak individuals or for individuals under bad weather conditions (Ma et al. Reference Ma, Hua, Zhang, Guo, Zhao, Ma, Xue and Tang2011, Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013). Studies have also indicated that Red Knots in the northern Yellow Sea take on large amounts of fuel that support both their northward migratory flight and their breeding ground activities (Hua et al. Reference Hua, Piersma and Ma2013). These results highlight the importance of conserving refuelling sites in the northern Yellow Sea. The temporary stop-over sites in the southern Yellow Sea are also important for maintaining a stable population as they provide “stepping stones” for weak individuals and for birds experiencing bad weather (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013).
There are also interspecific differences in the spatial distribution of refuelling sites for shorebirds in the Yellow Sea. Although species that migrate via “long-distance jumps” (such as Great Knots and Red Knots; Piersma Reference Piersma1987) are likely to use only the northern Yellow Sea for their refuelling sites during northward migration, those species that migrate via “short-distance hops and skips” may require more refuelling sites in the southern and middle Yellow Sea. Moreover, habitat and food conditions affect the use of refuelling sites. For example, although both Red and Great Knots mainly consume bivalves in the northern Yellow Sea, the Red Knots concentrate in a small area on the Luannan Coast (Yang et al. Reference Yang, Chen, Barter, Piersma, Zhou, Li and Zhang2011) and are uncommon at other sites along the eastern and northern coasts of the Yellow Sea. Great Knots, however, are dominant in the Yalu Estuary and Shuangtaizi Estuary in the northern Yellow Sea and also form large flocks on the Luannan Coast (Barter Reference Barter2002, Chen Reference Chen2006). This might be related to the different sizes of bivalves on the tidal flats among sites: being small, the Red Knots prefer small bivalves that are dominant along the Luannan Coast, while the larger Great Knots prefer the larger bivalves that are dominant in the Yalu Estuary but they can also forage on small bivalves (Yang et al. Reference Yang, Chen, Ma, Hua, van Gils, Zhang and Piersma2013, H. B. Peng and Z. J. Ma pers. obs.). Curlew Sandpipers Calidris ferruginea concentrate on the Luannan Coast, perhaps because the large area of saltworks there provides them with suitable foraging habitat (Yang Reference Yang2006). Those habitat and food specialists might suffer more seriously from habitat loss and degradation than those generalists (Rogers and Gosbell Reference Rogers and Gosbell2006, MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012, Yang et al. Reference Yang, Chen, Ma, Hua, van Gils, Zhang and Piersma2013).
As indicated in the previous paragraph, understanding the habitat and food conditions at different sites is helpful for understanding the spatial distribution of shorebirds. Except for a few sites (Zhu et al. Reference Zhu, Jing, Gan and Ma2007, Yang et al. Reference Yang, Chen, Ma, Hua, van Gils, Zhang and Piersma2013, Choi et al. Reference Choi, Battley, Potter, Ma and Liu2014b), however, such information is unavailable for the Yellow Sea. Although there have been extensive surveys on the macrobenthos along the coasts of the Yellow Sea, most of these surveys have focused on economic species, which might be unavailable to shorebirds. Moreover, reclamation, pollution, eutrophication, and overexploitation over the past two decades have seriously damaged the benthic community along the Yellow Sea coast, and this damage has been both direct and indirect; indirect damage results from changes in hydrology, sedimentation, and trophic levels (Choi et al. Reference Choi, Lee, Lim, Walton and Park2010). This damage to the benthic community has affected, and will continue to affect, use of the Yellow Sea by shorebirds. Understanding habitat and food conditions at different sites also helps in predicting the effects of ongoing and future environmental changes on shorebirds.
5) Maintaining and expanding surveys on shorebirds and their habitats
In order for conservation to be effective, research on demography and the annual cycle is essential to demonstrate where and when the population occurs in the flyway during the year. Although shorebird counts have indicated that a total of 37 sites in the Yellow Sea region support at least one shorebird species that meets the 1% criterion of Ramsar sites (Figure 1), those data were gathered from shorebird surveys since 1990s; some sites have not been visited again in recent decades. Because reclamation and development have dramatically changed the coastline of the Yellow Sea (MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012, Ma et al. Reference Ma, Melville, Liu, Chen, Yang, Ren, Zhang, Piersma and Li2014, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014), intensive development at some sites might have weakened their function as shorebird habitats. Regular surveys of shorebirds are important to evaluate conservation effectiveness at important sites (e.g. Ramsar sites and Important Bird Areas) and to take conservation action to reverse the disadvantageous changes.
With the rapid development of birdwatching in mainland China since the year 2000, an increasing number of birdwatchers have travelled to the coast and counted shorebirds during the migration season (Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013). Although information is lacking about shorebirds during the southward migration in China up to the early 2000s (Barter Reference Barter2002, Chen Reference Chen2006), local birdwatchers and volunteers have since provided a large amount of information concerning the use of stopping sites by shorebirds in the Yellow Sea during both the northward and southward migration (e.g., China Coastal Waterbird Census Group 2009, 2011). For example, more than 100 individuals of the Critically Endangered Spoon-billed Sandpiper Eurynorhynchus pygmeus were recorded at Rudong in October 2011, 2012, and 2013, suggesting that this region has been a stable stop-over site during the southward migration (Tong et al. Reference Tong, Zhang, Li, Zockler and Clark2012, J. Li pers. comm.). Shorebird surveys during the southward migration at the Yalu Estuary detected at least 13 species whose numbers were > 1% of their total flyway population estimates, including a peak number of 7,486 Far Eastern Curlews, equivalent to 23% of the total flyway population (Bai et al. Reference Bai, Chen and Choi2012). This highlights the importance of the Yalu Estuary for shorebirds during their southward migration.
In contrast, the information about migratory shorebirds on intertidal wetlands in North Korea is quite limited. In light of the rapid loss of these wetlands in China and South Korea, the intertidal wetlands in North Korea, which have remained stable in area over the past three decades (Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014), might be increasingly important for the conservation of shorebirds along the EAAF.
In order to explain the population changes of shorebirds at stopping sites, one of the key issues is to track habitat changes. The rapid development of remote sensing techniques provides an effective tool for understanding land use and land cover changes over extensive areas and in the long-term (e.g. Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014). Monitoring habitat condition involves both abiotic and biotic factors, e.g. changes in the tidal area caused by reclamation, sedimentation, and erosion, vegetation area and composition (including the spread of invasive plants), macrobenthos that provide food for shorebirds, and pollutants at stopping sites (see below). All these might affect shorebirds directly or indirectly.
It is also important to gather the data on shorebirds and their habitats into a single database which is accessible to researchers, birdwatchers, conservationists and policy makers along the flyway. Comprehensive analysis of the data from long-term surveys at different stopping sites will be helpful in understanding the spatial and temporal changes in shorebirds associated with regional environmental changes, which are unlikely to cease in the near future.
6) Identifying threats to shorebirds beyond habitat loss by reclamation
Although the loss of intertidal wetlands in the Yellow Sea by reclamation is recognised as the most critical threat to migratory shorebirds along the EAAF (Moores et al. Reference Moores, Rogers, Kim, Hassell, Gosbell, Kim and Park2008, Amano et al. Reference Amano, Székely, Koyama, Amano and Sutherland2010, Wilson et al. Reference Wilson, Kendall, Fuller, Milton and Possingham2011), there are other threats. Pollution is a potentially serious, but overlooked, threat to shorebirds in the Yellow Sea. Many studies have indicated the adverse effects of organic and non-organic pollutants and bioaccumulation of pollutants amplifies the adverse effects on long-lived birds (Rowe Reference Rowe2008). Although the eastern coast of the Yellow Sea is relatively free of pollution (Hong et al. Reference Hong, Yim, Shim, Li and Oh2006), the northern and north-eastern coasts of China have experienced rapid industrial and agricultural development, resulting in substantial pollution of intertidal wetlands (Academic Divisions of CAS 2010, Zhang et al. Reference Zhang, Yu, Xu, Cao, Liu and Su2012). Recent data indicate that China’s coastal wetlands have suffered from serious pollution and that Bohai Bay in the north-western Yellow Sea has been particularly affected (SOA 2013). For example, the level of persistent organic pollutants in shellfish exceeded the quality control standards of the U.S. Environmental Protection Agency at most sampling sites in Bohai Bay (Fan et al. Reference Fan, Chen, Liu, Lin, Xu, Hu and Tao2008). Shorebirds consume large amounts of macrobenthos for fuel deposition, and some species double their body mass during their stay in the Yellow Sea (Hua et al. Reference Hua, Piersma and Ma2013, Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013). Pollutants will be taken up along with nutrients when shorebirds feed. Because shorebirds are long-lived (many recaptured individuals are > 10 years old), the effects of pollutants and their bioaccumulation cannot be ignored.
Along China’s coasts, the exotic smooth cordgrass, which originated on the east coast of America and which was intentionally introduced into China in 1979, has spread rapidly through the intertidal wetlands over the past three decades and has occupied 25,000 ha in the Yellow Sea region (2007 data; Zuo et al. Reference Zuo, Liu, Zhao, Wang and Liang2009). Because cordgrass stands are dense, they hinder shorebird movements and the spread of cordgrass has therefore caused a net loss of shorebird habitat (Gan et al. Reference Gan, Cai, Choi, Ma and Chen2009). Cordgrass continues to spread on the intertidal wetlands, and such spread further adds to the losses caused by reclamation. Although there have been some trials of cordgrass removal from tidal flats (Ding et al. Reference Ding, Xu, Chen and Tang2011, GFN 2014), the effectiveness of these trials remain to be seen.
Another threat is the increase in human activity on the coast, which disturbs the foraging and roosting of shorebirds and thus decreases their fuel deposition efficiency at refuelling sites. The effects of human disturbance at refuelling sites might be especially serious when food is insufficient. In addition, poaching still occurs in the Yellow Sea region and especially in China, although hunting has been legally prohibited since the 1980s and has probably declined in recent years. Moreover, accidental by-catch of shorebirds in fishing nets on the intertidal flats frequently occurs in some regions (Z. J. Ma pers. obs.).
Yet another threat to shorebirds that use the Yellow Sea region for refuelling during migration is the loss of tidal flats driven by reduced sedimentation from major rivers because of dams and barrages (CCICED 2011, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014). Moreover, a rise in the level of the Yellow Sea (Iwamura et al. Reference Iwamura, Possingham, Chadès, Minton, Murray, Rogers, Treml and Fuller2013, Reference Iwamura, Fuller and Possingham2014) would further exacerbate the loss of available intertidal wetlands caused by reclamation and erosion.
Conclusions
Bird surveys conducted since the 1980s have revealed the importance of the Yellow Sea for migratory shorebirds in the EAAF (Barter Reference Barter2002, Chen Reference Chen2006, Moores Reference Moores2006). Recent international and regional cooperation among shorebird researchers has increased our understanding of the migration ecology of shorebirds (Rogers et al. Reference Rogers, Yang, Hassell, Boyle, Rogers, Chen, Zhang and Piersma2010, Battley et al. Reference Battley, Warnock, Tibbitts, Gill, Piersma, Hassell, Douglas, Mulcahy, Gartrell, Schuckard, Melville and Riegen2012, Ma et al. Reference Ma, Hua, Peng, Choi, Battley, Zhou, Chen, Ma, Jia, Xue, Bai, Wu, Feng and Tang2013, Yang et al. Reference Yang, Chen, Ma, Hua, van Gils, Zhang and Piersma2013). Results from these studies provide a foundation for designing conservation measures, some of which have been put into practice; these protective measures include the establishment of protected areas and the designation of sites as “wetlands of international importance” (Chen et al. Reference Chen2006). However, threats to shorebirds are numerous and may increase in future (MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012, Ma et al. Reference Ma, Melville, Liu, Chen, Yang, Ren, Zhang, Piersma and Li2014, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014). To counter these threats and to reverse the decline of shorebird populations, ornithologists and conservationists must work together to clarify the problem for the public and for policy-makers and to provide solid evidence in support of effective conservation actions. Besides the six key issues mentioned in this paper, some examples in other flyway can be helpful (e.g. Davidson et al. Reference Davidson, Stroud, Rothwell and Pienkowski1998, Bart et al. Reference Bart, Andres, Brown, Donaldson, Harrington, Johnston, Jones, Morrison, Skagen, Ralph and Rich2005, Sutherland et al. Reference Sutherland, Alves, Amano, Chang, Davidson, Finlayson, Gill, Gill, González, Gunnarsson, Kleijn, Spray, Székely and Thompson2012).
In addition to providing stopping sites for millions of shorebirds in the EAAF, the intertidal wetlands in the Yellow Sea support a rich, internationally shared biodiversity and provide enormous ecosystem services (MacKinnon et al. Reference MacKinnon, Verkuil and Murray2012, Murray et al. Reference Murray, Clemens, Phinn, Possingham and Fuller2014). Conservation of intertidal habitats will not only benefit shorebirds but will also benefit society as a whole by ensuring that intertidal wetlands provide the valuable ecosystem services required for sustainable development.
Acknowledgements
This paper owes a great debt to the late Mark Barter for his pioneering work on the importance of the Yellow Sea for migratory shorebirds. This work was financially supported by the National Basic Research Program of China (2013CB430404) and the National Natural Science Foundation of China (30670269, 31071939 and 31272334). We thank Theunis Piersma and David S. Melville for enlightening discussions and three anonymous referees for their constructive comments on an earlier version.





